Analysis of Whole-Genome Sequences of Pathogenic Gram-Positive and Gram-Negative Isolates from the Same Hospital Environment to Investigate Common Evolutionary Trends Associated with Horizontal Gene Exchange, Mutations and DNA Methylation Patterning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Bacterial Pathogens and Antibiotic Resistance Detection
2.2. DNA Extraction
2.3. Genome Sequencing and Bioinformatic Analysis
2.4. Data Availability
3. Results
3.1. Bacterial Isolates Used in This Study
3.2. Genotyping of Isolates
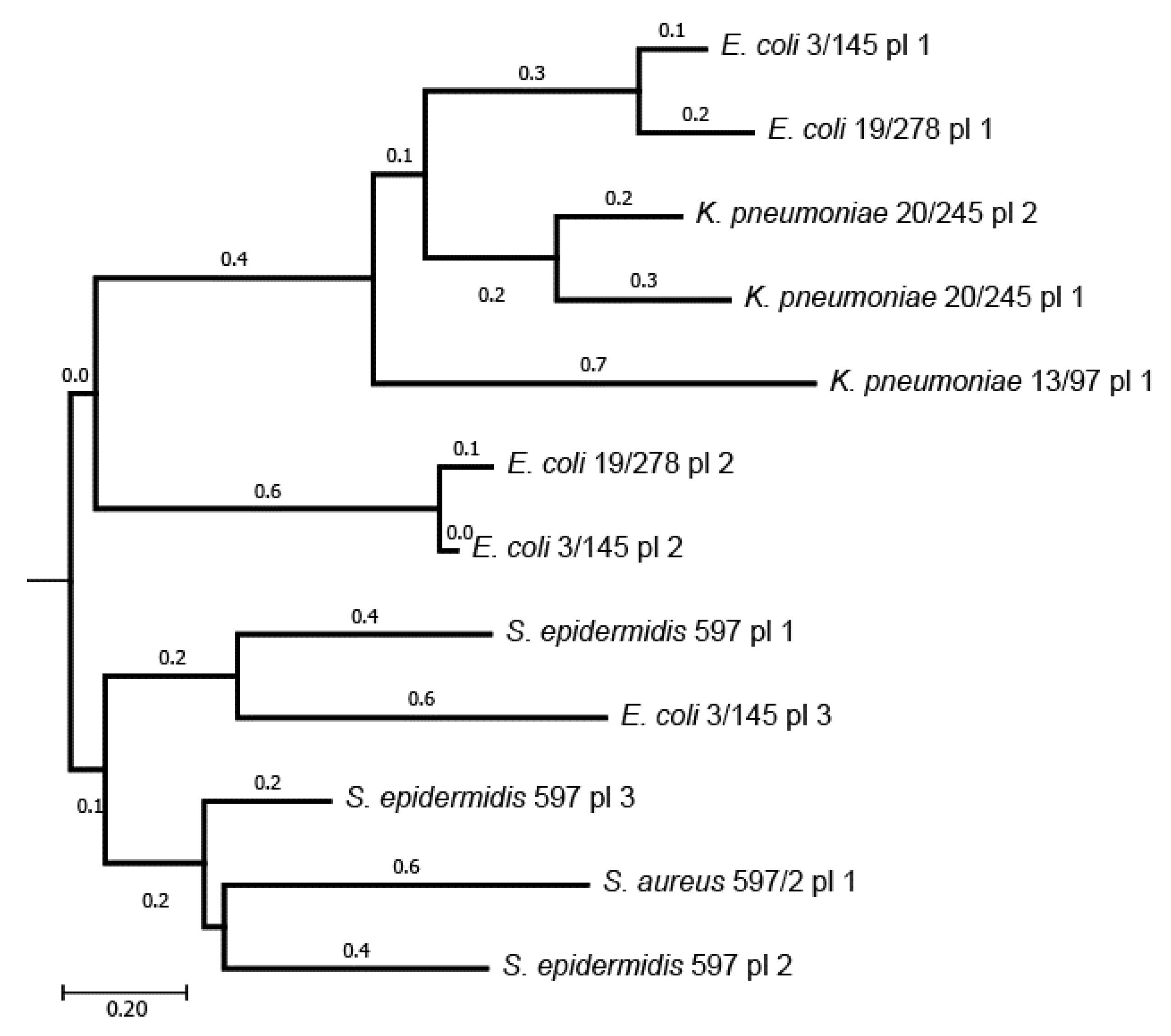
3.3. Plasmids and Genomic Islands of the Sequenced Isolates
3.4. Distribution of Antibiotic Resistance Determinants
3.4.1. Antibiotic Resistance Mutations
| Protein | E. coli | K. pneumoniae | P. aeruginosa | Staphylococcus | Associated Resistance | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3/145 | 19/278 | 13/97 | 20/245 | 7/157 | 16/222 | 9/195 | 597/2 | 598 | ||
| MarR | G103S, Y137H | S3N, G103S, Y137H | G103D, Y137Q | G103D, Y137Q | Broad spectrum [46] | |||||
| NalC | S209R, G71E | S209R, G71E | Broad spectrum [50,58] | |||||||
| MexS | V73A | V73A | Broad spectrum [50,58] | |||||||
| GrlA | I45M | Ciprofloxacin [57] | ||||||||
| GlpT | E448K | A100V | A100V, V213I | Fosfomycin [47,56] | ||||||
| CyaA | S352T | |||||||||
| UhpT | E350Q | E350Q | E350Q | |||||||
| MurA | E291D, T396N | D278E, E291D | Fosfomycin [59] | |||||||
| ParC | S80I | Fluoroquinolones [60] | ||||||||
| ParE | L416F | |||||||||
| GyrA | S83L, D87N | T83I | T83I | |||||||
| PBP3 | D350N, S357N | D350N, S357N | D350N, S357N | D350N, S357N | Beta-lactams [61] | |||||
| QseB | L71R | Aminoglycosides [51] | ||||||||
| EF-Tu | R234F | R234F | R234F | R234F | Elfamycins and other peptide synthesis inhibitors [45] | |||||
| SoxR | G74R | G74R | G74R | Broad spectrum [49] | ||||||
3.4.2. Antibiotic Resistance Genes
3.5. Distribution of Virulence Genetic Determinants
3.5.1. Virulence Factors of E. coli
3.5.2. Virulence Factors of K. pneumoniae
3.5.3. Virulence Factors of P. aeruginosa
3.5.4. Virulence Factors of the Gram-Positive Isolates
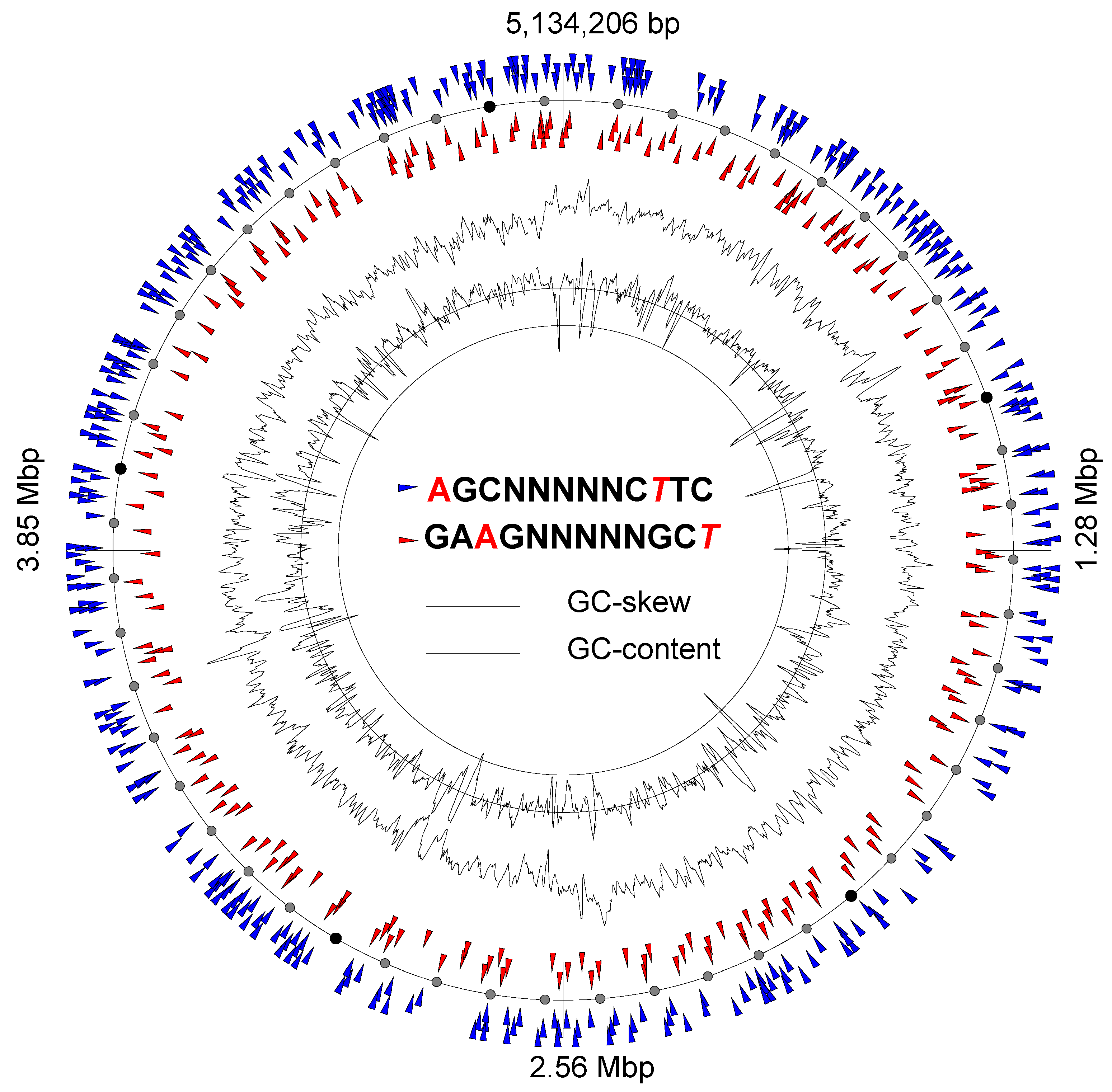
3.6. Genome Methylation Patterns and Restriction–Modification Systems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, M.; Sartelli, M.; McKimm, J.; Abu Bakar, M. Health care-associated infections—An overview. Infect. Drug. Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [Green Version]
- Boyd, S.; Vasudevan, A.; Moore, L.; Brewer, C.; Gilchrist, M.; Costelloe, C.; Gordon, A.; Holmes, A. Validating a prediction tool to determine the risk of nosocomial multidrug-resistant Gram-negative bacilli infection in critically ill patients: A retrospective case-control study. J. Glob. Antimicrob. Resist. 2020, 22, 826–831. [Google Scholar] [CrossRef]
- Ozenen, G.; Bal, Z.; Umit, Z.; Avcu, G.; Tekin, D.; Kurugol, Z.; Cilli, F.; Ozkinay, F. Nosocomial non-fermentative Gram negative bacteria bloodstream infections in children; Risk factors and clinical outcomes of carbapenem resistance. J. Infect. Chemother. 2021, 27, 729–735. [Google Scholar] [CrossRef]
- Khan, H.; Ahmad, A.; Mehboob, R. Nosocomial infections and their control strategies. Asian Pac. J. Trop. Biomed. 2015, 5, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D. Nosocomial infections and infection control. Medicine 2017, 45, 629–633. [Google Scholar] [CrossRef]
- Walana, W.; Bobzah, B.; Kuugbee, E.; Acquah, S.; Ezekiel, V.; Yabasin, I.; Abdul-Mumin, A.; Ziem, J. Staphylococcus aureus nasal carriage among healthcare workers, inpatients and caretakers in the Tamale Teaching Hospital, Ghana. Sci. Afr. 2020, 8, e00325. [Google Scholar] [CrossRef]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwardson, S.; Cairs, C. Nosocomial infections in the ICU. Anaesth. Intensive Care Med. 2019, 20, 14–18. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef]
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial resistance in bacteria: Mechanisms, evolution, and persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Lax, S.; Gilbert, J. Hospital-associated microbiota and implications for nosocomial infections. Trends Mol. Med. 2015, 21, 427–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wawire, S.A.; Reva, O.N.; O’Brien, T.J.; Figueroa, W.; Dinda, V.; Shivoga, W.A.; Welch, M. Virulence and antimicrobial resistance genes are enriched in the plasmidome of clinical Escherichia coli isolates compared with wastewater isolates from western Kenya. Infect. Genet. Evol. 2021, 91, 104784. [Google Scholar] [CrossRef] [PubMed]
