Routine Surveillance of SARS-CoV-2 Serostatus in Pediatrics Allows Monitoring of Humoral Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Antibody Procedures and Analyses
2.3. Clinical Data
2.4. Statistics
3. Results
3.1. Participants
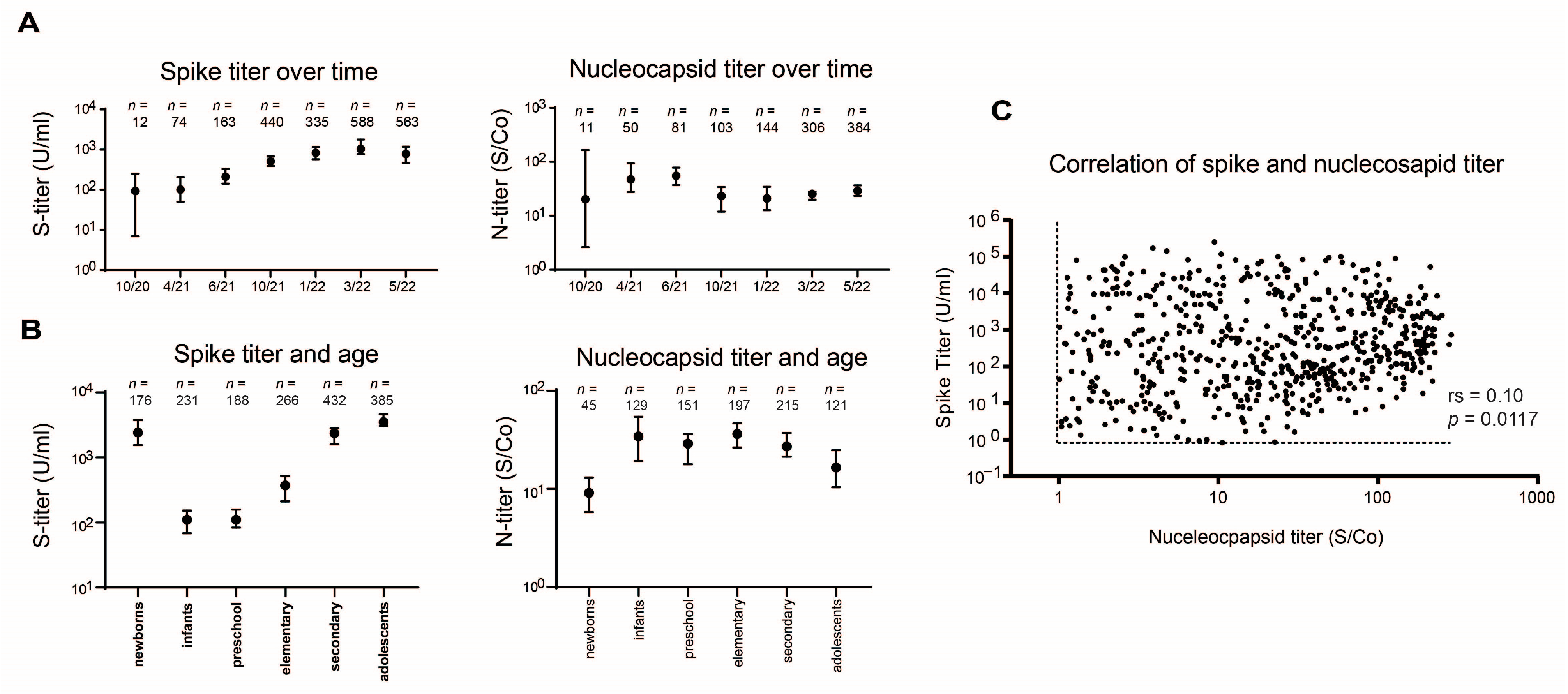
3.2. Seroprevalence Monitoring
3.3. Antibody Levels Monitoring
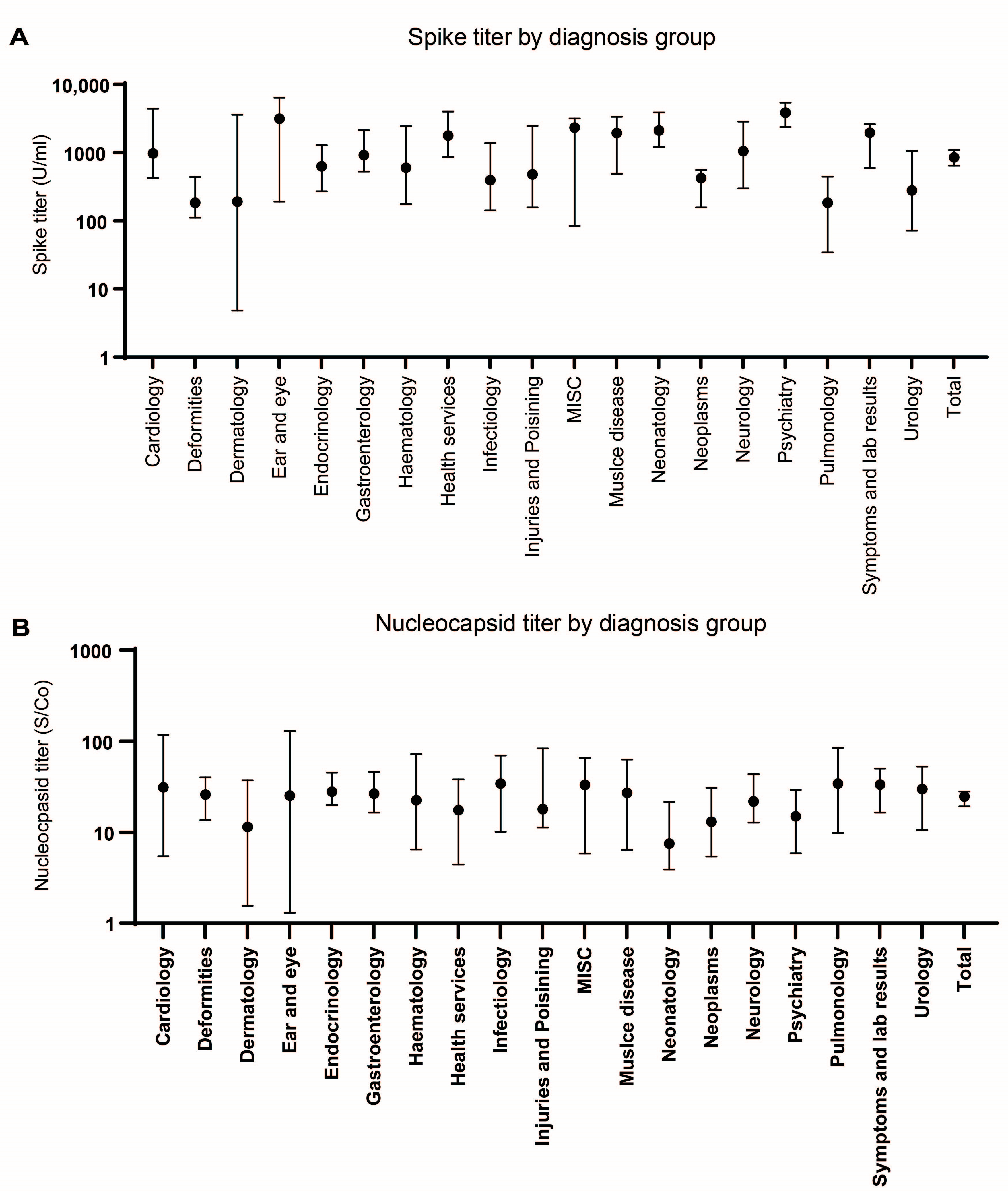
3.4. Seroprevalence According to Diagnostic Groups
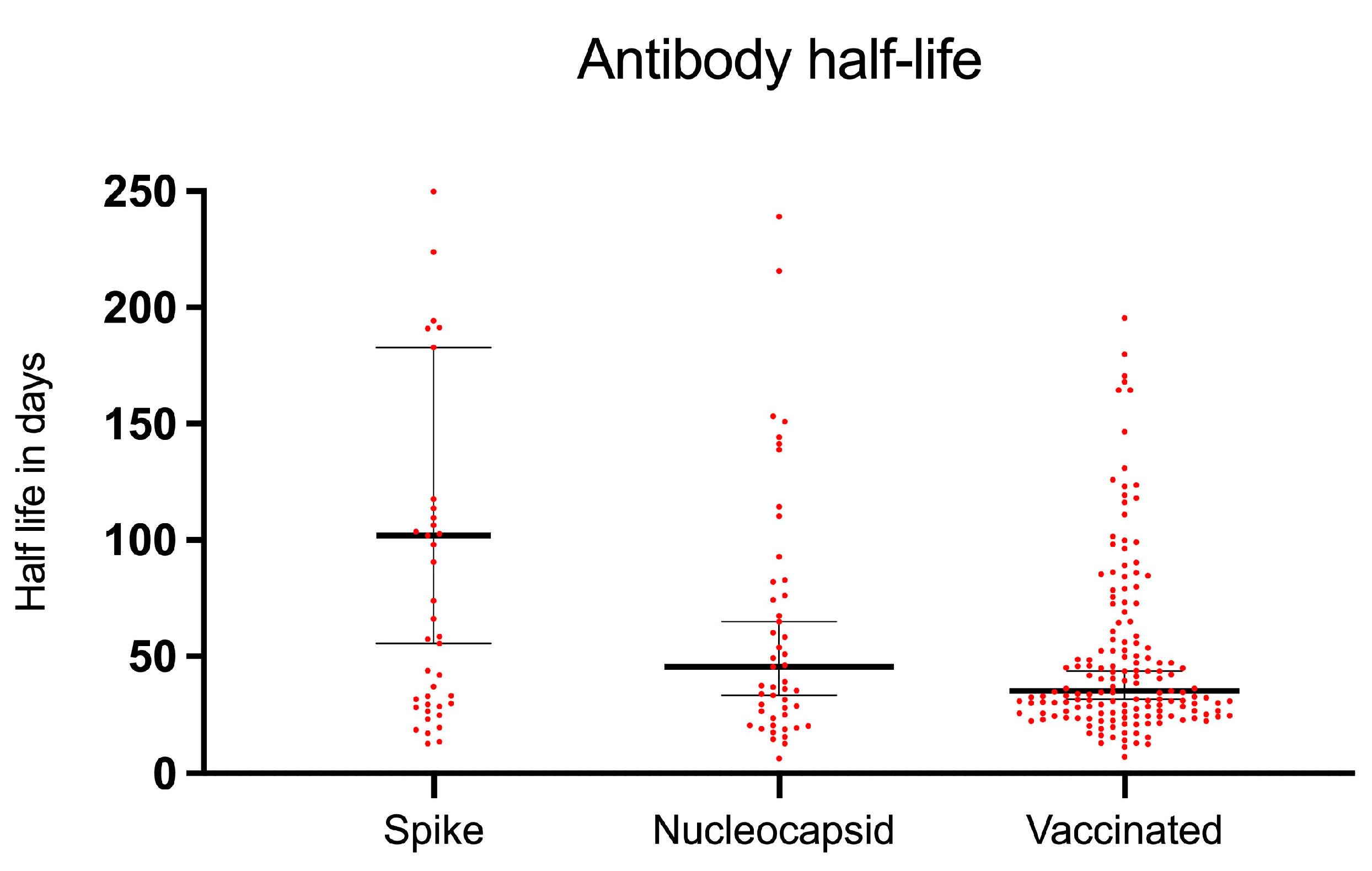
3.5. SARS-CoV-2 Antibody Decay
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arsenault, C.; Gage, A.; Kim, M.K.; Kapoor, N.R.; Akweongo, P.; Amponsah, F.; Aryal, A.; Asai, D.; Awoonor-Williams, J.K.; Ayele, W.; et al. COVID-19 and resilience of healthcare systems in ten countries. Nat. Med. 2022, 28, 1314–1324. [Google Scholar] [CrossRef]
- Pellegrino, R.; Chiappini, E.; Licari, A.; Galli, L.; Marseglia, G.L. Prevalence and clinical presentation of long COVID in children: A systematic review. Eur. J. Pediatr. 2022, 181, 3995–4009. [Google Scholar] [CrossRef] [PubMed]
- Heiss, R.; Tan, L.; Schmidt, S.; Regensburger, A.P.; Ewert, F.; Mammadova, D.; Buehler, A.; Vogel-Claussen, J.; Voskrebenzev, A.; Rauh, M.; et al. Pulmonary Dysfunction after Pediatric COVID-19. Radiology 2023, 306, e221250. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO COVID-19 Dashboard. Available online: https://covid19.who.int (accessed on 19 July 2023).
