The Use of Rapid COVID-19 Antigen Test in the Emergency Department as a Decision-Support Tool
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Setting
2.3. Study Period
2.4. Study Population
2.5. Triage Strategy
2.6. Sample Collection and Analysis
2.7. Statistical Analysis
3. Results
3.1. ED Visitations
3.2. Risk Reduction—Red ED
3.3. Risk Reduction—Green ED
3.4. Sensitivity, Specificity, PPV, NPV
3.5. Duration of ED Admission
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Israel Ministry of Health. COVID-19 Data Dashboard. 29 June 2022. Available online: https://datadashboard.health.gov.il/COVID-19/general (accessed on 29 June 2022).
- Fernandes, M.; Vieira, S.; Leite, F.; Palos, C.; Finkelstein, S.; Sousa, J. Clinical Decision Support Systems for Triage in the Emergency Department using Intelligent Systems: A Review. Artif. Intell. Med. 2020, 102, 101762. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sun, H.; He, Y.; Yang, J.; Li, X.; Taparia, K.; Zheng, B. Personal protective equipment in COVID-19: Impacts on health performance, work-related injuries, and measures for prevention. J. Occup. Environ. Med. 2021, 63, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Yamayoshi, S.; Sakai-Tagawa, Y.; Koga, M.; Akasaka, O.; Nakachi, I.; Koh, H.; Maeda, K.; Adachi, E.; Saito, M.; Nagai, H.; et al. Comparison of rapid antigen tests for COVID-19. Viruses 2020, 12, 1420. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Laboratory Testing of 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases: Interim Guidance. Updated 17 January 2020. Available online: https://apps.who.int/iris/handle/10665/330676 (accessed on 13 February 2022).
- Centers for Disease Control and Prevention. Interim Guidance for Antigen Testing for SARS-CoV-2. Updated 9 September 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html#:~:text=If%20there%20is%20an%20outbreak,frequent%20than%20serial%20NAAT%20testing (accessed on 30 September 2021).
- Levy, Y.; Frenkel Nir, Y.; Ironi, A.; Englard, H.; Regev-Yochay, G.; Rahav, G.; Afek, A.; Grossman, E. Emergency Department Triage in the Era of COVID-19: The Sheba Medical Center Experience. Isr. Med. Assoc. J. IMAJ 2020, 22, 470–475. [Google Scholar] [PubMed]
- Regev-Yochay, G.; Kriger, O.; Mina, M.J.; Beni, S.; Rubin, C.; Mechnik, B.; Hason, S.; Biber, E.; Nadaf, B.; Kreiss, Y.; et al. Real-world performance of SARS-CoV-2 antigen rapid diagnostic tests in various clinical settings. Infect. Control Hosp. Epidemiol. 2022, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Patel, M.; Charlett, A.; Lopez Bernal, J.; Saliba, V.; Ellis, J.; Ladhani, S.; Zambon, M.; Gopal, R. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Eurosurveillance 2020, 25, 2001483. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Spencer, E.A.; Brassey, J.; Heneghan, C. Viral cultures for COVID-19 infectivity assessment—A systematic review (update 4). Clin. Infect. Dis. 2021, 73, e3884–e3899. [Google Scholar] [CrossRef] [PubMed]
- Rickman, H.M.; Rampling, T.; Shaw, K.; Martinez-Garcia, G.; Hail, L.; Coen, P.; Shahmanesh, M.; Shin, G.Y.; Nastouli, E.; Houlihan, C.F. Nosocomial transmission of coronavirus disease 2019: A retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin. Infect. Dis. 2020, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Q.; He, Y.; Liu, L.; Ma, X.; Wei, X.; Jiang, N.; Liang, L.; Zheng, Y.; Ma, L.; et al. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur. Respir. J. 2020, 55, 2000544. [Google Scholar] [CrossRef] [PubMed]
