Adenoviral Vector Codifying for TNF as a Co-Adjuvant Therapy against Multi-Drug-Resistant Tuberculosis
Abstract
:1. Introduction
2. Material and Methods
2.1. Adenoviral Vectors and Determination of Dose
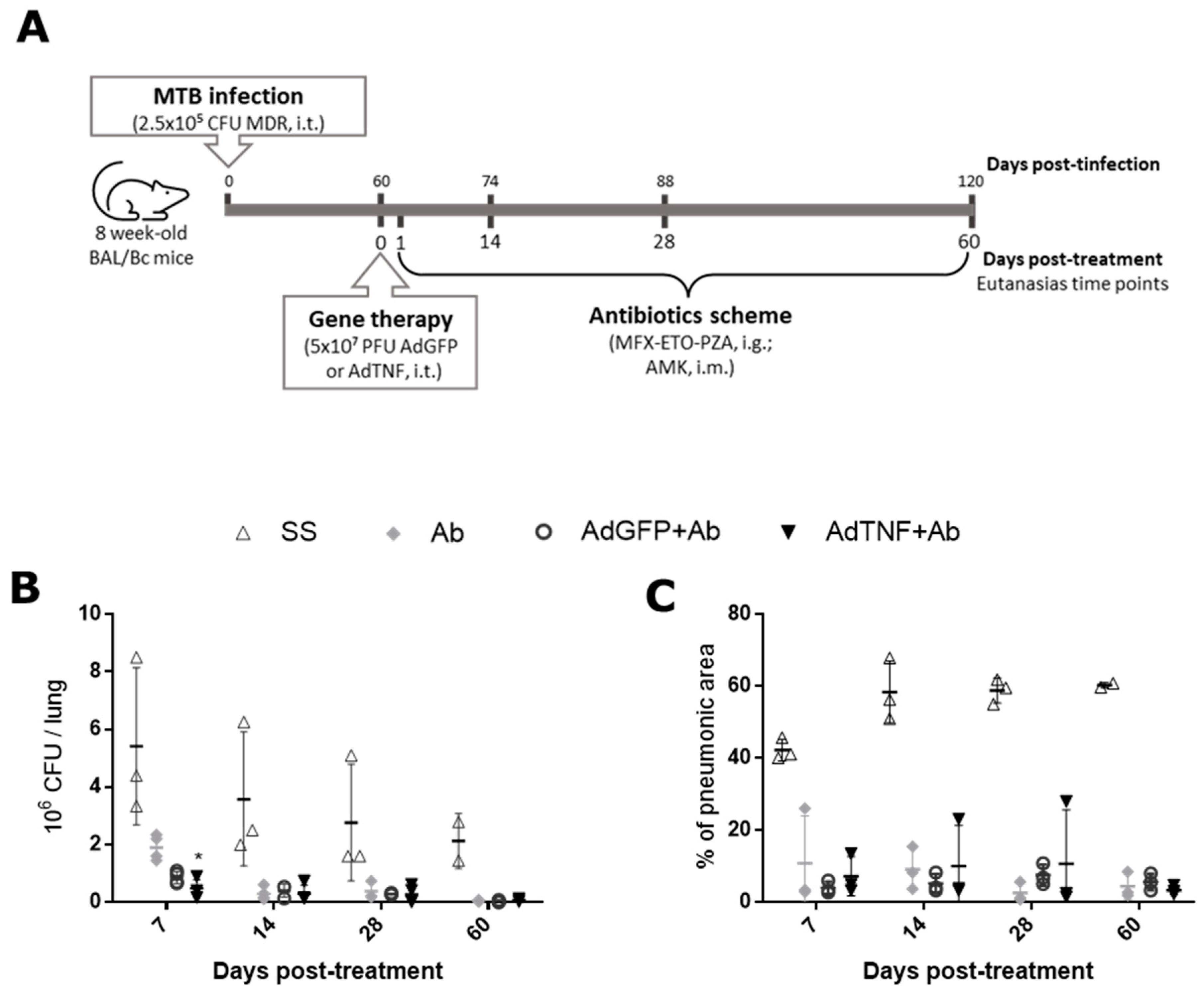
2.2. Experimental Model of Progressive Pulmonary TB and Gene Therapy Administration
2.3. Bacterial Load Quantification in Lungs
2.4. Quantitative Polymerase Chain Reaction (qPCR)
2.5. Histologic Analysis of Pneumonia, Granuloma and Immunohistochemistry
2.6. Statistical Analysis
2.7. Ethical Approval
3. Results
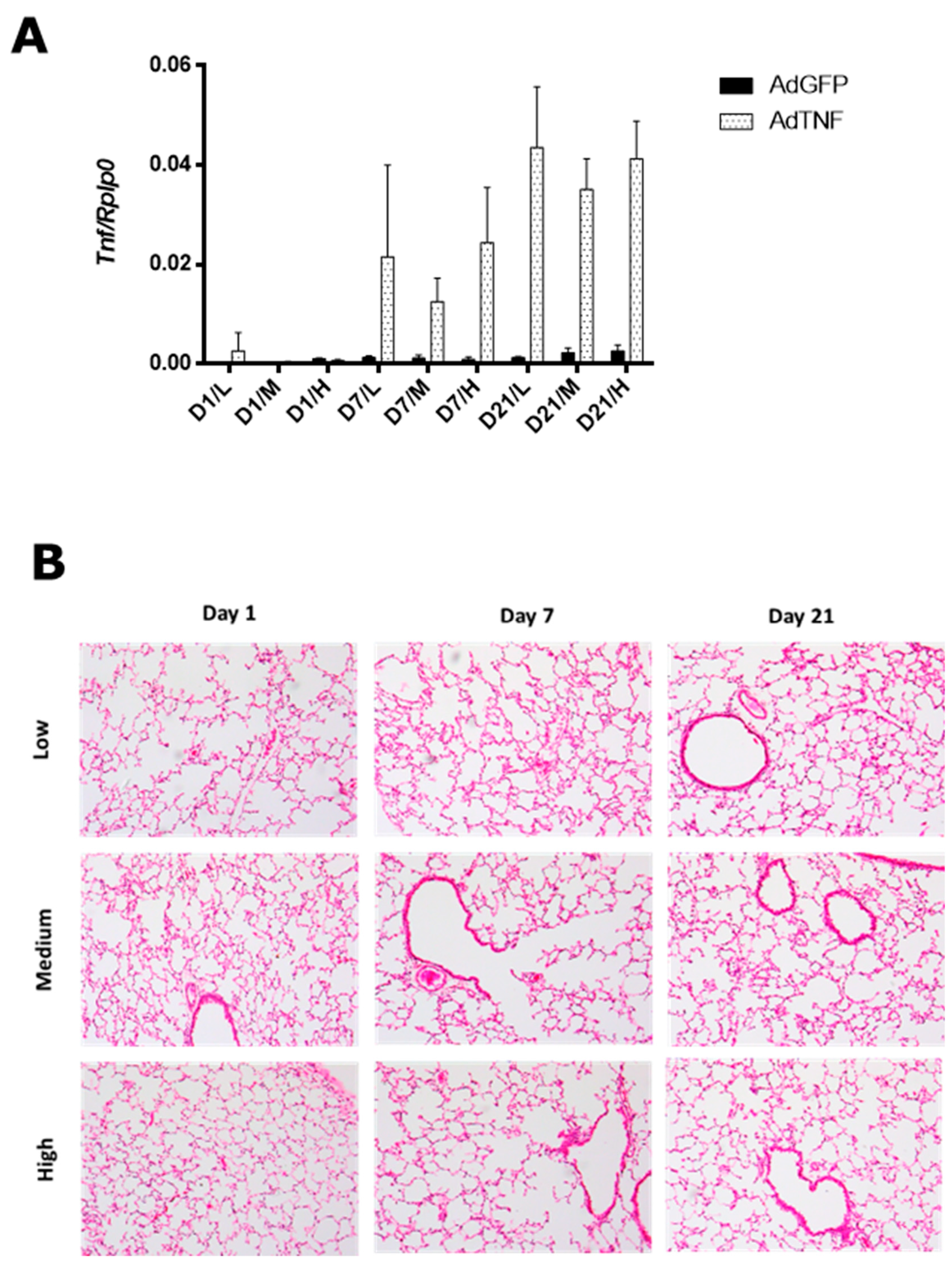
3.1. AdTNF Dose Determination for Gene Therapy
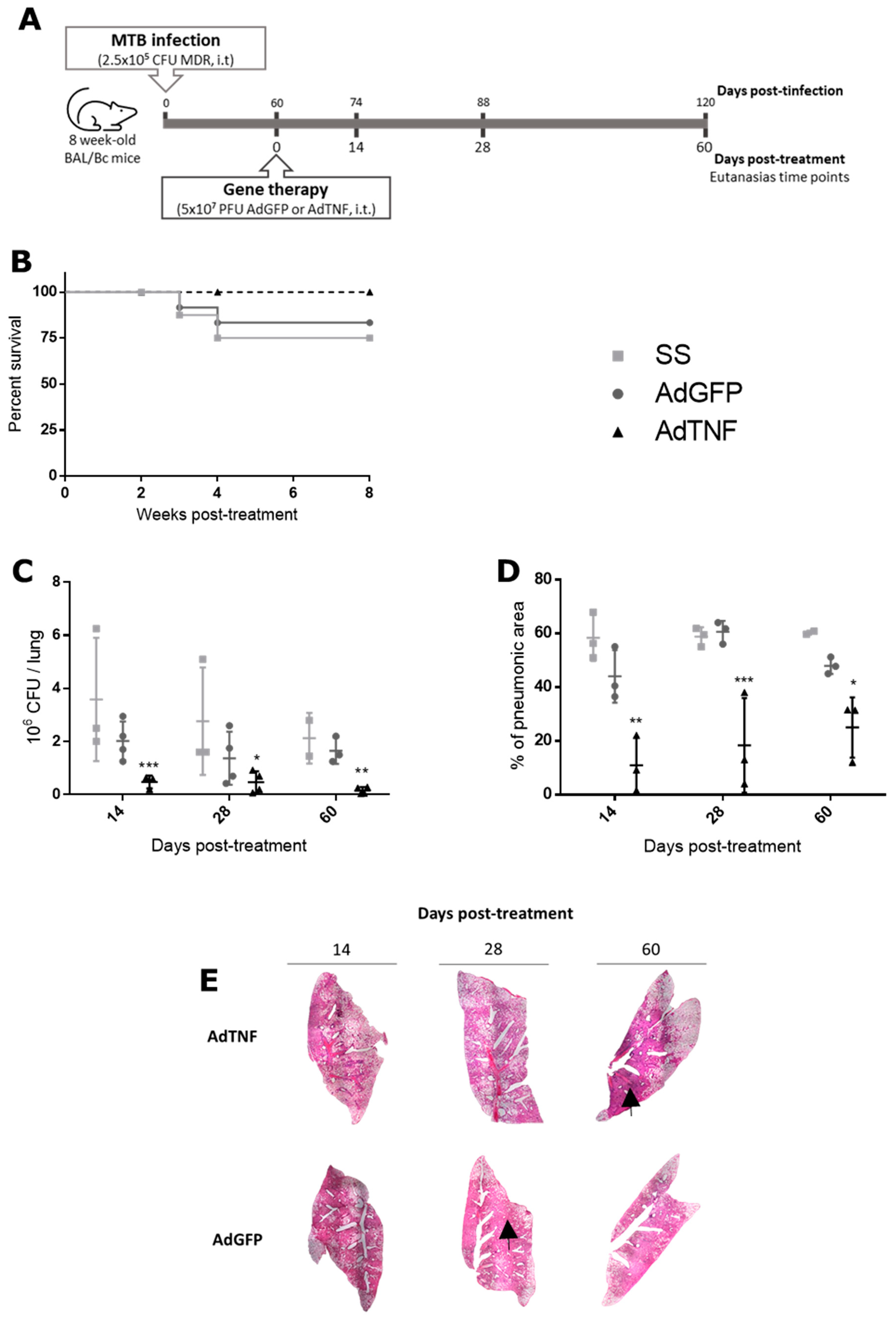
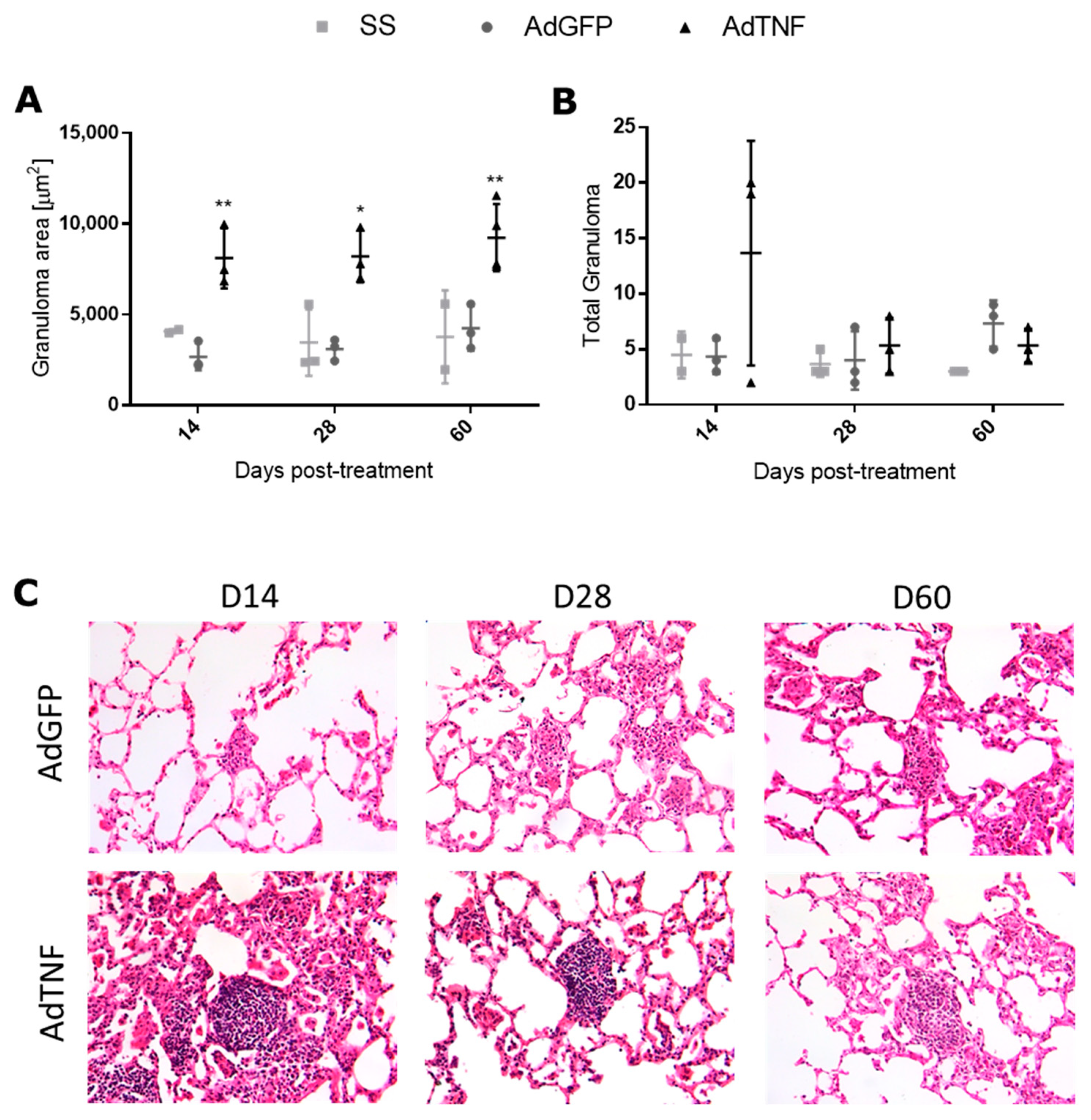
3.2. Therapeutic Effect of AdTNF Administration in a Chronic Phase of a Murine Model of Progressive MDR-TB
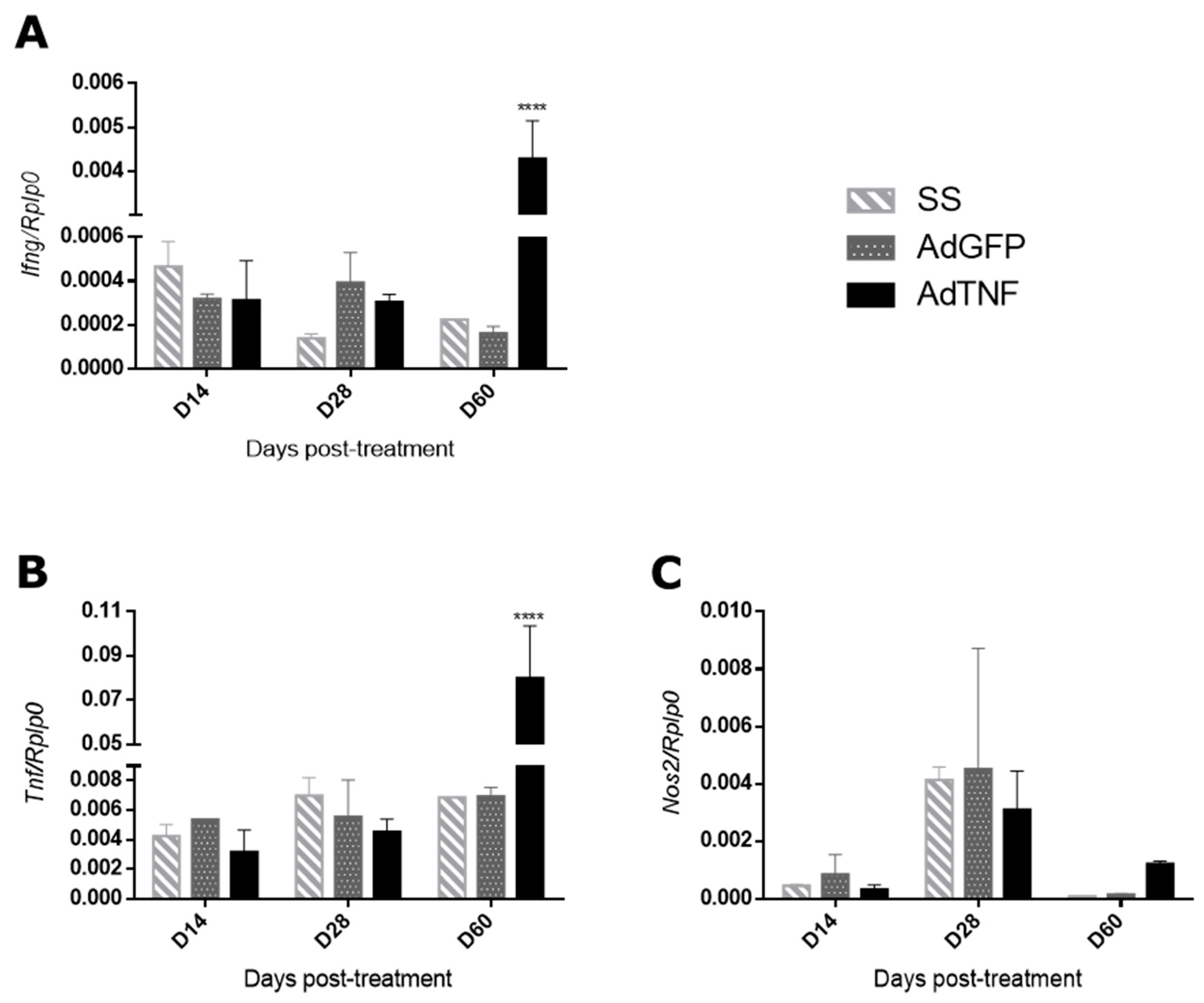
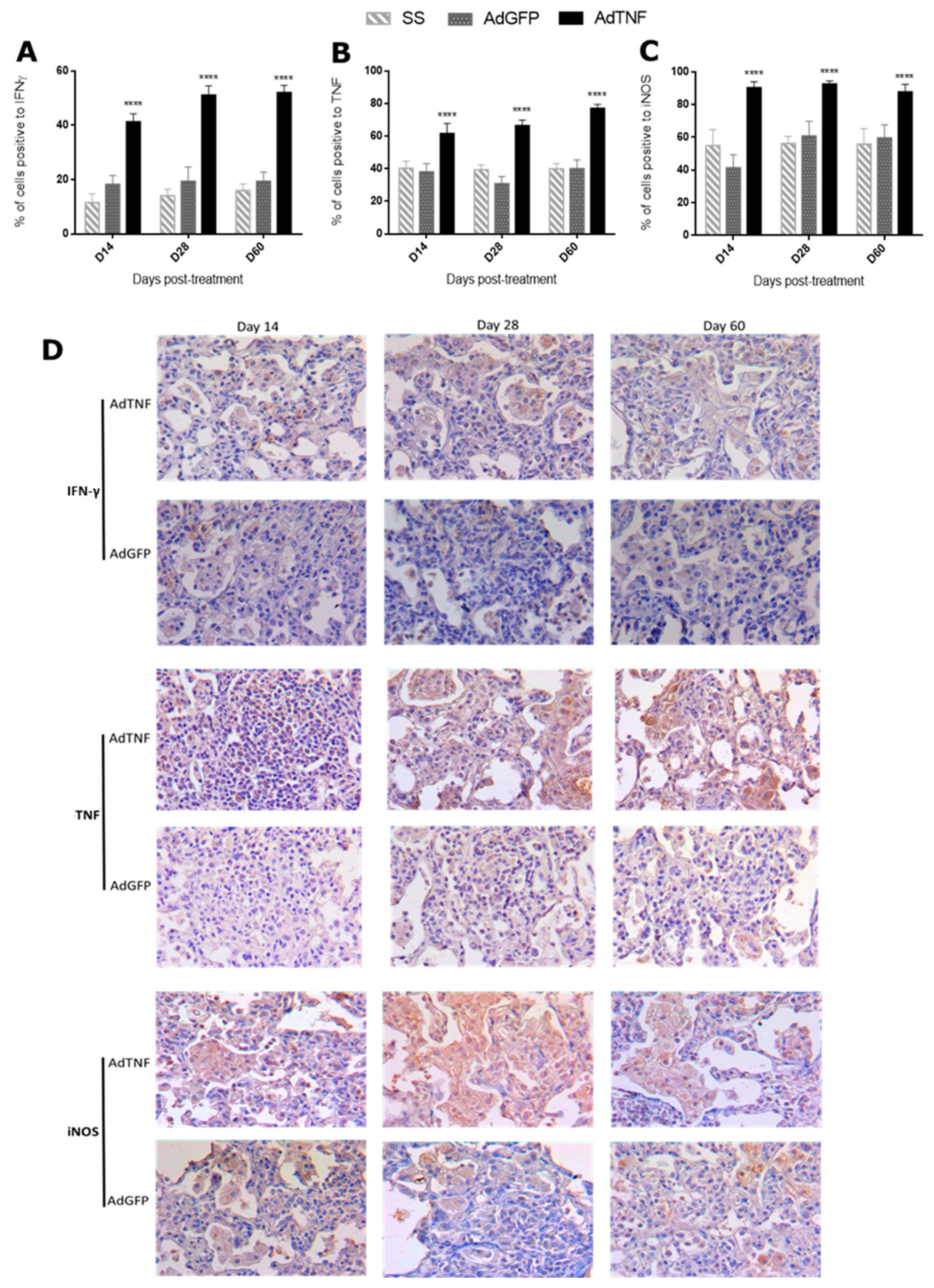
3.3. Immune Regulation by AdTNF Treatment in a Murine Model of Progressive MDR-TB
3.4. The Effect of AdTNF and Second-Line Antibiotics Administration in a Murine Model of Progressive MDR-TB
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2022; ISBN 9788527729833. [Google Scholar]
- Suárez, I.; Fünger, S.M.; Rademacher, J.; Fätkenheuer, G.; Kröger, S.; Rybniker, J. The Diagnosis and Treatment of Tuberculosis. Dtsch. Arztebl. Int. 2019, 116, 729–735. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P.R. The Immune Response in Tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.Y.; Huang, Y.S.; Lin, H.H.; Luo, S.F.; McCann, F.; McNamee, K.; Clanchy, F.; Williams, R. TNFR Signalling and Its Clinical Implications. Cytokine 2018, 101, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Fotin-Mleczek, M.; Henkler, F.; Samel, D.; Reichwein, M.; Hausser, A.; Parmryd, I.; Scheurich, P.; Schmid, J.A.; Wajant, H. Apoptotic Crosstalk of TNF Receptors: TNF-R2-Induces Depletion of TRAF2 and IAP Proteins and Accelerates TNF-R1-Dependent Activation of Caspase-8. J. Cell Sci. 2002, 115, 2757–2770. [Google Scholar] [CrossRef]
- Siegmund, D.; Kums, J.; Ehrenschwender, M.; Wajant, H. Activation of TNFR2 Sensitizes Macrophages for TNFR1-Mediated Necroptosis. Cell Death Dis. 2016, 7, e2375. [Google Scholar] [CrossRef] [PubMed]
- Kalliolias, G.D.; Ivashkiv, L.B.; Program, T.D. TNF Biology, Pathogenic Mechanisms and Emerging Therapeutic Strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, K.P.; Ganguli, G.; Naik, S.K.; Sonawane, A. Mycobacterium tuberculosis EsxL Induces TNF-α Secretion through Activation of TLR2 Dependent MAPK and NF-ΚB Pathways. Mol. Immunol. 2021, 130, 133–141. [Google Scholar] [CrossRef]
- Flynn, J.L.; Goldstein, M.M.; Chan, J.; Triebold, K.J.; Pfeffersps, K.; Lowensteln, C.J.; Schreiber, R. Tumor Necrosis Factor-Alfa Is Required in the Protective Immune Response Against Mycobacterium tuberculosis in Mice. Immunity 1995, 2, 561–572. [Google Scholar] [CrossRef]
- Keane, J.; Gershon, S.; Wise, R.P.; Mirabile-Levens, E.; Kasznica, J.; Schwieterman, W.D.; Siegel, J.N.; Braun, M.M. Tuberculosis Associated with Infliximab, a Tumor Necrosis Factor Alpha-Neutralizing Agent. N. Engl. J. Med. 2001, 345, 1098–1104. [Google Scholar] [CrossRef]