- Viderman, D.; Khamzina, Y.; Kaligozhin, Z.; Khudaibergenova, M.; Zhumadilov, A.; Crape, B.; Azizan, A. An observational case study of hospital associated infections in a critical care unit in Astana, Kazakhstan. Antimicrob. Resist. Infect. Control 2018, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Viderman, D.; Brotfain, E.; Khamzina, Y.; Kapanova, G.; Zhumadilov, A.; Poddighe, D. Bacterial resistance in the intensive care unit of developing countries: Report from a tertiary hospital in Kazakhstan. J. Glob. Antimicrob. Resist. 2019, 17, 35–38. [Google Scholar] [CrossRef]
- Kaliyeva, S.S.; Lavrinenko, A.V.; Tishkambayev, Y.; Zhussupova, G.; Issabekova, A.; Begesheva, D.; Simokhina, N. Microbial Landscape and Antibiotic Susceptibility Dynamics of Skin and Soft Tissue Infections in Kazakhstan 2018–2020. Antibiotics 2022, 11, 659. [Google Scholar] [CrossRef]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids. Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef]
- Leimbach, A. ecoli_VF_collection: v0.1.; Zenodo: Geneva, Switzerland, 2016. [Google Scholar] [CrossRef]
- Korotetskiy, I.S.; Ardak, B.; Jumagaziyeva, A.B.; Kerimzhanova, B.; Reva, O.N.; Shilov, S.V.; Kuznetsova, T.; Zubenko, N.; Korotetskaya, N.; Bekmukhamedova, A.; et al. Complete genome sequences of Gram-negative opportunistic pathogens isolated in hospitals in Almaty, Kazakhstan. Microbiol. Resour. Announc. 2021, 10, e00974-21. [Google Scholar] [CrossRef]
- Korotetskiy, I.S.; Jumagaziyeva, A.B.; Kerimzhanova, B.; Reva, O.N.; Shilov, S.V.; Kuznetsova, T.V.; Ivanova, L.; Korotetskaya, N.; Bekmukhamedova, A.; Satyigankyzy, G.; et al. Complete genome sequences of Gram-positive opportunistic pathogens isolated from hospitals in Almaty, Kazakhstan. Microbiol. Resour. Announc. 2022, 11, e00093-22. [Google Scholar] [CrossRef]
- Ferraro, M.J. Performance Standards for Antimicrobial Disk Susceptibility Tests; NCCLS: Wayne, PA, USA, 2000. [Google Scholar]
- Hon, T.; Mars, K.; Young, G.; Tsai, Y.C.; Karalius, J.W.; Landolin, J.M.; Maurer, N.; Kudrna, D.; Hardigan, M.A.; Steiner, C.C.; et al. Highly accurate long-read HiFi sequencing data for five complex genomes. Sci. Data 2020, 7, 399. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, Y.; Ermini, L.; Wang, H.; Carty, K.; Cheung, M.S. LongQC: A quality control tool for third generation sequencing long read data. G3 Genes Genomes Genet. 2020, 10, 1193–1196. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [Green Version]
- Schwengers, O.; Barth, P.; Falgenhauer, L.; Hain, T.; Chakraborty, T.; Goesmann, A. Platon: Identification and characterization of bacterial plasmid contigs in short-read draft assemblies exploiting protein sequence-based replicon distribution scores. Microb. Genom. 2020, 6, mgen000398. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Moreira, B.; Vinuesa, P. GET_HOMOLOGUES, a versatile software package for scalable and robust microbial pangenome analysis. Appl. Environ. Microbiol. 2013, 79, 7696–7701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, H.; Zhao, S.; Hsu, C.H.; McDermott, P.F.; et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob. Agents Chemother. 2019, 63, e00483-19. [Google Scholar] [CrossRef] [Green Version]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids. Res. 2020, 8, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Padmanabhan, B.R.; Diene, S.M.; Lopez-Rojas, R.; Kempf, M.; Landraud, L.; Rolain, L.L. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob. Agents Chemother. 2014, 58, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Doster, E.; Lakin, S.M.; Dean, C.J.; Wolfe, C.; Young, J.G.; Boucher, C.; Belk, K.E.; Noyes, N.R.; Morley, P.S. MEGARes 2.0: A database for classification of antimicrobial drug, biocide and metal resistance determinants in metagenomic sequence data. Nucleic Acids. Res. 2020, 489, D561–D569. [Google Scholar] [CrossRef]