- Onsongo, S.N.; Otieno, K.; van Duijn, S.; Adams, E.; Omollo, M.; Odero, I.A.; K’Oloo, A.; Houben, N.; Milimo, E.; Aroka, R.; et al. Field performance of NowCheck rapid antigen test for SARS-CoV-2 in Kisumu County, Western Kenya. medRxiv 2021. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Zarbiv, G.; Kabiri, D.; Porat, S.; Sompolinsky, Y.; Reubinoff, B.; Benenson, S.; Oster, Y. Rapid antigen detection testing for universal screening for severe acute respiratory syndrome coronavirus 2 in women admitted for delivery. Am. J. Obstet. Gynecol. 2021, 224, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Sluimer, J.; Goderski, G.; van den Brink, S.; Broeders, M.; Rahamat-Langendoen, J.; Then, E.; Wijsman, L.; Wolters, F.; van de Bovenkamp, J.; Melchers, W.J.; et al. Multi-center evaluation of cepheid Xpert® Xpress SARS-CoV-2/flu/RSV molecular point-of-care test. J. Clin. Virol. Plus 2021, 1, 100042. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.S.; Rothman, R.E.; Carroll, K.; Mostafa, H.H.; Ghobadi, K.; Smith, A.; Martinez, D.; Shaw-Saliba, K.; Klein, E.; Levin, S. Targeted rapid testing for SARS-CoV-2 in the emergency department is associated with large reductions in uninfected patient exposure time. J. Hosp. Infect. 2021, 107, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Merrick, B.; Noronha, M.; Batra, R.; Douthwaite, S.; Nebbia, G.; Snell, L.B.; Pickering, S.; Galao, R.P.; Whitfield, J.; Jahangeer, A.; et al. Real-world deployment of lateral flow SARS-CoV-2 antigen detection in the emergency department to provide rapid, accurate and safe diagnosis of COVID-19. Infect. Prev. Pract. 2021, 3, 100186. [Google Scholar] [CrossRef] [PubMed]
- Brody, B.D.; Shi, Z.; Shaffer, C.; Eden, D.; Wyka, K.; Parish, S.J.; Alexopoulos, G.S.; Nazario, H.; Russ, M.J.; Kanellopoulos, D. Universal COVID-19 testing and a three-space triage protocol is associated with a nine-fold decrease in possible nosocomial infections in an inpatient psychiatric facility. Psychiatry Res. 2021, 302, 114036. [Google Scholar] [CrossRef]
- Turcato, G.; Zaboli, A.; Pfeifer, N.; Sibilio, S.; Tezza, G.; Bonora, A.; Ciccariello, L.; Ausserhofer, D. Rapid antigen test to identify COVID-19 infected patients with and without symptoms admitted to the Emergency Department. Am. J. Emerg. Med. 2022, 51, 92–97. [Google Scholar] [CrossRef]
- Bond, K.A.; Smith, B.; Gardiner, E.; Liew, K.C.; Williams, E.; Walsham, N.; Putland, M.; Williamson, D.A. Utility of SARS-CoV-2 rapid antigen testing for patient triage in the emergency department: A clinical implementation study in Melbourne, Australia. Lancet Reg. Health-West. Pac. 2022, 25, 100486. [Google Scholar] [PubMed]
- Diel, R.; Nienhaus, A. Point-of-care COVID-19 antigen testing in German emergency rooms—A cost-benefit analysis. Pulmonology 2022, 28, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Symptoms of COVID-19. Updated 22 February 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 30 September 2021).
- World Health Organization. Coronavirus Disease (COVID-19) Symptoms. Available online: https://www.who.int/health-topics/coronavirus#tab=tab_3 (accessed on 30 September 2021).
