Interaction between Illite and a Pseudomonas stutzeri-Heavy Oil Biodegradation Complex
Abstract
1. Introduction
| No. | Clay Mineral | Clay Source/ Treatment Method | Degradation Duration 1 | Substrate | Effect | Mechanism of Influence | Aerobic/Anaerobic | Degrader | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mix | Collected | 56 d | Crude oil | Stimulation for SHs, Neutral for AHs | Increase biological accessibility | Aerobic | Microbial community | [19] |
| 2 | Kao | Purchased | 24 d | Heavy oil in the environment | Stimulation | C-O-Na-Si stimulates metabolism | Aerobic | Microbial community | [20] |
| 3 | Mon | Collected | 105 d | Heavy oil in the environment | Stimulation | Stimulate growth and buffer pH | Aerobic | Pseudomonas aeruginosa + Microbial community | [21] |
| Kao | |||||||||
| 4 | Mon | Collected | 36 mon | Heavy oil in the environment | Stimulation | Stimulate growth and buffer pH, C-O-Na-Si stimulates metabolism | Aerobic | Microbial community | [22] |
| Mon | Stimulation | ||||||||
| Kao-low | Stimulation | ||||||||
| Kao-high | Overall inhibition, inhibition for SHs and AHs, stimulation for Rs and As | Low SSA and CEC | |||||||
| 5 | Ver | Purchased | 20 d | Naphthalene, Anthracene | Stimulation | Protect from toxicity | Aerobic | Microbial community | [23] |
| 6 | Mon | Purchased | 60 d | Crude oil | Stimulation | Adsorbent | Aerobic + Anaerobic | Microbial community | [24] |
| Mon-Org | Modified by DDDMA bromide | Neutral | Poor adsorption | ||||||
| Mon-Acid | HCl modified | Neutral | Poor adsorption | ||||||
| Mon-Na | NaCl modified | Stimulation | Adsorbent | ||||||
| Mon-K | KCl modified | Neutral | Poor adsorption | ||||||
| Mon-Ca | CaCl2 modified | Stimulation | Poor adsorption | ||||||
| Mon-Fe | FeCl3 modified | Stimulation | Poor adsorption | ||||||
| 7 | Mon | Purchased | 21 d | SHs in crude oil | Stimulation | High SSA | Aerobic + Anaerobic | Microbial community | [25] |
| Mon-Acid | HCl modified | Inhibition | Low pH | ||||||
| Mon-Org | DDDMA bromide modified | Inhibition | Adsorption is blocked and local bridging effect are weakened | ||||||
| Pal | Collected | Stimulation | High SSA | ||||||
| Pal-Acid | HCl modified | Inhibition | Low pH | ||||||
| Sap | Collected | Neutral | / | ||||||
| Sap-Acid | HCl modified | Inhibition | Low pH | ||||||
| Sap-Org | DDDMA bromide modified | Inhibition | / | ||||||
| Kao | Purchased | Inhibition | No local bridging effect, Low SSA | ||||||
| Kao-Acid | HCl modified | Inhibition | Low pH | ||||||
| 8 | Mon-Na | Purchased | 60 d | Crude oil | Stimulation | High SSA and CEC | Aerobic + Anaerobic | Microbial community | [26] |
| Mon-Org | Modified by DDDMA bromide | Inhibition | Hydrophobicity | ||||||
| Sap | Collected | Stimulation | High SSA and CEC | ||||||
| Sap-Org | Modified by DDDMA bromide | Neutral | Hydrophobicity | ||||||
| 9 | Kao | Purchased | 60 d | Crude oil | Inhibition | Low SSA and CEC | Aerobic + Anaerobic | Microbial community | [27] |
| Pal | Collected | Stimulation | High SSA and CEC | ||||||
| Sap | Collected | Neutral | / | ||||||
| Mon | Purchased | Stimulation | High SSA and CEC | ||||||
| Kao-Acid | HCl modified | Inhibition | Reduce pH to form biological toxicity | ||||||
| Pal-Acid | HCl modified | Inhibition | |||||||
| Sap-Acid | HCl modified | Inhibition | |||||||
| Mon-Acid | HCl modified | Inhibition | |||||||
| 10 | Mon | Purchased | 60 d | Crude oil | Stimulation | / | Aerobic + Anaerobic | Microbial community | [28] |
| Mon-Na | NaCl modified | Stimulation | SSA, CEC | ||||||
| Mon-K | KCl modified | Inhibition | Adsorbent | ||||||
| Mon-Mg | MgCl2 modified | / | / | ||||||