- Joensen, K.G.; Tetzschner, A.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef] [Green Version]
- Harmsen, D.; Claus, H.; Witte, W.; Rothgänger, J.; Claus, H.; Turnwald, D.; Vogel, U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting using a novel software for spa-repeat determination and database management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef] [Green Version]
- Bezuidt, O.; Lima-Mendez, G.; Reva, O.N. SeqWord Gene Island Sniffer: A program to study the lateral genetic exchange among bacteria. World Acad. Sci. Eng. Technol. 2009, 22, 1169–1174. [Google Scholar]
- Pierneef, R.; Bezuidt, O.; Reva, O.N. Optimization and practical use of composition based approaches towards identification and collection of genomic islands and their ontology in prokaryotes. Procedia Comput. Sci. 2015, 1, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Pierneef, R.; Cronje, L.; Bezuidt, O.; Reva, O.N. Pre_GI: A global map of ontological links between horizontally transferred genomic islands in bacterial and archaeal genomes. Database 2015, 2015, bav058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezuidt, O.; Pierneef, R.; Mncube, K.; Lima-Mendez, G.; Reva, O.N. Mainstreams of horizontal gene exchange in enterobacteria: Consideration of the outbreak of enterohemorrhagic E. coli O104:H4 in Germany in 2011. PLoS ONE 2011, 6, e25702. [Google Scholar] [CrossRef]
- Reva, O.N.; Korotetskiy, I.S.; Joubert, M.; Shilov, S.V.; Jumagaziyeva, A.B.; Suldina, N.A.; Ilin, A.I. The effect of iodine-containing nano-micelles, FS-1, on antibiotic resistance, gene expression and epigenetic modifications in the genome of multidrug resistant MRSA strain Staphylococcus aureus ATCC BAA-39. Front. Microbiol. 2020, 11, 581660. [Google Scholar] [CrossRef]
- Catalán-Nájera, J.C.; Garza-Ramos, U.; Barrios-Camacho, H. Hypervirulence and hypermucoviscosity: Two different but complementary Klebsiella spp. phenotypes? Virulence 2017, 8, 1111–1123. [Google Scholar] [CrossRef] [Green Version]
- Remya, P.; Shanthi, M.; Sekar, U. Occurrence and characterization of hyperviscous K1 and K2 serotype in Klebsiella pneumoniae. J. Lab. Phys. 2018, 10, 283–288. [Google Scholar] [CrossRef]
- Wang, L.; Shen, D.; Wu, H.; Ma, Y. Resistance of hypervirulent Klebsiella pneumoniae to both intracellular and extracellular killing of neutrophils. PLoS One 2017, 12, e0173638. [Google Scholar] [CrossRef] [Green Version]
- Ruppitsch, W.; Indra, A.; Stöger, A.; Mayer, B.; Stadlbauer, S.; Wewalka, G.; Allerberger, F. Classifying spa types in complexes improves interpretation of typing results for methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2006, 44, 2442–2448. [Google Scholar] [CrossRef] [Green Version]
- Carattoli, A.; Hasman, H. PlasmidFinder and In Silico pMLST: Identification and Typing of Plasmid Replicons in Whole-Genome Sequencing (WGS). In Horizontal Gene Transfer. Methods in Molecular Biology, Vol. 2075; De la Cruz, F., Ed.; Humana: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Humphrey, S.; Fillol-Salom, A.; Quiles-Puchalt, N.; Ibarra-Chávez, R.; Haag, A.F.; Chen, J.; Penadés, J.R. Bacterial chromosomal mobility via lateral transduction exceeds that of classical mobile genetic elements. Nat. Commun. 2021, 12, 6509. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, S.M.; Brown, N.E.; Goldberg, J.B. Elfamycins: Inhibitors of elongation factor-Tu. Mol. Microbiol. 2017, 106, 22–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, M. Molecular Characterization of Multidrug-Resistant Escherichia coli Isolates in Azerbaijan Hospitals. Microb. Drug. Resist. 2019, 25, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Mowlaboccus, S.; Daley, D.A.; Shoby, P.; Coombs, G.W. Molecular confirmation of Escherichia coli classified as fosfomycin-resistant by the revised EUCAST MIC breakpoint. Pathology 2022, 54, 965–966. [Google Scholar] [CrossRef]
- Mendes, A.C.; Rodrigues, C.; Pires, J.; Amorim, J.; Ramos, M.H.; Novais, Â.; Peixe, L. Importation of fosfomycin resistance fosA3 gene to Europe. Emerg. Infect. Dis. 2016, 22, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Koutsolioutsou, A.; Peña-Llopis, S.; Demple, B. Constitutive soxR mutations contribute to multiple-antibiotic resistance in clinical Escherichia coli isolates. Antimicrob. Agents Chemother. 2005, 49, 2746–2752. [Google Scholar] [CrossRef] [Green Version]