- Harris, J.; Keane, J. How Tumour Necrosis Factor Blockers Interfere with Tuberculosis Immunity. Clin. Exp. Immunol. 2010, 161, 1–9. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, E.H.; Lee, M.; Jung, I.; Ahn, S.S. Risk of Cancer, Tuberculosis and Serious Infections in Patients with Ankylosing Spondylitis, Psoriatic Arthritis, and Psoriasis Treated with IL-17 and TNF-α Inhibitors: A Nationwide Nested Case-Control Analysis. Clin. Exp. Rheumatol. 2023, 41, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Olivier, M.; Gros, P.; Barrera, L.F.; García, L.F. TNF-α and IL-10 Modulate the Induction of Apoptosis by Virulent Mycobacterium tuberculosis in Murine Macrophages. J. Immunol. 1999, 162, 6122–6131. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Espinosa, O.; Hernández-Bazán, S.; Francisco-Cruz, A.; Mata-Espinosa, D.; Barrios-Payán, J.; Marquina-Castillo, B.; López-Casillas, F.; Carretero, M.; del Río, M.; Hernández-Pando, R. Gene Therapy Based in Antimicrobial Peptides and Proinflammatory Cytokine Prevents Reactivation of Experimental Latent Tuberculosis. Pathog. Dis. 2016, 74, ftw075. [Google Scholar] [CrossRef] [PubMed]
- Mata-Espinosa, D.A.; Mendoza-Rodríguez, V.; Aguilar-León, D.; Rosales, R.; López-Casillas, F.; Hernández-Pando, R. Therapeutic Effect of Recombinant Adenovirus Encoding Interferon-Gamma in a Murine Model of Progressive Pulmonary Tuberculosis. Mol. Ther. 2008, 16, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bazán, S.; Mata-Espinosa, D.; Lozano-Ordaz, V.; Ramos-Espinosa, O.; Barrios-Payán, J.; López-Casillas, F.; Pando, R.H. Immune Regulatory Effect of Osteopontin Gene Therapy in a Murine Model of Multidrug Resistant Pulmonary Tuberculosis. Hum. Gene Ther. 2022, 33, 1037–1051. [Google Scholar] [CrossRef]
- Hernandez-Pando, R.; Orozco, H.; Honour, J.; Silva, P.; Leyva, R.; Rook, G.A.W. Adrenal Changes in Murine Pulmonary Tuberculosis; a Clue to Pathogenesis? FEMS Immunol. Med. Microbiol. 1995, 12, 63–72. [Google Scholar] [CrossRef]
- Douglas, J.T. Adenoviral Vectors for Gene Therapy. Mol. Biotechnol. 2007, 36, 71–80. [Google Scholar] [CrossRef]
- Cooper, A.M.; Mayer-Barber, K.D.; Sher, A. Role of Innate Cytokines in Mycobacterial Infection. Mucosal Immunol. 2011, 4, 252–260. [Google Scholar] [CrossRef]
- Liebenberg, D.; Gordhan, B.G.; Kana, B.D. Drug Resistant Tuberculosis: Implications for Transmission, Diagnosis, and Disease Management. Front. Cell. Infect. Microbiol. 2022, 12, 943545. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, D. Adenoviral Vector-Based Strategies against Infectious Disease and Cancer. Hum. Vaccines Immunother. 2016, 12, 2064–2074. [Google Scholar] [CrossRef]
- Tatsis, N.; Ertl, H.C.J. Adenoviruses as Vaccine Vectors. Mol. Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A.; Smith, A.J.; Ali, R.R. The Future Looks Brighter After 25 Years of Retinal Gene Therapy. Hum. Gene Ther. 2017, 28, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Ricobaraza, A.; Gonzalez-Aparicio, M.; Mora-Jimenez, L.; Lumbreras, S.; Hernandez-Alcoceba, R. High-Capacity Adenoviral Vectors: Expanding the Scope of Gene Therapy. Int. J. Mol. Sci. 2020, 21, 3643. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.C. Update on Adenovirus and Its Vectors. J. Gen. Virol. 2000, 81, 2573–2604. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An Overview of Signals, Mechanisms and Functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-Gamma (IFN-γ): Exploring Its Implications in Infectious Diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Prim. 2016, 2, 16076. [Google Scholar] [CrossRef]
- Tsao, T.C.Y.; Huang, C.C.; Chiou, W.K.; Yang, P.Y.; Hsieh, M.J.; Tsao, K.C. Levels of Interferon-γ and Interleukin-2 Receptor-α for Bronchoalveolar Lavage Fluid and Serum Were Correlated with Clinical Grade and Treatment of Pulmonary Tuberculosis. Int. J. Tuberc. Lung Dis. 2002, 6, 720–727. [Google Scholar]
- Vankayalapati, R.; Wizel, B.; Weis, S.E.; Klucar, P.; Shams, H.; Samten, B.; Barnes, P.F. Serum Cytokine Concentrations Do Not Parallel Mycobacterium tuberculosis—Induced Cytokine Production in Patients with Tuberculosis. Clin. Infect. Dis. 2003, 36, 24–28. [Google Scholar] [CrossRef]
- Roberts, T.; Beyers, N.; Aguirre, A.; Walzl, G. Immunosuppression during Active Tuberculosis Is Characterized by Decreased Interferon- Gamma Production and CD25 Expression with Elevated Forkhead Box P3, Transforming Growth Factor- Beta, and Interleukin-4 MRNA Levels. J. Infect. Dis. 2007, 195, 870–878. [Google Scholar] [CrossRef]