| Mon-Ca | CaCl2 modified | Stimulation | SSA, CEC | ||||||
| Mon-Zn | ZnCl2 modified | Inhibition | Adsorbent | ||||||
| Mon-Al | AlCl3 modified | Inhibition | Increase acidity | ||||||
| Mon-Cr | CrCl3 modified | Inhibition | Adsorb and increase acidity | ||||||
| Mon-Fe | FeCl3 modified | Stimulation | CEC | ||||||
| 11 | Mon | Purchased | 60 d | Phenanthrene and dibenzothiophene compounds | Stimulation | / | Aerobic + Anaerobic | Microbial community | [29] |
| Mon-Acid | HCl modified | Inhibition | |||||||
| Mon-Org | Modified by DDDMA bromide | Stimulation | |||||||
| Mon-Na | NaCl modified | Stimulation | |||||||
| Mon-K | KCl modified | Inhibition | |||||||
| Mon-Ca | CaCl2 modified | Stimulation | |||||||
| Mon-Zn | ZnCl2 modified | Inhibition | |||||||
| Mon-Cr | CrCl3 modified | Inhibition | |||||||
| Mon-Fe | FeCl3 modified | Stimulation | |||||||
| 12 | Mon-Na | NaCl modified | 60 d | AHs in crude oil | Stimulation | SSA, CEC | Aerobic | Microbial community | [30] |
| Mon-K | KCl modified | Inhibition | Adsorption and hydrophobic siloxane surface exposure | ||||||
| Mon-Mg | MgCl2 modified | Stimulation | High SSA and CEC, Local bridging effect | ||||||
| Mon-Ca | CaCl2 modified | Stimulation | SSA, CEC and local bridging effect | ||||||
| Mon-Zn | ZnCl2 modified | Inhibition | Adsorbed aromatic hydrocarbons | ||||||
| Mon-Al | AlCl3 modified | Inhibition | Low SSA | ||||||
| Mon-Cr | CrCl3 modified | Inhibition | Low SSA | ||||||
| Mon-Fe | FeCl3 modified | Stimulation | High SSA | ||||||
| 13 | Bentonite-Surf + Acid | Surfactant and palmitic acid modified | 21 d | Phenanthrene and cadmium contaminated soil | Stimulation | Adsorb cadmium to reduce toxicity | Aerobic | Microbial community | [31] |
| Bentonite-Surf | Surfactant modified | Stimulation | |||||||
| Bentonite | Purchased | Stimulation | |||||||
| 14 | Calcium bentonite | Collected | 30 d/60 d | Crude oil in the environment | Stimulation | High SSA | Aerobic + Anaerobic | Microbial community | [32] |
| Fuller soil | Collected | Stimulation | |||||||
| Kao | Collected | Stimulation | |||||||
| Eutrophic bentonite | Mixed with nutrients containing nitrogen, phosphorus and potassium | Stimulation | Fixed nutrients | ||||||
| Eutrophic fuller soil | Stimulation | ||||||||
| Eutrophic kaolinite | Stimulation | ||||||||
| 15 | Pal | Purchased | 5 d | Phenanthrene(C14) | Stimulation | Stimulate biofilm formation and accommodate extracellular enzymes | Aerobic | Burkholderia sartisoli | [33] |
| Pal-Ther | Thermal modification | Stimulation | Reduced cooperation with the phenanthrene | ||||||
| 16 | Mon | Collected | 21 d | Phenanthrene(C14) | Stimulation | High SSA and CEC | Aerobic | Burkholderia sartisoli RP007 + Microbial community | [34] |
| Mon-Acid | HCl modified | Stimulation | Element release, increase SSA and CEC | ||||||
| Mon-Alk | NaOH modified | Stimulation | |||||||
| Pal | Purchased | Stimulation | High SSA and CEC | ||||||
| Pal-Acid | HCl modified | Stimulation | Element release, increase SSA and CEC | ||||||
| Pal-Alk | NaOH modified | Stimulation | |||||||
| 17 | Mon | Purchased | / | Crude oil | Stimulation | Stimulate contact with nutrients | Aerobic + Anaerobic | Microbial community | [35] |
| Sap | Collected | Stimulation | Increase nutrient utilization | ||||||
| Mon-Org | Modified by didecyl dimethyl ammonium bromide | Inhibition for LMW AHs | Adsorbent | ||||||
| Sap-Org | Inhibition for LMW AHs and stimulation for phenanthrene | Adsorbent | |||||||
| 18 | Mon | Purchased | 21 d | AHs in crude oil | Stimulation | High SSA and CEC | Aerobic + Anaerobic | Microbial community | [36] |
| Sap | Collected | Stimulation | |||||||