- Amsalu, A.; Sapula, S.A.; De Barros Lopes, M.; Hart, B.J.; Nguyen, A.H.; Drigo, B.; Turnidge, J.; Leong, L.E.; Venter, H. Efflux pump-driven antibiotic and biocide cross-resistance in Pseudomonas aeruginosa isolated from different ecological niches: A case study in the development of multidrug resistance in environmental hotspots. Microorganisms 2020, 8, 1647. [Google Scholar] [CrossRef]
- Schniederjans, M.; Koska, M.; Häussler, S. Transcriptional and mutational profiling of an aminoglycoside-resistant Pseudomonas aeruginosa small-colony variant. Antimicrob. Agents Chemother. 2017, 61, e01178-17. [Google Scholar] [CrossRef] [Green Version]
- Knopp, M.; Andersson, D.I. Predictable phenotypes of antibiotic resistance mutations. mBio 2018, 9, e00770-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Dzink-Fox, J.L.; Chen, M.; Levy, S.B. Genetic characterization of highly fluoroquinolone-resistant clinical Escherichia coli strains from China: Role of acrR mutations. Antimicrob. Agents Chemother. 2001, 45, 1515–1521. [Google Scholar] [CrossRef] [Green Version]
- Novais, A.; Rodrigues, C.; Branquinho, R.; Antunes, P.; Grosso, F.; Boaventura, L.; Ribeiro, G.; Peixe, L. Spread of an OmpK36-modified ST15 Klebsiella pneumoniae variant during an outbreak involving multiple carbapenem-resistant Enterobacteriaceae species and clones. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3057–3063. [Google Scholar] [CrossRef]
- Humphries, R.M.; Hemarajata, P. Resistance to ceftazidime-avibactam in Klebsiella pneumoniae due to porin mutations and the increased expression of KPC-3. Antimicrob. Agents Chemother. 2017, 61, e00537-17. [Google Scholar] [CrossRef] [Green Version]
- Mosime, L.B.; Newton-Foot, M.; Nel, P. Fosfomycin resistance in community-acquired urinary pathogens from Western Cape, South Africa. S. Afr. J. Infect. Dis. 2022, 37, 321. [Google Scholar] [CrossRef]
- Gordon, N.C.; Price, J.R.; Cole, K.; Everitt, R.; Morgan, M.; Finney, J.; Kearns, A.M.; Pichon, B.; Young, B.; Wilson, D.J.; et al. Prediction of Staphylococcus aureus antimicrobial resistance by whole-genome sequencing. J. Clin. Microbiol. 2014, 52, 1182–1191. [Google Scholar] [CrossRef] [Green Version]
- Braz, V.S.; Furlan, J.P.; Fernandes, A.F.; Stehling, E.G. Mutations in NalC induce MexAB-OprM overexpression resulting in high level of aztreonam resistance in environmental isolates of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2016, 363, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin-Tóth, J.; Kovács, E.; Tóthpál, A.; Juhász, J.; Forró, B.; Bányai, K.; Havril, K.; Horváth, A.; Ghidán, Á.; Dobay, O. Whole genome sequencing of coagulase positive staphylococci from a dog-and-owner screening survey. PLoS ONE 2021, 16, e0245351. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Yang, Y.; Li, F.; Li, X.; Liu, H.; Fazilani, S.A.; Guo, W.; Xu, G.; Zhang, X. The prevalence and mechanism of fluoroquinolone resistance in Escherichia coli isolated from swine farms in China. BMC Vet. Res. 2020, 16, 258. [Google Scholar] [CrossRef]
- Avershina, E.; Sharma, P.; Taxt, A.M.; Singh, H.; Frye, S.A.; Paul, K.; Kapil, A.; Naseer, U.; Kaur, P.; Ahmad, R. AMR-Diag: Neural network based genotype-to-phenotype prediction of resistance towards β-lactams in Escherichia coli and Klebsiella pneumoniae. Comput. Struct. Biotechnol. J. 2021, 19, 1896–1906. [Google Scholar] [CrossRef]
- Phuc Nguyen, M.C.; Woerther, P.L.; Bouvet, M.; Andremont, A.; Leclercq, R.; Canu, A. Escherichia coli as reservoir for macrolide resistance genes. Emerg. Infect. Dis. 2009, 15, 1648–1650. [Google Scholar] [CrossRef] [PubMed]
- Pages, J.M.; Lavigne, J.P.; Leflon-Guibout, V.; Marcon, E.; Bert, F.; Noussair, L.; Nicolas-Chanoine, M.H. Efflux pump, the masked side of beta-lactam resistance in Klebsiella pneumoniae clinical isolates. PLoS ONE 2009, 4, e4817. [Google Scholar] [CrossRef] [Green Version]