- Balikó, Z.; Szereday, L.; Szekeres-Bartho, J. Th2 Biased Immune Response in Cases with Active Mycobacterium tuberculosis Infection and Tuberculin Anergy. FEMS Immunol. Med. Microbiol. 1998, 22, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Chanthaphavong, R.S.; Loughran, P.A.; Lee, T.Y.S.; Scott, M.J.; Billiar, T.R. A Role for CGMP in Inducible Nitric-Oxide Synthase (INOS)-Induced Tumor Necrosis Factor (TNF) α-Converting Enzyme (TACE/ADAM17) Activation, Translocation, and TNF Receptor 1 (TNFR1) Shedding in Hepatocytes. J. Biol. Chem. 2012, 287, 35887–35898. [Google Scholar] [CrossRef] [PubMed]
- Burney, S.; Caulfield, J.L.; Niles, J.C.; Wishnok, J.S.; Tannenbaum, S.R. The Chemistry of DNA Damage from Nitric Oxide and Peroxynitrite. Mutat. Res. Mol. Mech. Mutagen. 1999, 424, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Scanga, C.A.; Mohan, V.P.; Tanaka, K.; Alland, D.; Flynn, J.L.; Chan, J. The Inducible Nitric Oxide Synthase Locus Confers Protection against Aerogenic Challenge of Both Clinical and Laboratory Strains of Mycobacterium tuberculosis in Mice. Infect. Immun. 2001, 69, 7711–7717. [Google Scholar] [CrossRef]
- Ramakrishnan, L. Revisiting the Role of the Granuloma in Tuberculosis. Nat. Rev. Immunol. 2012, 12, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.K.; Choung, R.S.; Hyun, J.J.; Kim, S.Y.; Jung, S.W.; Koo, J.S.; Lee, S.W.; Choi, J.H.; Kim, H.; Lee, H.S.; et al. Incidences of Serious Infections and Tuberculosis among Patients Receiving Anti-Tumor Necrosis Factor-α Therapy. Yonsei Med. J. 2014, 55, 442–448. [Google Scholar] [CrossRef]