| Pal | Purchased | Stimulation | Channel structure | ||||||
| Kao | Purchased | Inhibition | Influence of impurities | ||||||
| Mon-Acid | HCl modified | Inhibition | Decrease pH | ||||||
| Sap-Acid | HCl modified | Inhibition | |||||||
| Pal-Acid | HCl modified | Inhibition | |||||||
| Kao-Acid | HCl modified | Inhibition | |||||||
| 19 | Kao | Purchased | 48 h | Phenanthrene | Stimulation | Silicon/oxygen atoms stimulate biological effects | Aerobic | Sphingomonas sp. GY2B | [37] |
| Quartz | Purchased | Stimulation | |||||||
| 20 | Nontronite | Collected | 37 d | Crude oil | Stimulation | Stimulate ion exchange and nutrient absorption | Aerobic | Alcanivorax borkumensis | [38] |
| 21 | Bentonite | Purchased | 70 d | AHs and cadmium contaminated soil | Stimulation | Adsorption of heavy metals | Aerobic + Anaerobic | Microbial community | [39] |
| Bentonite- Surf | Modified by Arquad | Stimulation | Improve biological activity | ||||||
| Bentonite- Surf + Acid | Modified by Arquad and palmitic acid | Stimulation | Adsorb cadmium to reduce toxicity | ||||||
| 22 | Pal | Collected | 2 mon | Crude oil contaminated soil | Neutral | / | Aerobic | Microbial community | [40] |
| Pal-Org | Modified by DDTMA bromide | Neutral | |||||||
| 23 | Illite | Purchased | 56 d | Heavy oil | Inhibition for all SHs and 50 AHs, stimulation for 45 AHs | Adsorption and cation-π | Aerobic | Pseudomonas stutzeri | This study |
2. Materials and Methods
2.1. Materials
2.1.1. Illite
2.1.2. Strain Pseudomonas stutzeri L1SHX-3X
2.1.3. Heavy Oil
2.2. Methods
2.2.1. Experiment on the Interaction between Illite and P. stutzeri-Heavy Oil Complex
2.2.2. Measurements of Particle Size and SSA of Illite
2.2.3. Measurements of Viscosity and Density of Heavy Oil
2.2.4. Gene Sequence Analysis of In Situ Microorganisms in Heavy Oil
2.2.5. Gas Chromatography Analysis
2.2.6. Measurements of pH, Conductivity, and Redox Potential
2.2.7. Fractions Analysis of Heavy Oil
2.2.8. Gas Chromatography–Mass Spectrometry Analysis of Saturated and Aromatic Hydrocarbons
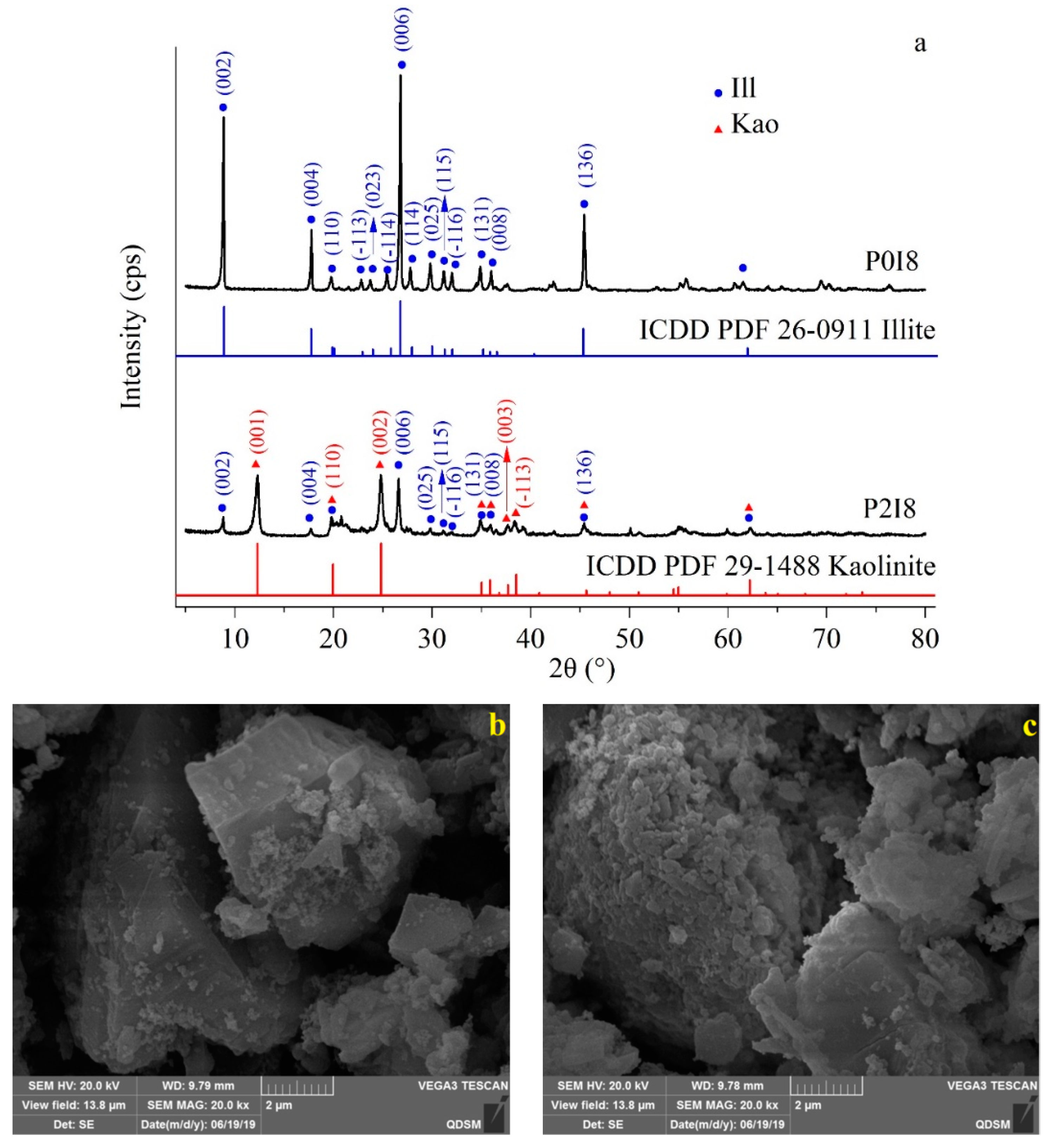
2.2.9. X-ray Diffraction Analysis of Illite
2.2.10. Scanning Electron Microscopy Analysis of Illite
3. Results and Discussion
3.1. Effect of Illite on Heavy Oil and In Situ Microorganisms in Heavy Oil
3.2. Illite Effect on P. stutzeri-Heavy Oil Complex
3.2.1. Illite Effect on Activity of P. stutzeri
3.2.2. Illite Inhibition of Saturated Hydrocarbons Biodegradation
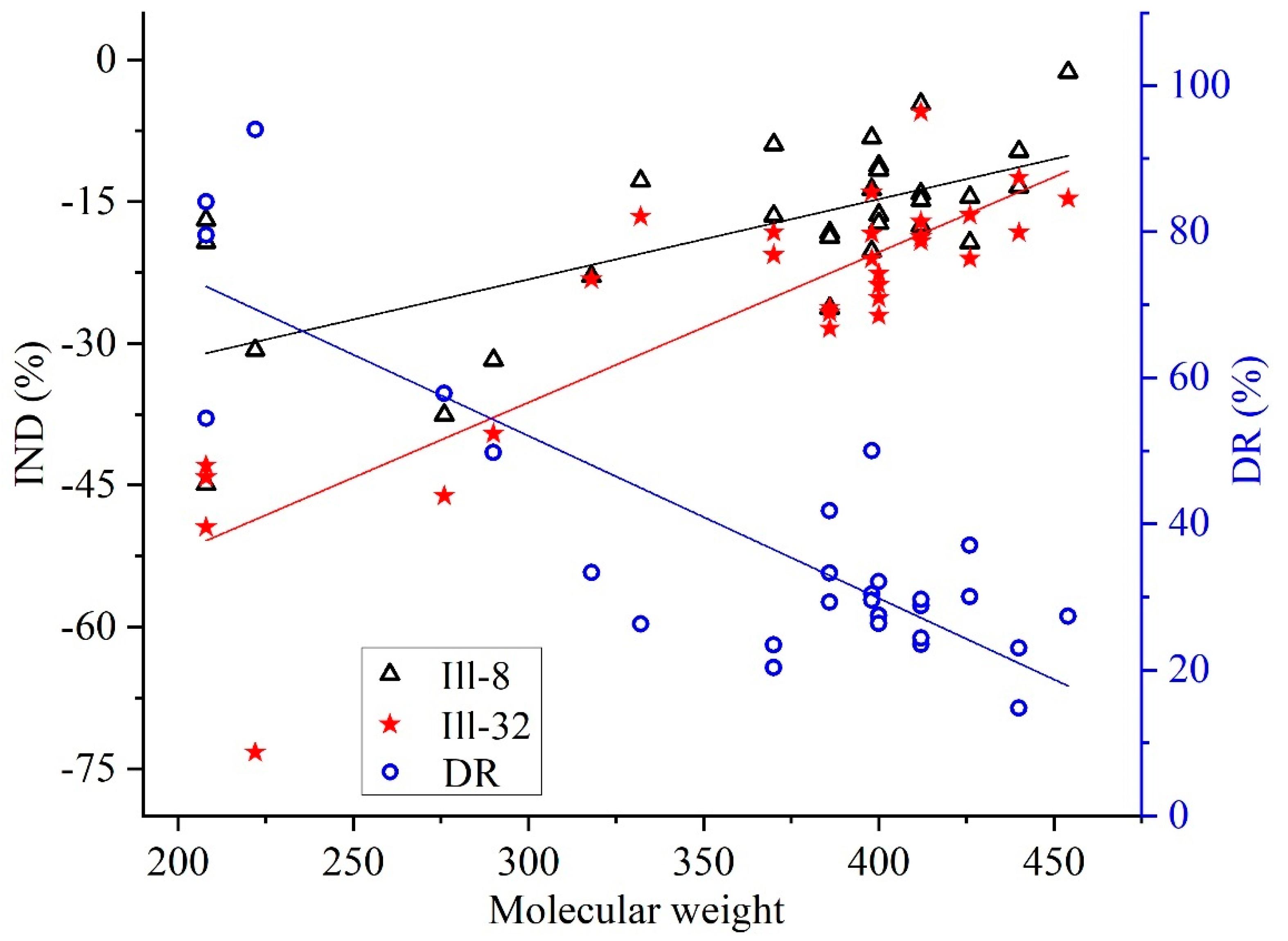
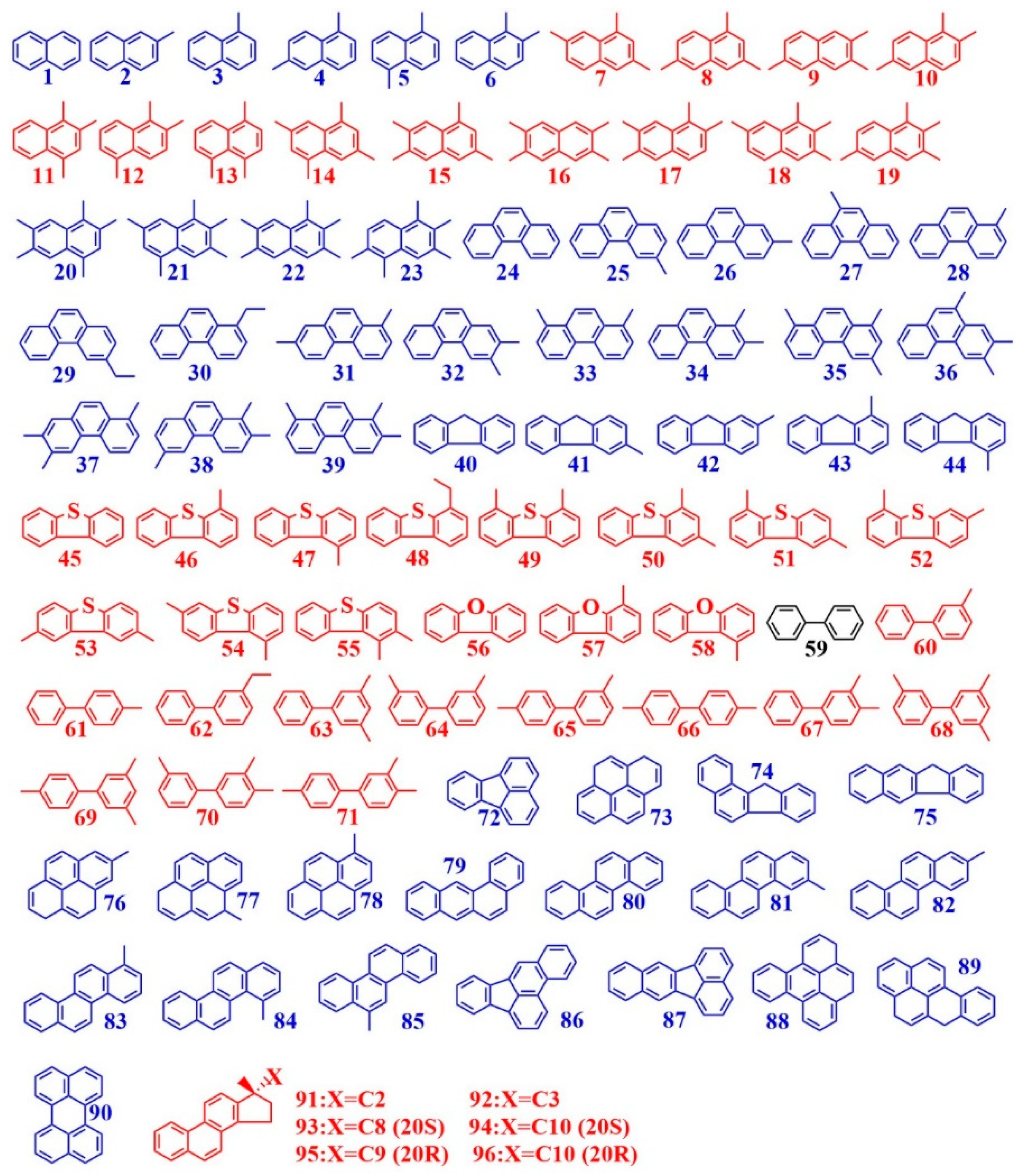
3.2.3. Two Effects of Illite on Aromatic Hydrocarbons Biodegradation
3.3. Kaolinization of Illite in P. stutzeri-Heavy Oil Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Q.; Sun, Z.; Forsling, W.; Tang, H. Adsorption of Copper at Aqueous Illite Surfaces. J. Colloid Interface Sci. 1997, 187, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Macht, F.; Eusterhues, K.; Pronk, G.J.; Totsche, K.U. Specific surface area of clay minerals: Comparison between atomic force microscopy measurements and bulk-gas (N2) and -liquid (EGME) adsorption methods. Appl. Clay Sci. 2011, 53, 20–26. [Google Scholar] [CrossRef]
- Seabaugh, J.L.; Dong, H.; Kukkadapu, R.K.; Eberl, D.D.; Morton, J.P.; Kim, J. Microbial reduction of fe(III) in the Fithian and Muloorina illites: Contrasting extents and rates of bioreduction. Clays Clay Miner. 2006, 54, 67–79. [Google Scholar] [CrossRef]
- Chang, P.-H.; Li, Z.; Jean, J.-S.; Jiang, W.-T.; Wang, C.-J.; Lin, K.-H. Adsorption of tetracycline on 2:1 layered non-swelling clay mineral illite. Appl. Clay Sci. 2012, 67–68, 158–163. [Google Scholar] [CrossRef]