- Domenech-Sanchez, A.; Hernandez-Alles, S.; Martinez-Martinez, L.; Benedi, V.J.; Alberti, S. Identification and characterization of a new porin gene of Klebsiella pneumoniae: Its role in β-Lactam antibiotic resistance. J. Bacteriol. 1999, 181, 2726–2732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surleac, M.; Czobor Barbu, I.; Paraschiv, S.; Popa, L.I.; Gheorghe, I.; Marutescu, L.; Popa, M.; Sarbu, I.; Talapan, D.; Nita, M.; et al. Whole genome sequencing snapshot of multi-drug resistant Klebsiella pneumoniae strains from hospitals and receiving wastewater treatment plants in Southern Romania. PLoS One 2020, 15, e0228079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chávez-Jacobo, V.M.; Hernández-Ramírez, K.C.; Romo-Rodríguez, P.; Pérez-Gallardo, R.V.; Campos-García, J.; Gutiérrez-Corona, J.F.; García-Merinos, J.P.; Meza-Carmen, V.; Silva-Sánchez, J.; Ramírez-Díaz, M.I. CrpP is a novel ciprofloxacin-modifying enzyme encoded by the Pseudomonas aeruginosa pUM505 plasmid. Antimicrob. Agents Chemother. 2018, 62, e02629-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subedi, D.; Vijay, A.K.; Kohli, G.S.; Rice, S.A.; Willcox, M. Comparative genomics of clinical strains of Pseudomonas aeruginosa strains isolated from different geographic sites. Sci. Rep. 2018, 8, 15668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobel, M.L.; Hocquet, D.; Cao, L.; Plesiat, P.; Poole, K. Mutations in PA3574 (nalD) lead to increased MexAB-OprM expression and multidrug resistance in laboratory and clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 1782–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, A.M.; Martins, K.B.; Silva, V.R.; Mondelli, A.L.; Cunha, M.D. Correlation of phenotypic tests with the presence of the blaZ gene for detection of beta-lactamase. Braz. J. Microbiol. 2017, 48, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-PLoSkonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Eto, D.S.; Jones, T.A.; Sundsbak, J.L.; Mulvey, M.A. Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog. 2007, 7, e100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangel, D.E.; Marín-Medina, N.; Castro, J.E.; González-Mancera, A.; Forero-Shelton, M. Observation of bacterial type I pili extension and contraction under fluid flow. PLoS ONE 2013, 8, e65563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagan, E.C.; Mobley, H.L.T. Uropathogenic Escherichia coli outer membrane antigens expressed during urinary tract infection. Infect. Immun. 2007, 8, 3941–3949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.T.; Chang, H.Y.; Lai, Y.C.; Pan, C.C.; Tsai, S.F.; Peng, H.L. Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumoniae CG43. Gene 2004, 337, 189–198. [Google Scholar] [CrossRef]
- Parrott, A.M.; Shi, J.; Aaron, J.; Green, D.; Whittier, S.; Wu, F. Detection of multiple hypervirulent Klebsiella pneumonia strains in a New York City hospital through screening of virulence genes. Clin. Microbiol. Infect. 2020, 4, 583–589. [Google Scholar] [CrossRef]
- Shon, A.S.; Bajwa, R.P.; Russo, T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 2013, 4, 107–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, T.A.; Olson, R.; Macdonald, U.; Metzger, D.; Maltese, L.M.; Drake, E.J.; Gulick, A.M. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect. Immun. 2014, 82, 2356–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyres, K.L.; Lam, M.M.C.; Holt, K.E. Population genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef]
- Nougayrède, J.P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J.; Dobrindt, U.; Oswald, E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef]
- Sarris, P.F.; Zoumadakis, C.; Panopoulos, N.J.; Scoulica, E.V. Distribution of the putative type VI secretion system core genes in Klebsiella spp. Infect. Genet. Evol. 2011, 11, 157–166. [Google Scholar] [CrossRef]
- Azam, M.W.; Khan, A.U. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug. Discov. Today. 2019, 24, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Feltman, H.; Schulert, G.; Khan, S.; Jain, M.; Peterson, L.; Hauser, A.R. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 2001, 147, 2659–2669. [Google Scholar] [CrossRef] [Green Version]
- Moser, C.; Jensen, P.Ø.; Thomsen, K.; Kolpen, M.; Rybtke, M.; Lauland, A.S.; Trøstrup, H.; Tolker-Nielsen, T. Immune responses to Pseudomonas aeruginosa biofilm infections. Front. Immunol. 2021, 12, 625597. [Google Scholar] [CrossRef]