- Ehlers, S.; Kutsch, S.; Ehlers, E.M.; Benini, J.; Pfeffer, K. Lethal Granuloma Disintegration in Mycobacteria-Infected TNFRp55−/− Mice Is Dependent on T Cells and IL-12. J. Immunol. 2000, 165, 483–492. [Google Scholar] [CrossRef]
- Hernández-Pando, R.; Orozcoe, H.; Sampieri, A.; Pavón, L.; Velasquillo, C.; Larriva-Sahd, J.; Alcocer, J.M.; Madrid, M.V. Correlation between the Kinetics of Th1, Th2 Cells and Pathology in a Murine Model of Experimental Pulmonary Tuberculosis. Immunology 1996, 89, 26–33. [Google Scholar] [PubMed]
- Flynn, J.A.L.; Chan, J. Immune Cell Interactions in Tuberculosis. Cell 2022, 185, 4682–4702. [Google Scholar] [CrossRef] [PubMed]
- Sia, J.K.; Rengarajan, J. Immunology of Mycobacterium tuberculosis Infections. Microbiol. Spectr. 2019, 7, 1–37. [Google Scholar] [CrossRef]
- Roca, F.J.; Whitworth, L.J.; Prag, H.A.; Murphy, M.P.; Ramakrishnan, L. TNF Induces Pathogenic Mitochondrial ROS in Tuberculosis through Reverse Electron Transport. Science 2022, 376, eabh2841. [Google Scholar] [CrossRef] [PubMed]
- Wilby, K.J.; Hussain, F.N. A Review of Clinical Pharmacokinetic and Pharmacodynamic Relationships and Clinical Implications for Drugs Used to Treat Multi-Drug Resistant Tuberculosis. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Francisco-Cruz, A.; Mata-Espinosa, D.; Estrada-Parra, S.; Xing, Z.; Hernández-Pando, R. Immunotherapeutic Effects of Recombinant Adenovirus Encoding Granulocyte-Macrophage Colony-Stimulating Factor in Experimental Pulmonary Tuberculosis. Clin. Exp. Immunol. 2013, 171, 283–297. [Google Scholar] [CrossRef]