- O’Brien, S.C.; Cole, L.D.; Albanese, B.A.; Mahon, A.; Knight, V.; Williams, N.; Severson, R.; Burakoff, A.; Alden, N.B.; Dominguez, S.R. SARS-CoV-2 Seroprevalence Compared with Confirmed COVID-19 Cases among Children, Colorado, USA, May–July 2021. Emerg. Infect. Dis. 2023, 29, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Colman, E.; Puspitarani, G.A.; Enright, J.; Kao, R.R. Ascertainment rate of SARS-CoV-2 infections from healthcare and community testing in the UK. J. Theor. Biol. 2023, 558, 111333. [Google Scholar] [CrossRef] [PubMed]
- Hippich, M.; Sifft, P.; Zapardiel-Gonzalo, J.; Bohmer, M.M.; Lampasona, V.; Bonifacio, E.; Ziegler, A.G. A public health antibody screening indicates a marked increase of SARS-CoV-2 exposure rate in children during the second wave. Med 2021, 2, 571–572. [Google Scholar] [CrossRef]
- Wachter, F.; Regensburger, A.P.; Antonia Sophia, P.; Knieling, F.; Wagner, A.L.; Simon, D.; Hoerning, A.; Woelfle, J.; Uberla, K.; Neubert, A.; et al. Continuous monitoring of SARS-CoV-2 seroprevalence in children using residual blood samples from routine clinical chemistry. Clin. Chem. Lab. Med. 2022, 60, 941–951. [Google Scholar] [CrossRef]
- Offergeld, R.; Preußel, K.; Zeiler, T.; Aurich, K.; Baumann-Baretti, B.I.; Ciesek, S.; Corman, V.M.; Dienst, V.; Drosten, C.; Görg, S.; et al. Monitoring the SARS-CoV-2 Pandemic: Prevalence of Antibodies in a Large, Repetitive Cross-Sectional Study of Blood Donors in Germany—Results from the SeBluCo Study 2020–2022. Pathogens 2023, 12, 551. [Google Scholar] [CrossRef]
- Are, E.B.; Song, Y.; Stockdale, J.E.; Tupper, P.; Colijn, C. COVID-19 endgame: From pandemic to endemic? Vaccination, reopening and evolution in low- and high-vaccinated populations. J. Theor. Biol. 2023, 559, 111368. [Google Scholar] [CrossRef]
- Contreras, S.; Iftekhar, E.N.; Priesemann, V. From emergency response to long-term management: The many faces of the endemic state of COVID-19. Lancet Reg. Health Eur. 2023, 30, 100664. [Google Scholar] [CrossRef]
- Adams, K.; Tastad, K.J.; Huang, S.; Ujamaa, D.; Kniss, K.; Cummings, C.; Reingold, A.; Roland, J.; Austin, E.; Kawasaki, B.; et al. Prevalence of SARS-CoV-2 and Influenza Coinfection and Clinical Characteristics Among Children and Adolescents Aged <18 Years Who Were Hospitalized or Died with Influenza—United States, 2021–2022 Influenza Season. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- The Federal Ministry of Health. Current Vaccination Status. Available online: https://impfdashboard.de (accessed on 19 July 2023).