- Li, S.; He, H.; Tao, Q.; Zhu, J.; Tan, W.; Ji, S.; Yang, Y.; Zhang, C. Kaolinization of 2:1 type clay minerals with different swelling properties. Am. Miner. 2020, 105, 687–696. [Google Scholar] [CrossRef]
- Sergeyev, Y.M.; Grabowska-Olszewska, B.; Osipov, V.I.; Sokolov, V.N.; Kolomenski, Y.N. The classification of microstructures of clay soils. J. Microsc. 1980, 120, 237–260. [Google Scholar] [CrossRef]
- US Department of Interior and US Geological Survey. Heavy Oil and Natural Bitumen: Strategic Petroleum Resources. 2003; pp. 1–2. Available online: https://pubs.usgs.gov/fs/fs070-03/fs070-03.pdf (accessed on 12 December 2020).
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide: Biomarkers and Isotopes in Petroleum Exploration and Earth History; Cambridge University Press: Cambridge, UK, 2005; pp. 560–579. [Google Scholar]
- China National Energy Administration. Classification of Oil Reservoir, SY/T6169-2021; Petroleum Industry Press: Bijing, China, 2021. (In Chinese)
- Rahman, P.; Thahira-Rahman, J.; Lakshmanaperumalsamy, P.; Banat, I. Towards efficient crude oil degradation by a mixed bacterial consortium. Bioresour. Technol. 2002, 85, 257–261. [Google Scholar] [CrossRef]
- Nnabuife, O.O.; Ogbonna, J.C.; Anyanwu, C.; Ike, A.C.; Eze, C.N.; Enemuor, S.C. Mixed bacterial consortium can hamper the efficient degradation of crude oil hydrocarbons. Arch. Microbiol. 2022, 204, 306. [Google Scholar] [CrossRef]
- Lalucat, J.; Bennasar, A.; Bosch, R.; Garcia-Valdes, E.; Palleroni, N.J. Biology of Pseudomonas stutzeri. Microbiol. Mol. Biol. Rev. 2006, 70, 510–547. [Google Scholar] [CrossRef]
- Li, H.; Chen, G.; Zhang, Y.; Xu, H.; Jin, H.; Zhang, C. Isolation and identification of high-efficiency petroleum-degrading bacteria and their degradation characteristics. J. Harbin Inst. Technol. 2007, 39, 1664–1669. (In Chinese) [Google Scholar]
- Celik, G.Y.; Aslim, B.; Beyatli, Y. Enhanced crude oil biodegradation and rhamnolipid production by Pseudomonas stutzeri strain G11 in the presence of Tween-80 and Triton X-100. J. Environ. Biol. 2008, 29, 867–870. [Google Scholar] [PubMed]
- Hao, J.; Gao, L.; Wu, C.; Zhang, H.; Wang, Y.; Chen, Z.; Zhao, X. Isolation and optimization of a crude-oil-degrading bacteria Pseudomonas stutzeri TH-31. Chin. J. Environ. Eng. 2015, 9, 1771–1777. [Google Scholar]
- Baquiran, J.P.; Thater, B.; Songco, K.; Crowley, D. Characterization of Culturable PAH and BTEX Degrading Bacteria from Heavy Oil of the Rancho La Brea Tarpits. Polycycl. Aromat. Compd. 2012, 32, 600–614. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, H.; Edelmann, R.E.; Zeng, Q.; Agrawal, A. Coupling of Fe(II) oxidation in illite with nitrate reduction and its role in clay mineral transformation. Geochim. Cosmochim. Acta 2017, 200, 353–366. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, W.; Zhang, X.; Zhu, M.; Tan, W. Characteristics of bio-desilication and bio-flotation of Paenibacillus mucilaginosus BM-4 on aluminosilicate minerals. Int. J. Miner. Process. 2017, 168, 40–47. [Google Scholar] [CrossRef]
- Weise, A.M.; Lee, K. The effect of clay-oil flocculation on natural oil degradation. Int. Oil Spill Conf. Proc. 1997, 1997, 955–956. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Tazaki, K. How kaolinite plays an essential role in remediating oil-polluted seawater. Clay Miner. 2005, 40, 481–491. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Tazaki, K.; Asada, R.; Kogure, K. Interaction between clay minerals and hydrocarbon-utilizing indigenous microorganisms in high concentrations of heavy oil: Implications for bioremediation. Clay Miner. 2005, 40, 105–114. [Google Scholar] [CrossRef]