- McCaslin, C.A.; Petrusca, D.N.; Poirier, C.; Serban, K.A.; Anderson, G.G.; Petrache, I. Impact of alginate-producing Pseudomonas aeruginosa on alveolar macrophage apoptotic cell clearance. J. Cyst. Fibros. 2015, 14, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stacey, S.D.; Pritchett, C.L. Pseudomonas aeruginosa AlgU contributes to posttranscriptional activity by increasing rsmA expression in a mucA22 strain. J. Bacteriol. 2016, 198, 1812–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostroff, R.M.; Vasil, A.I.; Vasil, M.L. Molecular comparison of a nonhemolytic and a hemolytic phospholipase C from Pseudomonas aeruginosa. J. Bacteriol. 1990, 172, 5915–5923. [Google Scholar] [CrossRef] [Green Version]
- Cornelis, P.; Dingemans, J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front. Cell. Infect. Microbiol. 2013, 3, 75. [Google Scholar] [CrossRef] [Green Version]
- Bultreys, A.; Gheysen, I.; De Hoffmann, E. Yersiniabactin production by Pseudomonas syringae and Escherichia coli, and description of a second yersiniabactin locus evolutionary group. Appl. Environ. Microbiol. 2006, 72, 3814–3825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Dinges, M.M.; Orwin, P.M.; Schlievert, P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000, 13, 16–34. [Google Scholar] [CrossRef]
- Bien, J.; Sokolova, O.; Bozko, P. Characterization of virulence factors of Staphylococcus aureus: Novel function of known virulence factors that are implicated in activation of airway epithelial proinflammatory response. J. Pathog. 2011, 2011, 601905. [Google Scholar] [CrossRef] [Green Version]
- Hammerschmidt, S.; Bethe, G.; Remane, P.H.; Chhatwal, G.S. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun. 1999, 67, 1683–1687. [Google Scholar] [CrossRef]
- Jedrzejas, M.J. Pneumococcal virulence factors: Structure and function. Microbiol. Mol. Biol. Rev. 2001, 65, 187–207. [Google Scholar] [CrossRef] [Green Version]
- Shaper, M.; Hollingshead, S.K.; Benjamin, W.H.; Briles, D.E. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin. Infect. Immun. 2004, 72, 5031–5040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steel, H.C.; Cockeran, R.; Anderson, R.; Feldman, C. Overview of community-acquired pneumonia and the role of inflammatory mechanisms in the immunopathogenesis of severe pneumococcal disease. Mediat. Inflamm. 2013, 2013, 490346. [Google Scholar] [CrossRef] [PubMed]
- Mellroth, P.; Daniels, R.; Eberhardt, A.; Rönnlund, D.; Blom, H.; Widengren, J.; Normark, S.; Henriques-Normark, B. LytA, major autolysin of Streptococcus pneumoniae, requires access to nascent peptidoglycan. J. Biol. Chem. 2012, 287, 11018–11029. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Liu, S.; Zhang, Y.; Zhang, W. Role of DNA methylation in persister formation in uropathogenic E. coli. Microbiol. Res. 2020, 246, 126709. [Google Scholar] [CrossRef]
- Spadar, A.; Perdigão, J.; Phelan, J.; Charleston, J.; Modesto, A.; Elias, R.; De Sessions, P.E.; Hibberd, M.L.; Campino, S.; Duarte, A.; et al. Methylation analysis of Klebsiella pneumoniae from Portuguese hospitals. Sci. Rep. 2021, 11, 6491. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.R.; Ross, C.A.; Jain, S.; Shapiro, R.S.; Gutierrez, A.; Belenky, P.; Li, H.; Collins, J.J. A role for the bacterial GATC methylome in antibiotic stress survival. Nat. Genet. 2016, 48, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Phillips, Z.N.; Husna, A.U.; Jennings, M.P.; Seib, K.L.; Atack, J.M. Phasevarions of bacterial pathogens-phase-variable epigenetic regulators evolving from restriction-modification systems. Microbiology 2019, 165, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Price, V.J.; Huo, W.; Sharifi, A.; Palmer, K.L. CRISPR-Cas and restriction-modification act additively against conjugative antibiotic resistance plasmid transfer in Enterococcus faecalis. mSphere 2016, 1, e00064-16. [Google Scholar] [CrossRef] [Green Version]

| Strain Name | Resistance | Isolation Metadata | Replicons | Length (bp) | GenBank Acc |
|---|---|---|---|---|---|
| Escherichia coli SCAID WND2-2021 (3/145) | AMP, AMX, AZM *, E, OXA | Swabbed wound discharge | Chromosome | 5,134,206 | CP082827 |
| Plasmid 1 | 139,267 | CP082828 | |||
| Plasmid 2 | 106,249 | CP082829 | |||
| Escherichia coli SCAID URN1-2021 (19/278) | AMP, AMX, CEF, CTR, E, OXA | Urine, patient with pyelonephritis | Plasmid 3 | 32,040 | CP082830 |