- Leone, V.; Meisinger, C.; Temizel, S.; Kling, E.; Gerstlauer, M.; Fruhwald, M.C.; Burkhardt, K. Longitudinal change in SARS-CoV-2 seroprevalence in 3- to 16-year-old children: The Augsburg Plus study. PLoS ONE 2022, 17, e0272874. [Google Scholar] [CrossRef]
- Tsang, H.W.; Chua, G.T.; To, K.K.W.; Wong, J.S.C.; Tu, W.; Kwok, J.S.Y.; Wong, W.H.S.; Wang, X.; Zhang, Y.; Rosa Duque, J.S.; et al. Assessment of SARS-CoV-2 Immunity in Convalescent Children and Adolescents. Front. Immunol. 2021, 12, 797919. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Nyhoff, L.E.; Zarnitsyna, V.I.; Moreno, A.; Manning, K.; Linderman, S.; Burrell, A.R.; Stephens, K.; Norwood, C.; Mantus, G.; et al. Infants and young children generate more durable antibody responses to SARS-CoV-2 infection than adults. medRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Engels, G.; Hecker, K.; Forster, J.; Toepfner, N.; Hick, E.; Pietsch, F.; Heuschmann, P.; Berner, R.; Hartel, C.; Kurzai, O.; et al. High Seroprevalence of SARS-CoV-2 in Preschool Children in July 2022. Dtsch. Arztebl. Int. 2022, 119, 771–772. [Google Scholar] [CrossRef] [PubMed]
- Ott, R.; Achenbach, P.; Ewald, D.A.; Friedl, N.; Gemulla, G.; Hubmann, M.; Kordonouri, O.; Loff, A.; Marquardt, E.; Sifft, P.; et al. SARS-CoV-2 Seroprevalence in Preschool and School-Age Children-Population Screening Findings from January 2020 to June 2022. Dtsch. Arztebl. Int. 2022, 119, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, R.; Sepidarkish, M.; Mollalo, A.; Parsa, H.; Mahjour, S.; Safarpour, F.; Almukhtar, M.; Mechaal, A.; Chemaitelly, H.; Sartip, B.; et al. SARS-CoV-2 seroprevalence in children worldwide: A systematic review and meta-analysis. EClinicalMedicine 2023, 56, 101786. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, A.K.; Juno, J.A.; Wang, J.J.; Selva, K.J.; Reynaldi, A.; Tan, H.X.; Lee, W.S.; Wragg, K.M.; Kelly, H.G.; Esterbauer, R.; et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat. Commun. 2021, 12, 1162. [Google Scholar] [CrossRef]
- Mantus, G.; Nyhoff, L.E.; Edara, V.V.; Zarnitsyna, V.I.; Ciric, C.R.; Flowers, M.W.; Norwood, C.; Ellis, M.; Hussaini, L.; Manning, K.E.; et al. Pre-existing SARS-CoV-2 immunity influences potency, breadth, and durability of the humoral response to SARS-CoV-2 vaccination. Cell Rep. Med. 2022, 3, 100603. [Google Scholar] [CrossRef]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef]
- Loesche, M.; Karlson, E.W.; Talabi, O.; Zhou, G.; Boutin, N.; Atchley, R.; Loevinsohn, G.; Chang, J.B.P.; Hasdianda, M.A.; Okenla, A.; et al. Longitudinal SARS-CoV-2 Nucleocapsid Antibody Kinetics, Seroreversion, and Implications for Seroepidemiologic Studies. Emerg. Infect. Dis. 2022, 28, 1859–1862. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.M.; Dodd, R.Y.; Crowder, L.A.; Notari, E.P.; Stramer, S.L. Trajectory and Demographic Correlates of Antibodies to SARS-CoV-2 Nucleocapsid in Recently Infected Blood Donors, United States. Emerg. Infect. Dis. 2023, 29, 1323–1329. [Google Scholar] [CrossRef] [PubMed]

| All Periods | M1 | M2 | M3 | M4 | |