- Warr, L.N.; Perdrial, J.N.; Lett, M.-C.; Heinrich-Salmeron, A.; Khodja, M. Clay mineral-enhanced bioremediation of marine oil pollution. Appl. Clay Sci. 2009, 46, 337–345. [Google Scholar] [CrossRef]
- Froehner, S.; Da Luz, E.C.; Maceno, M. Enhanced Biodegradation of Naphthalene and Anthracene by Modified Vermiculite Mixed with Soil. Water Air, Soil Pollut. 2009, 202, 169–177. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Head, I.M.; Manning, D.A.C. Effect of modified montmorillonites on the biodegradation and adsorption of biomarkers such as hopanes, steranes and diasteranes. Environ. Sci. Pollut. Res. 2013, 20, 8881–8889. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Jones, M.D.; Head, I.M.; Manning, D.A.C.; Fialips, C.I. Biodegradation of crude oil saturated fraction supported on clays. Biogeochemistry 2013, 25, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Ugochukwu, U.C.; Manning, D.A.; Fialips, C.I. Microbial degradation of crude oil hydrocarbons on organoclay minerals. J. Environ. Manag. 2014, 144, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Ugochukwu, U.C.; Jones, M.D.; Head, I.M.; Manning, D.A.; Fialips, C.I. Effect of acid activated clay minerals on biodegradation of crude oil hydrocarbons. Int. Biodeterior. Biodegrad. 2014, 88, 185–191. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Jones, M.D.; Head, I.M.; Manning, D.A.; Fialips, C.I. Biodegradation and adsorption of crude oil hydrocarbons supported on “homoionic” montmorillonite clay minerals. Appl. Clay Sci. 2014, 87, 81–86. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Head, I.M.; Manning, D.A.C. Biodegradation and adsorption of C1- and C2-phenanthrenes and C1- and C2-dibenzothiophenes in the presence of clay minerals: Effect on forensic diagnostic ratios. Biogeochemistry 2013, 25, 515–527. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Manning, D.A.; Fialips, C.I. Effect of interlayer cations of montmorillonite on the biodegradation and adsorption of crude oil polycyclic aromatic compounds. J. Environ. Manag. 2014, 142, 30–35. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Mandal, A.; Naidu, R. Heavy metal-immobilizing organoclay facilitates polycyclic aromatic hydrocarbon biodegradation in mixed-contaminated soil. J. Hazard. Mater. 2015, 298, 129–137. [Google Scholar] [CrossRef]
- Warr, L.N.; Friese, A.; Schwarz, F.; Schauer, F.; Portier, R.J.; Basirico, L.M.; Olson, G.M. Experimental study of clay-hydrocarbon interactions relevant to the biodegradation of the Deepwater Horizon oil from the Gulf of Mexico. Chemosphere 2016, 162, 208–221. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Naidu, R. Bacterial mineralization of phenanthrene on thermally activated palygorskite: A 14C radiotracer study. Sci. Total Environ. 2016, 579, 709–717. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Rusmin, R.; Naidu, R. Mild acid and alkali treated clay minerals enhance bioremediation of polycyclic aromatic hydrocarbons in long-term contaminated soil: A 14C-tracer study. Environ. Pollut. 2017, 223, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Ugochukwu, U.C.; Fialips, C.I. Removal of crude oil polycyclic aromatic hydrocarbons via organoclay-microbe-oil interactions. Chemosphere 2017, 174, 28–38. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Fialips, C.I. Crude oil polycyclic aromatic hydrocarbons removal via clay-microbe-oil interactions: Effect of acid activated clay minerals. Chemosphere 2017, 178, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Wu, P.; Ruan, B.; Zhang, Y.; Lai, X.; Yu, L.; Li, Y.; Dang, Z. Differential regulation of phenanthrene biodegradation process by kaolinite and quartz and the underlying mechanism. J. Hazard. Mater. 