| Chromosome | 5,168,688 | CP082824 | |||
| Klebsiella pneumoniae SCAID PHRX1-2021 (13/97) | AMP, AMX, AZM, E, OXA | Swab from pharynx, patient with pneumonia | Plasmid 1 | 108,070 | CP082825 |
| Plasmid 2 | 86,164 | CP082826 | |||
| Chromosome | 5,498,275 | CP082805 | |||
| Plasmid | 217,781 | CP082806 | |||
| Klebsiella pneumoniae SCAID PHRX2-2021 (20/245) | AMP, AMX, E, OXA | Swab from pharynx, patient with pneumonia | Chromosome | 5,319,600 | CP082796 |
| Plasmid 1 | 162,135 | CP082797 | |||
| Plasmid 2 | 95,203 | CP082798 | |||
| Pseudomonas aeruginosa SCAID TST1-2021 (7/157) | AMP, AMX, AZM *, CEF, CTR *, E, IPM, OXA | Swab from a tracheostomy tube after an operation | Chromosome | 7,173,620 | CP082823 |
| Pseudomonas aeruginosa SCAID PLC1-2021 (16/222) | AMP, AMX, AZM, CEF, E, OXA | Pleural cavity during an operation | Chromosome | 7,124,329 | CP082821 |
| Pseudomonas aeruginosa SCAID WND1-2021 (9/195) | AMP, AMX, CTR, CEF, E, IPM *, OXA | Swabbed wound discharge | Chromosome | 7,093,992 | CP082822 |
| Streptococcus pneumoniae SCAID PHRX1-2021 | AMP, E, OXA | Swab from pharynx, patient with pneumonia | Chromosome | 2,098,200 | CP082820 |
| Staphylococcus epidermidis SCAID OTT1-2021 (597) | AMP, AMX, AZM, E | Otitis, swab from ear | Chromosome | 2,099,244 | CP082816 |
| Plasmid 1 | 24,456 | CP082817 | |||
| Plasmid 2 | 24,520 | CP082818 | |||
| Plasmid 3 | 13,203 | CP082819 | |||
| Staphylococcus aureus SCAID OTT1-2021 (597/2) | AMP, AMX, OXA | Otitis, swab from ear | Chromosome | 2,737,085 | CP082813 |
| Plasmid 1 | 33,923 | CP082814 | |||
| Staphylococcus aureus SCAID WND1-2021 (598) | AMP, AMX | Swabbed wound discharge | Chromosome | 2,889,511 | CP082815 |
| Strain | GeneLocation | Type of Antibiotic Resistance Mechanism | |||||
|---|---|---|---|---|---|---|---|
| Beta- Lactamases | Antibiotic Inactivation | Target Alteration | Target Replacement or Protection | Efflux Pump | Reduced Permeability to Antibiotic | ||
| E. coli 3/145 | Chr | 3 | 0 | 6 | 0 | 30 | 1 |
| GIs | 0 | 0 | 1 | 0 | 6 | 0 | |
| Pl | 1 | 3 | 1 | 1 | 2 | 0 | |
| E. coli 19/278 | Chr | 2 | 0 | 5 | 0 | 35 | 1 |
| GIs | 0 | 0 | 1 | 0 | 4 | 0 | |
| Pl | 2 | 2 | 1 | 2 | 1 | 0 | |
| K. pneumoniae 13/97 | Chr | 2 | 1 | 2 | 0 | 13 | 1 |
| GIs | 0 | 0 | 0 | 0 | 3 | 0 | |
| Pl | 0 | 0 | 0 | 0 | 0 | 0 | |
| K. pneumoniae 20/245 | Chr | 1 | 2 | 2 | 0 | 15 | 3 |
| GIs | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pl | 0 | 0 | 0 | 0 | 0 | 0 | |
| P. aeruginosa 7/157 | Chr | 1 | 1 | 6 | 0 | 41 | 2 |
| GIs | 0 | 4 | 0 | 0 | 1 | 0 | |
| P. aeruginosa 9/195 | Chr | 1 | 3 | 6 | 0 | 43 | 0 |
| GIs | 0 | 2 | 0 | 0 | 2 | 2 | |
| P. aeruginosa 16/222 | Chr | 1 | 2 | 4 | 0 | 41 | 2 |
| GIs | 0 | 2 | 1 | 0 | 0 | 0 | |
| S. pneumoniae PHRX1-2021 | Chr | 0 | 0 | 1 | 0 | 3 | 0 |
| GIs | 0 | 0 | 0 | 0 | 0 | 0 | |
| S. epidermidis 597 | Chr | 1 | 1 | 0 | 0 | 1 | 0 |
| GIs | 0 | 0 | 0 | 1 | 0 | 0 | |
| Pl | 0 | 0 | 0 | 0 | 0 | 0 | |
| S. aureus 597/2 | Chr | 0 | 0 | 0 | 0 | 5 | 0 |
| GIs | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pl | 0 | 0 | 0 | 0 | 0 | 0 | |
| S. aureus 598 | Chr | 1 | 1 | 0 | 0 | 4 | 0 |
| GIs | 0 | 0 | 0 | 0 | 0 | 0 | |
| Strain | Core Chromosome | GIs | Plasmids |
|---|---|---|---|
| E. coli 3/145 | 131 | GI#1: 3 genes; GI#4: 14 genes; GI#5: 1 gene; GI#6: 6 genes; GI#9: 3 genes; GI#11: 5 genes; GI#13: 3 genes; GI#14: 3 genes; GI#15: 3 genes; GI#16: 1 gene; GI#17: 2 genes; GI#19: 7 genes; GI#20: 8 genes; GI#21: 1 gene; GI#23: 3 genes; TOTAL: 63 | pl_1: 7 genes; pl_2: 6 genes; pl_3: 1 gene; TOTAL: 14 |
| E. coli 19/278 | 122 | GI#1: 3 genes; GI#2: 2 genes; GI#4: 28 genes; GI#5: 5 genes; GI#9: 4 genes; GI#11: 11 gene; GI#12: 3 genes; GI#13: 2 genes; GI#14: 3 genes; GI#17: 7 genes; GI#18: 4 genes; GI#19: 2 genes; GI#20: 9 genes; TOTAL: 83 | pl_1: 7 genes; pl_2: 5 genes; TOTAL: 12 |
| K. pneumoniae 13/97 | 111 | GI#5: 24 genes; GI#6: 7 genes; GI#7: 7 genes; GI#8: 8 genes; GI#9: 2 genes; GI#14: 11 genes; GI#21: 2 genes; TOTAL: 61 | pl: 23 genes; TOTAL: 23 |
| K. pneumoniae 20/245 | 122 | GI#1: 2 genes; GI#4: 17 genes; GI#5: 7 genes; GI#6: 7 genes; GI#7: 8 genes; GI#13: 2 genes; TOTAL: 43 | pl_1: 21 genes; pl_2: 2 genes; TOTAL: 23 |