|---|---|---|---|---|---|
| Samples (n) | 3235 | 892 | 848 | 834 | 661 |
| Male (%) | 1683 (52%) | 466 (52%) | 453 (52%) | 433 (52%) | 331 (50%) |
| Female (%) | 1552 (48%) | 426 (48%) | 395 (47%) | 401 (48%) | 330 (50%) |
| Age in years mean (SD 1) | 8.12 (5.79) | 7.91 (5.79) | 8.24 (5.88) | 8.32 (5.82) | 7.96 (5.62) |
| Age in years median (IQR 2) | 7.88 (2.7–13.5) | 7.72 (2.4–13.0) | 8.29 (2.6–13.7) | 8.09 (2.8–13.8) | 7.55 (2.8–13.1) |
| Sample Period | Groups | Total n | S+ n | N+ n | Prevalence S+ | p-Value * | Prevalence N+ | p-Value * |
|---|---|---|---|---|---|---|---|---|
| Total | All | 3235 | 1926 | 937 | 59.54% | 28.96% | ||
| Male | 1683 | 978 | 504 | 58.11% 1 | 0.0920 1 | 29.95% | 0.2008 | |
| Female | 1552 | 948 | 433 | 61.08% 1 | 27.90% | |||
| Newborns | 263 | 176 | 45 | 66.92% 4 | 0.0106 4 | 17.11% | 0.0001 | |
| 0–2 | 595 | 261 | 138 | 43.87% 4 | 0.0001 | 23.19% | 0.0005 | |
| 3–5 | 477 | 225 | 166 | 47.17% 4 | 0.0001 | 34.80% | 0.0026 | |
| 6–9 | 596 | 307 | 216 | 51.51% 4 | 0.0001 | 36.24% | 0.0001 | |
| 10–14 | 768 | 503 | 237 | 65.49% 4 | 0.0001 | 30.86% | 0.1868 | |
| 15–17 | 536 | 454 | 135 | 84.70% 4 | 0.0001 | 25.19% | 0.0370 | |
| Nov. 2021 | All | 892 | 335 | 103 | 37.56% 2 | 0.0001 2 | 11.55% | 0.0012 |
| Male | 466 | 173 | 62 | 37.12% | 0.7824 | 13.30% | 0.0937 | |
| Female | 426 | 162 | 41 | 38.03% | 9.62% | |||
| Newborns | 99 | 49 | 6 | 49.49% | 0.0112 | 6.06% | 0.0931 | |
| 0–2 | 142 | 42 | 18 | 29.58% | 0.0374 | 12.68% | 0.6675 | |
| 3–5 | 138 | 36 | 19 | 26.09% | 0.0022 | 13.77% | 0.3849 | |
| 6–9 | 168 | 32 | 21 | 19.05% | 0.0001 | 12.50% | 0.6878 | |
| 10–14 | 203 | 73 | 21 | 35.96% | 0.6214 | 10.34% | 0.6178 | |
| 15–17 | 142 | 103 | 18 | 72.54% | 0.0001 | 12.68% | 0.6675 | |
| Jan. 2022 | All | 848 | 440 | 144 | 51.89% 2,3 | 0.0001 3 | 16.98% | 0.0001 |
| Male | 453 | 222 | 86 | 49.01% | 0.0738 | 18.98% | 0.0997 | |
| Female | 395 | 218 | 58 | 55.19% | 14.68% | |||
| Newborns | 75 | 43 | 9 | 57.33% | 0.3355 | 12.00% | 0.2624 | |
| 0–2 | 151 | 49 | 19 | 32.45% | 0.0001 | 12.58% | 0.1211 | |
| 3–5 | 117 | 38 | 22 | 32.48% | 0.0001 | 18.80% | 0.5958 | |
| 6–9 | 148 | 62 | 37 | 41.89% | 0.0086 | 25.00% | 0.0056 | |
| 10–14 | 208 | 122 | 31 | 58.65% | 0.0255 | 14.90% | 0.3960 | |
| 15–17 | 149 | 126 | 26 | 84.56% | 0.0001 | 17.45% | 0.9044 | |
| Mar. 2022 | All | 834 | 588 | 306 | 70.50% 3 | 0.0001 | 36.69% | 0.0001 |
| Male | 433 | 308 | 167 | 71.13% | 0.7043 | 38.57% | 0.2506 | |
| Female | 401 | 280 | 139 | 69.83% | 34.66% | |||
| Newborns | 48 | 44 | 14 | 91.67% | 0.0005 | 29.17% | 0.2848 | |
| 0–2 | 173 | 84 | 41 | 48.55% | 0.0001 | 23.70% | 0.0001 | |
| 3–5 | 115 | 62 | 50 | 53.91% | 0.0001 | 43.48% | 0.1180 | |
| 6–9 | 148 | 100 | 69 | 67.57% | 0.4266 | 46.62% | 0.0064 | |
| 10–14 | 198 | 160 | 84 | 80.81% | 0.0002 | 42.42% | 0.0631 | |
| 15–17 | 152 | 138 | 48 | 90.79% | 0.0001 | 31.58% | 0.1631 | |
| May 2022 | All | 661 | 563 | 384 | 85.17% | 58.09% | ||
| Male | 331 | 275 | 189 | 83.08% | 0.1546 | 57.10% | 0.6364 | |
| Female | 330 | 288 | 195 | 87.27% | 59.09% | |||