- Ramos-Espinosa, O.; Mata-Espinosa, D.; Francisco-Cruz, A.; López-Torres, M.O.; Hernández-Bazán, S.; Barrios-Payán, J.; Marquina-Castillo, B.; Carretero, M.; del Río, M.; Hernández-Pando, R. Immunotherapeutic Effect of Adenovirus Encoding Antimicrobial Peptides in Experimental Pulmonary Tuberculosis. J. Leukoc. Biol. 2021, 110, 951–963. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Bazán, S.; Mata-Espinosa, D.; Ramos-Espinosa, O.; Lozano-Ordaz, V.; Barrios-Payán, J.; López-Casillas, F.; Hernández-Pando, R. Adenoviral Vector Codifying for TNF as a Co-Adjuvant Therapy against Multi-Drug-Resistant Tuberculosis. Microorganisms 2023, 11, 2934. https://doi.org/10.3390/microorganisms11122934
Hernández-Bazán S, Mata-Espinosa D, Ramos-Espinosa O, Lozano-Ordaz V, Barrios-Payán J, López-Casillas F, Hernández-Pando R. Adenoviral Vector Codifying for TNF as a Co-Adjuvant Therapy against Multi-Drug-Resistant Tuberculosis. Microorganisms. 2023; 11(12):2934. https://doi.org/10.3390/microorganisms11122934
Chicago/Turabian StyleHernández-Bazán, Sujhey, Dulce Mata-Espinosa, Octavio Ramos-Espinosa, Vasti Lozano-Ordaz, Jorge Barrios-Payán, Fernando López-Casillas, and Rogelio Hernández-Pando. 2023. "Adenoviral Vector Codifying for TNF as a Co-Adjuvant Therapy against Multi-Drug-Resistant Tuberculosis" Microorganisms 11, no. 12: 2934. https://doi.org/10.3390/microorganisms11122934