2018, 349, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Warr, L.N.; Schlüter, M.; Schauer, F.; Olson, G.M.; Basirico, L.M.; Portier, R.J. Nontronite-enhanced biodegradation of Deepwater Horizon crude oil by Alcanivorax borkumensis. Appl. Clay Sci. 2018, 158, 11–20. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Faustorilla, M.V.; Naidu, R. Effect of surface-tailored biocompatible organoclay on the bioavailability and mineralization of polycyclic aromatic hydrocarbons in long-term contaminated soil. Environ. Technol. Innov. 2018, 10, 152–161. [Google Scholar] [CrossRef]
- Tolpeshta, I.I.; Erkenova, M.I. Effect of Palygorskite Clay, Fertilizers, and Lime on the Degradation of Oil Products in Oligotrophic Peat Soil under Laboratory Experimental Conditions. Eurasian Soil Sci. 2018, 51, 229–240. [Google Scholar] [CrossRef]
- Wang, G.F.; Wang, S.; Sun, W.; Zheng, S.L. Experimental Research on Bleaching of Illite by Acid Leaching. Bull. Chin. Ceram. Soc. 2016, 35, 1301–1305. (In Chinese) [Google Scholar]
- Tian, Y.; Wan, Y.-Y.; Mu, H.-M.; Dong, H.-L.; Briggs, B.; Zhang, Z.-H. Microbial Diversity in High-Temperature Heavy Oil Reservoirs. Geomicrobiol. J. 2019, 37, 59–66. [Google Scholar] [CrossRef]
- Mu, H.M.; Wan, Y.Y.; Wu, B.C.; Tian, Y.; Dong, H.L.; Xian, C.G.; Li, Y. A rapid change in microbial communities of the shale gas drilling fluid from 3548 m depth to the above-ground storage tank. Sci. Total. Environ. 2021, 784, 147009. [Google Scholar] [CrossRef]
- Wan, Y.Y.; Dong, H. Environmental Geomicrobiology Experiments; Petroleum Industry Press: Beijing, China, 2014; pp. 100–105. [Google Scholar]
- Van Niel, C.B. The culture, general physiology, morphology, and classification of the non-sulfur purple and brown bacteria. Bacteriol. Rev. 1944, 8, 1–118. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, Y.; Wu, G.; Li, Z.; Li, H.; Sui, H. Solvent extraction for heavy crude oil removal from contaminated soils. Chemosphere 2012, 88, 245–249. [Google Scholar] [CrossRef]
- Wan, Y.Y.; Du, W. New Approach Technologies for Prevention and Control of Petroleum Contaminated and Sediments; Petroleum Industry Press: Beijing, China, 2017; pp. 77–78. [Google Scholar]
- Raheem, A.S.A.; Hentati, D.; Bahzad, D.; Abed, R.M.; Ismail, W. Biocatalytic upgrading of unconventional crude oil using oilfield-inhabiting bacterial consortia. Int. Biodeterior. Biodegrad. 2022, 174, 105468. [Google Scholar] [CrossRef]
- Ransmark, E.; Svensson, B.; Svedberg, I.; Göransson, A.; Skoglund, T. Measurement of homogenisation efficiency of milk by laser diffraction and centrifugation. Int. Dairy J. 2019, 96, 93–97. [Google Scholar] [CrossRef]
- GB/T 30515-2014; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, 2014. Petroleum Products-Transparent and Opaque Liquids-Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. China Quality Inspection Press: Beijing, China, 2014. (In Chinese)
- GB/T 29617-2013; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, 2013. Determination of Density, Relative Density, and API Gravity of Iquids by Digital Density Meter. China Quality Inspection Press: Beijing, China, 2013. (In Chinese)
- Goecks, J.; Nekrutenko, A.; Taylor, J. Galaxy the Galaxy Team Galaxy: A comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 2010, 11, R86. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Li, L.; Wan, Y.Y.; Li, Z.G.; Luo, N.; Mu, H.M.; Li, W.H.; Zhang, Y. Interaction between in-situ oil reservoir microorganisms and minerals. J. China Univ. Pet. 2021, 45, 121–130. (In Chinese) [Google Scholar]