- de Michelena, P.; Torres, I.; Ramos-García, Á.; Gozalbes, V.; Ruiz, N.; Sanmartín, A.; Botija, P.; Poujois, S.; Huntley, D.; Albert, E.; et al. Real-life performance of a COVID-19 rapid antigen detection test targeting the SARS-CoV-2 nucleoprotein for diagnosis of COVID-19 due to the Omicron variant. J. Infect. 2022, 84, e64–e66. [Google Scholar] [CrossRef] [PubMed]
- Croft, L.D.; Liquori, M.; Ladd, J.; Day, H.; Pineles, L.; Lamos, E.; Arnold, R.; Mehrotra, P.; Fink, J.C.; Langenberg, P.; et al. The effect of contact precautions on frequency of hospital adverse events. Infect. Control Hosp. Epidemiol. 2015, 36, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
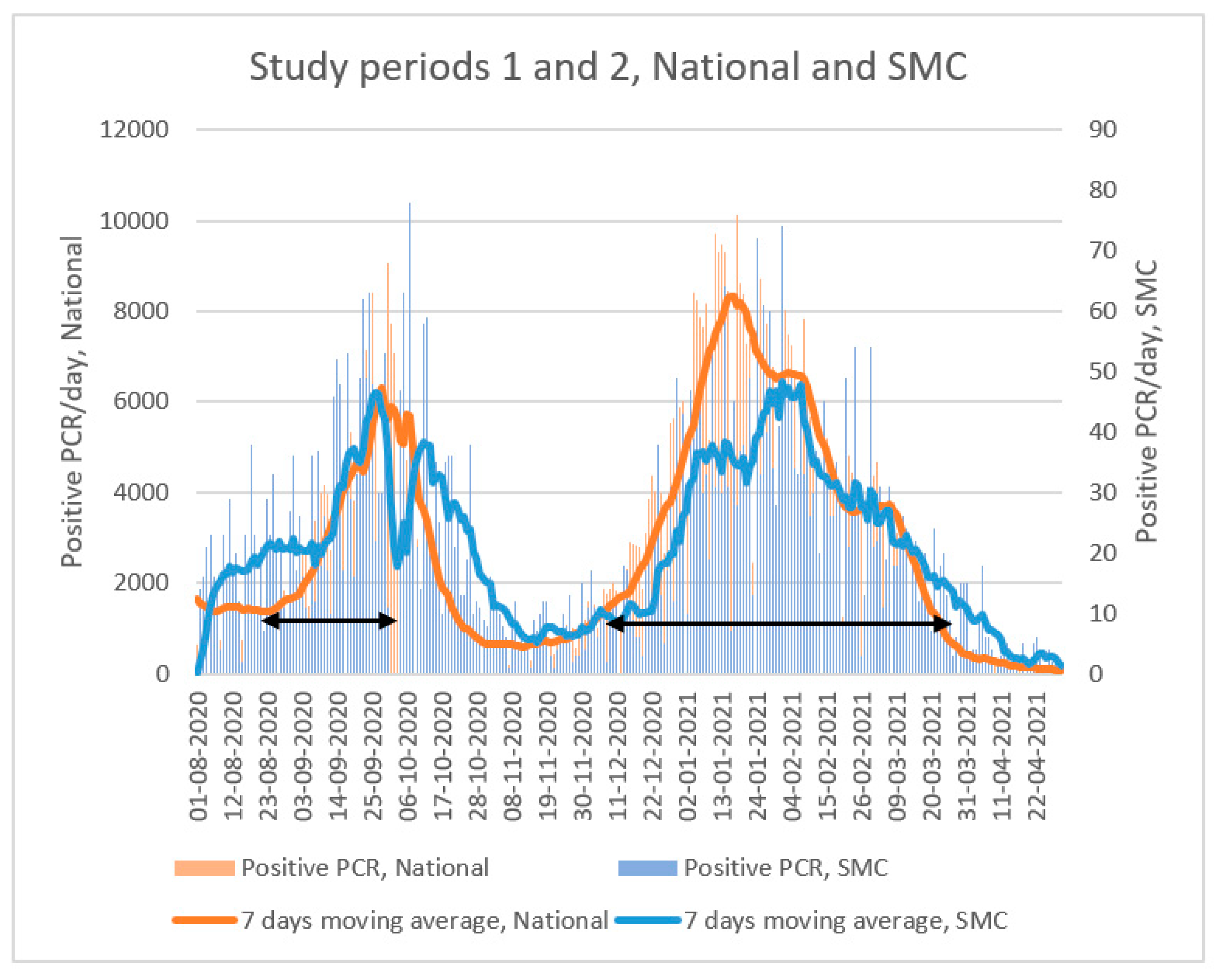
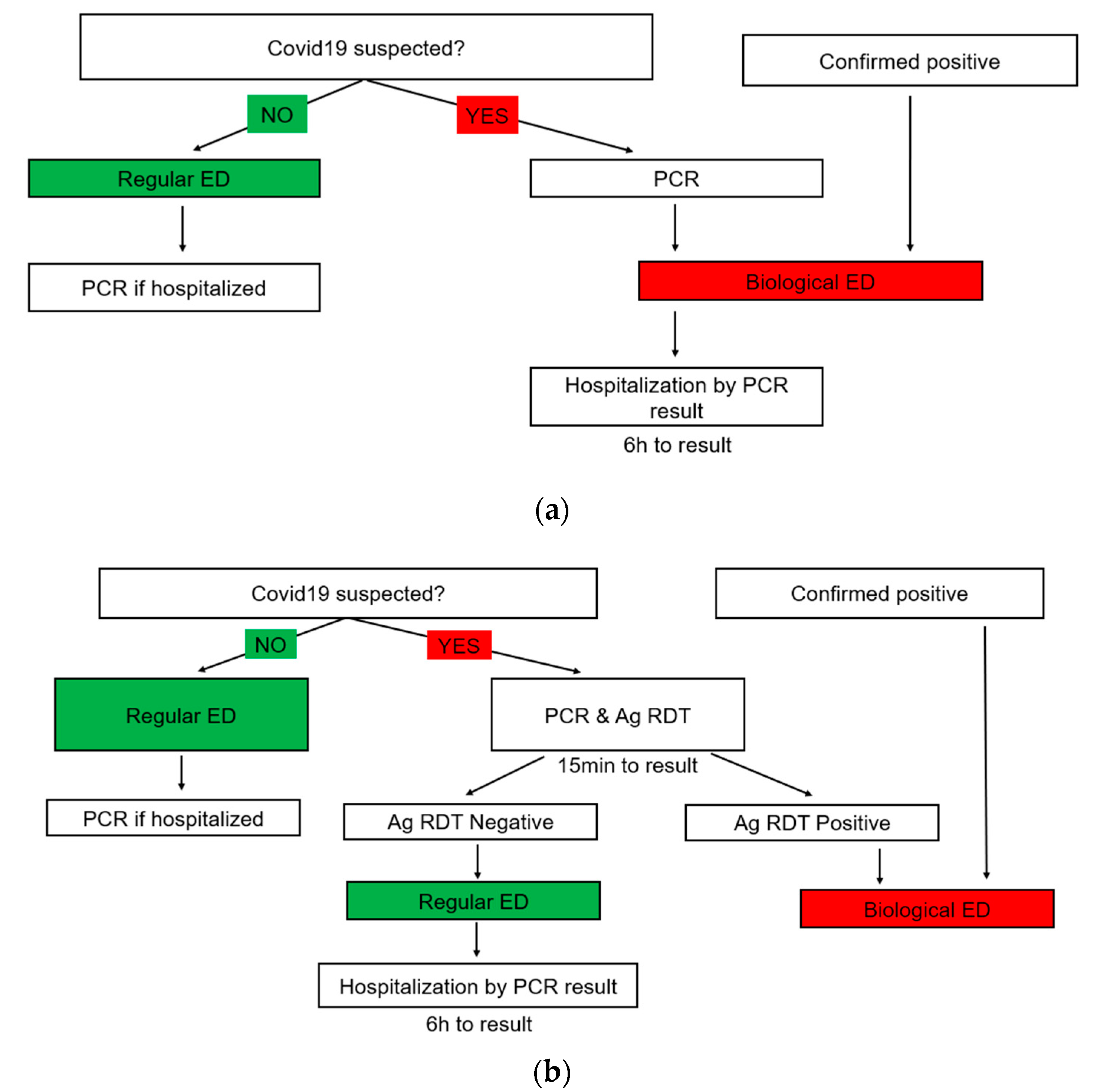
| Period 1 (21 August 2020–4 October 2020) | Period 2 (7 December 2020–21 March 2021) | |||
|---|---|---|---|---|
| N | % | N | % | |
| ED patients | 4659 | 13,648 | ||
| ED patients with PCR test result | 4511 | 13,592 | ||
| Average ED patient with PCR test result/day | 100.24 | 129.45 | ||
| Sex (Male) | 2442 | 54.1% | 7021 | 51.7% |
| Age—Mean | 61 | 61 | ||
| Age—Median (range) | 65 (18–104) | 66 (18–105) | ||