| P. aeruginosa 7/157 | 352 | GI#1: 2 genes; GI#2: 2 genes; GI#4: 1 gene; GI#6: 6 genes; GI#10: 1 gene; GI#17: 2 genes; GI#19: 1 gene; GI#20: 3 genes; GI#21: 2 genes; GI#26: 2 genes; GI#27: 1 gene; GI#28: 1 gene; TOTAL: 24 | NA |
| P. aeruginosa 9/195 | 358 | GI#2: 1 gene; GI#5: 1 gene; GI#6: 9 genes; GI#16: 1 gene; GI#17: 2 genes; GI#18: 5 genes; TOTAL: 19 | NA |
| P. aeruginosa 16/222 | 351 | GI#1: 2 genes; GI#2: 2 genes; GI#5: 1 gene; GI#6: 1 gene; GI#7: 7 genes; GI#17: 2 genes; GI#19: 1 gene; GI#20: 2 genes; GI#24: 5 genes; GI#28: 1 gene; TOTAL: 24 | NA |
| S. pneumoniae PHRX1-2021 | 50 | 0 | NA |
| S. epidermidis 597 | 35 | GI#1: 1 gene; GI#2: 1 gene; GI#6: 2 genes; TOTAL: 4 | pl_1: 2 genes; TOTAL: 2 |
| S. aureus 597/2 | 108 | GI#1: 3 genes; GI#6: 1 gene; GI#7: 3 genes; TOTAL: 7 | pl: 2 genes; TOTAL: 2 |
| S. aureus 598 | 102 | GI#1: 3 genes; GI#2: 2 genes; TOTAL: 5 | NA |
| Strain Name | Methylation Motifs * | Identified Restriction–Modification Systems (RMSs) † |
|---|---|---|
| E. coli 3/145 | GATC | Dam M (425828..426664); |
| AGCNNNNNCTTC | Type I EcoKI-like RMS (4380568..4384080); | |
| E. coli 19/278 | GATC | Dam M (395585..396421); |
| K. pneumoniae 13/97 | GATC | Dam M (394809..395609); |
| K. pneumoniae 20/245 | GATC | Dam M (383480..384307); |
| P. aeruginosa 7/157 | CAGNNNNNTGGG | Type III M (1806087..1807313); Type I RMS (5234163..5240525); Type I SM (5245219..5248468) |
| P. aeruginosa 16/222 | CAGNNNNNTGGG | Type III M (1810717..1811943); Type I RMS (5178388..5184750); Type I SM (5189444..5192693) |
| P. aeruginosa 9/195 | GACNNNNNTGCC | Type 1 RMS (5480535..5484830); |
| S. pneumoniae PHRX1-2021 | GATC | Dam methylase (425828..426664) |
| TCTAGA | Type II M XbaI (830119..831863); | |
| GAANNNNNNNNNTTYG | Type I SMR (450315..455692); Type I SMR (467913..473290); Type I MR (481404..486290); Type I SMR (494068..498918); Type I MR (506310..510980); Type I MR (516392..520416); Type I SMR (1272436..1278431); | |
| S. epidermidis 597 | GGTGA | Type IIS MR HphI (513688..515338) |
| S. aureus 597/2 | GWAGNNNNNNTAAA | Type I M (34076..35782); Type I R (179513..182302); Type I SM (411763..414571); |
| S. aureus 598 | GWAGNNNNNGAT GGANNNNNNNTCG | Type I R (164443..167232); Type I MS (402254..405035); Type I MS (1881251..1883954) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korotetskiy, I.S.; Shilov, S.V.; Kuznetsova, T.; Kerimzhanova, B.; Korotetskaya, N.; Ivanova, L.; Zubenko, N.; Parenova, R.; Reva, O.N. Analysis of Whole-Genome Sequences of Pathogenic Gram-Positive and Gram-Negative Isolates from the Same Hospital Environment to Investigate Common Evolutionary Trends Associated with Horizontal Gene Exchange, Mutations and DNA Methylation Patterning. Microorganisms 2023, 11, 323. https://doi.org/10.3390/microorganisms11020323
Korotetskiy IS, Shilov SV, Kuznetsova T, Kerimzhanova B, Korotetskaya N, Ivanova L, Zubenko N, Parenova R, Reva ON. Analysis of Whole-Genome Sequences of Pathogenic Gram-Positive and Gram-Negative Isolates from the Same Hospital Environment to Investigate Common Evolutionary Trends Associated with Horizontal Gene Exchange, Mutations and DNA Methylation Patterning. Microorganisms. 2023; 11(2):323. https://doi.org/10.3390/microorganisms11020323
Chicago/Turabian StyleKorotetskiy, Ilya S., Sergey V. Shilov, Tatyana Kuznetsova, Bahkytzhan Kerimzhanova, Nadezhda Korotetskaya, Lyudmila Ivanova, Natalya Zubenko, Raikhan Parenova, and Oleg N. Reva. 2023. "Analysis of Whole-Genome Sequences of Pathogenic Gram-Positive and Gram-Negative Isolates from the Same Hospital Environment to Investigate Common Evolutionary Trends Associated with Horizontal Gene Exchange, Mutations and DNA Methylation Patterning" Microorganisms 11, no. 2: 323. https://doi.org/10.3390/microorganisms11020323
APA StyleKorotetskiy, I. S., Shilov, S. V., Kuznetsova, T., Kerimzhanova, B., Korotetskaya, N., Ivanova, L., Zubenko, N., Parenova, R., & Reva, O. N. (2023). Analysis of Whole-Genome Sequences of Pathogenic Gram-Positive and Gram-Negative Isolates from the Same Hospital Environment to Investigate Common Evolutionary Trends Associated with Horizontal Gene Exchange, Mutations and DNA Methylation Patterning. Microorganisms, 11(2), 323. https://doi.org/10.3390/microorganisms11020323