- Du, W.D.; Wan, Y.Y.; Zhong, N.N.; Fei, J.J.; Zhang, Z.H. Current status of petroleum-contaminated soils and sediments. J. Wuhan Univ. 2011, 57, 311–322. [Google Scholar]
- SY/T5119-2016; China National Energy Administration, 2016. Analysis Method for Family Composition of Rock Extracts and Crude Oil. Petroleum Industry Press: Beijing, China, 2013. (In Chinese)
- Liu, Y.; Wan, Y.Y.; Zhu, Y.; Fei, C.; Shen, Z.; Ying, Y. Impact of Biodegradation on Polar Compounds in Crude Oil: Comparative Simulation of Biodegradation from Two Aerobic Bacteria Using Ultrahigh-Resolution Mass Spectrometry. Energy Fuels 2020, 34, 5553–5565. [Google Scholar] [CrossRef]
- Gu, J.D. Biodegradation testing: So many tests but very little new innovation. Appl. Environ. Biotechnol. 2016, 1, 92–95. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Y.Y.; Wang, C.; Ma, Z.; Liu, X.; Li, S. Biodegradation of n-alkanes in crude oil by three identified bacterial strains. Fuel 2020, 275, 117897. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Pevear, D.R.; Hill, R.J. Mineral Surface Control of Organic Carbon in Black Shale. Science 2002, 295, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.M.; Shaikh, S.M.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Masliyah, J. Role of fine clays in bitumen extraction from oil sands. AIChE J. 2004, 50, 1917–1927. [Google Scholar] [CrossRef]
- Du, W.; Wan, Y.Y.; Zhong, N.N.; Fei, J.J.; Zhang, Z.H.; Chen, L.J.; Hao, J.M. Status quo of soil petroleum contamination and evolution of bioremediation. Pet. Sci. 2011, 8, 502–514. [Google Scholar] [CrossRef]
- Wang, D.; Lin, J.; Lin, J.; Wang, W.; Li, S. Biodegradation of Petroleum Hydrocarbons by Bacillus subtilis BL-27, a Strain with Weak Hydrophobicity. Molecules 2019, 24, 3021. [Google Scholar] [CrossRef]
- Volkman, J.K.; Alexander, R.; Kagi, R.I.; Rowland, S.J.; Sheppard, P.N. Biodegradation of aromatic hydrocarbons in crude oils from the Barrow Sub-basin of Western Australia. Org. Geochem. 1984, 6, 619–632. [Google Scholar] [CrossRef]
- Fisher, S.J.; Alexander, R.; Kagi, R.I. Biodegradation of Alkylnaphthalenes in Sediments Adjacent to an Off-Shore Petroleum Production Platform. Polycycl. Aromat. Compd. 1996, 11, 35–42. [Google Scholar] [CrossRef]
- Bao, J.; Zhu, C. The effects of biodegradation on the compositions of aromatic hydrocarbons and maturity indicators in biodegraded oils from Liaohe Basin. Sci. China Ser. D Earth Sci. 2009, 52, 59–68. [Google Scholar] [CrossRef]
- van Aarssen, B.G.; Bastow, T.P.; Alexander, R.; Kagi, R.I. Distributions of methylated naphthalenes in crude oils: Indicators of maturity, biodegradation and mixing. Org. Geochem. 1999, 30, 1213–1227. [Google Scholar] [CrossRef]
- Cheng, X.; Hou, D.; Mao, R.; Xu, C. Severe biodegradation of polycyclic aromatic hydrocarbons in reservoired crude oils from the Miaoxi Depression, Bohai Bay Basin. Fuel 2018, 211, 859–867. [Google Scholar] [CrossRef]
- Zhu, D.; Herbert, B.E.; Schlautman, M.A.; Carraway, E.; Hur, J. Cation-π bonding: A new perspective on the sorption of polycyclic aromatic hydrocarbons to mineral surfaces. J. Environ. Qual. 2004, 33, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- SY/T5163-2018; China National Energy Administration, 2018. Analysis Method for Clay Minerals and Ordinary Non-Clay Minerals in Sedimentary Rocks by the X-ray Diffraction. Petroleum Industry Press: Beijing, China, 2018. (In Chinese)
- Hong, H.; Fang, Q.; Cheng, L.; Wang, C.; Churchman, G.J. Microorganism-induced weathering of clay minerals in a hydromorphic soil. Geochim. Cosmochim. Acta 2016, 184, 272–288. [Google Scholar] [CrossRef]