| Newborns | 41 | 40 | 16 | 97.56% | 0.0205 | 39.02% | 0.0137 | |
| 0–2 | 129 | 86 | 60 | 66.67% | 0.0001 | 46.51% | 0.0038 | |
| 3–5 | 107 | 89 | 75 | 83.18% | 0.5523 | 70.09% | 0.0073 | |
| 6–9 | 132 | 113 | 89 | 85.61% | 1.00000 | 67.42% | 0.0178 | |
| 10–14 | 159 | 148 | 101 | 93.08% | 0.0008 | 63.52% | 0.1177 | |
| 15–17 | 93 | 87 | 43 | 93.55% | 0.01160 | 46.24% | 0.0169 |
| Diagnosis Group | Prevalence S | p-Value * | Prevalence N | p-Value * | n | Median Age |
|---|---|---|---|---|---|---|
| Cardiology | 64.7% | 0.8609 | 31.4% | 0.7295 | 51 | 12.3 |
| Deformities | 56.4% | 0.4160 | 27.5% | 0.5059 | 273 | 2.0 |
| Dermatology | 42.9% | 0.4101 | 32.1% | 0.5266 | 28 | 5.9 |
| Ear and eye | 56.5% | 0.3466 | 26.1% | 0.4823 | 23 | 11.8 |
| Endocrinology | 59.3% | 0.4058 | 36.9% | 0.9156 | 290 | 9.2 |
| Gastroenterology | 68.8% | 0.8039 | 33.0% | 0.8068 | 176 | 11.6 |
| Hematology | 55.8% | 0.2244 | 28.3% | 0.5083 | 120 | 7.2 |
| Health services | 61.6% | 0.3857 | 14.1% | 0.5283 | 99 | 0.0 |
| Infectiology | 52.9% | 1.000 | 34.5% | 0.4043 | 87 | 5.8 |
| Injuries/poisoning | 59.7% | 0.3847 | 34.7% | 0.4609 | 72 | 6.0 |
| MISC | 100.0% | 0.2482 | 100.0% | 0.0657 | 3 | 5.3 |
| Muscle disease | 67.6% | 0.2840 | 28.4% | 0.8784 | 74 | 14.1 |
| Neonatology | 70.3% | 0.5067 | 17.2% | 1.000 | 145 | 0.0 |
| Neoplasms | 74.8% | 0.0001 | 42.1% | 0.2498 | 107 | 7.6 |
| Neurology | 58.8% | 0.5274 | 38.6% | 0.8973 | 114 | 9.1 |
| Psychiatry | 78.4% | 0.4834 | 33.3% | 0.2934 | 111 | 14.4 |
| Pulmonology | 39.7% | 0.2297 | 24.4% | 0.1871 | 131 | 2.7 |
| Symptoms/lab results | 58.9% | 0.0682 | 26.0% | 0.7186 | 192 | 11.3 |
| Urology | 54.5% | 0.5379 | 29.1% | 0.3556 | 110 | 8.4 |
| Total | 60.6% | 0.0035 | 30.1% | 0.5229 | 2206 | 7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wachter, F.; Knieling, F.; Raming, R.; Simon, D.; Woelfle, J.; Hoerning, A.; Neubert, A.; Rauh, M.; Regensburger, A.P. Routine Surveillance of SARS-CoV-2 Serostatus in Pediatrics Allows Monitoring of Humoral Response. Microorganisms 2023, 11, 2919. https://doi.org/10.3390/microorganisms11122919
Wachter F, Knieling F, Raming R, Simon D, Woelfle J, Hoerning A, Neubert A, Rauh M, Regensburger AP. Routine Surveillance of SARS-CoV-2 Serostatus in Pediatrics Allows Monitoring of Humoral Response. Microorganisms. 2023; 11(12):2919. https://doi.org/10.3390/microorganisms11122919
Chicago/Turabian StyleWachter, Felix, Ferdinand Knieling, Roman Raming, David Simon, Joachim Woelfle, André Hoerning, Antje Neubert, Manfred Rauh, and Adrian P. Regensburger. 2023. "Routine Surveillance of SARS-CoV-2 Serostatus in Pediatrics Allows Monitoring of Humoral Response" Microorganisms 11, no. 12: 2919. https://doi.org/10.3390/microorganisms11122919
APA StyleWachter, F., Knieling, F., Raming, R., Simon, D., Woelfle, J., Hoerning, A., Neubert, A., Rauh, M., & Regensburger, A. P. (2023). Routine Surveillance of SARS-CoV-2 Serostatus in Pediatrics Allows Monitoring of Humoral Response. Microorganisms, 11(12), 2919. https://doi.org/10.3390/microorganisms11122919