| Hospitalized | 3717 | 82.4% | 10,342 | 76.1% |
| Number of Ag tests | 0 | 7922 | ||
| Period 1 (21 August 2020–4 October 2020) | Period 2 (7 December 2020–21 March 2021) | |||
|---|---|---|---|---|
| Green | Red | Green | Red | |
| COVID-19 Positive ED patients | 1 (1%) | 6.8 (6.8%) | 1.7 (1.3%) | 6.7 (5.1%) |
| COVID-19 Positive Patients, CT < 30 | 0.6 (0.6%) | 5 (5%) | 0.7 (0.5%) | 3.9 (3%) |
| COVID-19 Negative Patients | 75.6 (76.4%) | 15.9 (15.8%) | 120.6 (93.2%) | 0.5 (0.4%) |
| Total ED Patients | 77.6 (77.4%) | 22.7 (22.6%) | 123.9 (94.5%) | 7.1 (5.5%) |
| Period 1 Mean ED Admission, Hours (95% CI) | Period 2 Mean ED Admission, Hours (95% CI) | % Change | Exposure Time Saved, Hours | |
|---|---|---|---|---|
| All Patients | 7 (6.9–7.1) | 6.7 (6.6–6.8) | 4.8 | 4621.3 |
| Green ED | 7.3 | 6.8 | 7.7 | 7192.1 |
| Red ED | 6.2 | 6 | 3.4 | 157.3 |
| Misassigned to green ED | 6.9 (5.8–8) | 6.7 (6.1–7.3) | 2.9 | 36.4 |
| Misassigned to green ED, CT < 30 | 5.9 (4.8–7) | 5.5 (4.8–6.1) | 6.5 | 26.6 |
| Misassigned to red ED | 6 (5.7–6.3) | 4.2 (3.6–4.8) | 30.2 | 90.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meltzer, L.; Amit, S.; Gilboa, M.; Tal, I.; Mechnik, B.; Irony, A.; Engelrad, H.; Epstein, A.; Frenkel-Nir, Y.; Levy, Y.; et al. The Use of Rapid COVID-19 Antigen Test in the Emergency Department as a Decision-Support Tool. Microorganisms 2023, 11, 284. https://doi.org/10.3390/microorganisms11020284
Meltzer L, Amit S, Gilboa M, Tal I, Mechnik B, Irony A, Engelrad H, Epstein A, Frenkel-Nir Y, Levy Y, et al. The Use of Rapid COVID-19 Antigen Test in the Emergency Department as a Decision-Support Tool. Microorganisms. 2023; 11(2):284. https://doi.org/10.3390/microorganisms11020284
Chicago/Turabian StyleMeltzer, Lilac, Sharon Amit, Mayan Gilboa, Ilana Tal, Bella Mechnik, Avi Irony, Hindi Engelrad, Avi Epstein, Yael Frenkel-Nir, Yuval Levy, and et al. 2023. "The Use of Rapid COVID-19 Antigen Test in the Emergency Department as a Decision-Support Tool" Microorganisms 11, no. 2: 284. https://doi.org/10.3390/microorganisms11020284
APA StyleMeltzer, L., Amit, S., Gilboa, M., Tal, I., Mechnik, B., Irony, A., Engelrad, H., Epstein, A., Frenkel-Nir, Y., Levy, Y., Kreiss, Y., & Regev-Yochay, G. (2023). The Use of Rapid COVID-19 Antigen Test in the Emergency Department as a Decision-Support Tool. Microorganisms, 11(2), 284. https://doi.org/10.3390/microorganisms11020284