- Fiore, S.; Dumontet, S.; Huertas, F.J.; Pasquale, V. Bacteria-induced crystallization of kaolinite. Appl. Clay Sci. 2011, 53, 566–571. [Google Scholar] [CrossRef]

| No. | First Appearance Location | Content | Abbreviation | Occurrence Number |
|---|---|---|---|---|
| 1 | Introduction | montmorillonite | Mon | 8 |
| 2 | kaolinite | Kao | 12 | |
| 3 | saponite | Sap | 5 | |
| 4 | palygorskite | Pal | 5 | |
| 5 | specific surface area | SSA | 10 | |
| 6 | cation exchange capacity | CEC | 9 | |
| 7 | Pseudomonas stutzeri-heavy oil system | PstHO | 25 | |
| 8 | saturated hydrocarbons | SHs | 35 | |
| 9 | aromatic hydrocarbons | AHs | 46 | |
| 10 | Materials and methods | modified Van Niel culture medium | MVN | 16 |
| 11 | gas chromatography | GC | 5 | |
| 12 | conductivity | σ | 9 | |
| 13 | redox potential | Eh | 9 | |
| 14 | resins | Rs | 4 | |
| 15 | asphaltenes | Aps | 4 | |
| 16 | saturated hydrocarbons, aromatic hydrocarbons, resins, and asphaltenes | SARA | 6 | |
| 17 | gas chromatography–mass spectrometry | GC–MS | 8 | |
| 18 | X-ray diffraction | XRD | 3 | |
| 19 | scanning electron microscope | SEM | 4 | |
| 20 | fraction content | FC | 3 | |
| 21 | the content difference of CO2, O2 and N2 between illite-PstHO and atmosphere | ΔG | 3 | |
| 22 | residual mass content | RMC | 11 | |
| 23 | degradation rate | DR | 4 | |
| 24 | influence degree | IND | 9 | |
| 25 | Results and discussion | Peters and Moldowan | PM | 2 |
| 26 | high ring number (≥4) aromatic hydrocarbons | HRAHs | 7 | |
| 27 | Supplemental Materials | 8 g illite | Ill-8 | 29 |
| 28 | 32 g illite | Ill-32 | 29 | |
| 29 | organic modified montmorillonite | Mon-Org | 18 | |
| 30 | acid modified montmorillonite | Mon-Acid | 29 | |
| 31 | sodium ion modified montmorillonite | Mon-Na | 10 | |
| 32 | potassium ion modified montmorillonite | Mon-K | 21 | |
| 33 | calcium ion modified montmorillonite | Mon-Ca | 21 | |
| 34 | iron ion modified montmorillonite | Mon-Fe | 21 | |
| 35 | acid modified palygorskite | Pal-Acid | 10 | |
| 36 | acid modified saponite | Sap-Acid | 8 | |
| 37 | organic modified saponite | Sap-Org | 3 | |
| 38 | acid modified kaolinite | Kao-Acid | 8 | |
| 39 | magnesium ion modified montmorillonite | Mon-Mg | 8 | |
| 40 | zinc ion modified montmorillonite | Mon-Zn | 9 | |
| 41 | aluminum ion modified montmorillonite | Mon-Al | 8 | |
| 42 | chromium ion modified montmorillonite | Mon-Cr | 9 |
| Group # | Volume of P. stutzeri Culture (mL) | Optical Density of Culture (Cell/mL) | Mass of Illite (g) | Mass of Heavy Oil (g) | Volume of MVN (mL) | Duration (d) | Number of Parallel Replicates |
|---|---|---|---|---|---|---|---|
| P0I0 | 0 | 108 | 0 | 0.50 | 40.0 | 56 | 3 |
| P0I8 | 0 | 8.00 | |||||
| P2I0 | 2.0 | 0.00 | |||||
| P2I8 | 2.0 | 8.00 | |||||
| P2I32 | 2.0 | 32.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wan, Y.Y.; Mu, H.M.; Shi, S.B.; Chen, J.F. Interaction between Illite and a Pseudomonas stutzeri-Heavy Oil Biodegradation Complex. Microorganisms 2023, 11, 330. https://doi.org/10.3390/microorganisms11020330
Li L, Wan YY, Mu HM, Shi SB, Chen JF. Interaction between Illite and a Pseudomonas stutzeri-Heavy Oil Biodegradation Complex. Microorganisms. 2023; 11(2):330. https://doi.org/10.3390/microorganisms11020330
Chicago/Turabian StyleLi, Lei, Yun Yang Wan, Hong Mei Mu, Sheng Bao Shi, and Jian Fa Chen. 2023. "Interaction between Illite and a Pseudomonas stutzeri-Heavy Oil Biodegradation Complex" Microorganisms 11, no. 2: 330. https://doi.org/10.3390/microorganisms11020330
APA StyleLi, L., Wan, Y. Y., Mu, H. M., Shi, S. B., & Chen, J. F. (2023). Interaction between Illite and a Pseudomonas stutzeri-Heavy Oil Biodegradation Complex. Microorganisms, 11(2), 330. https://doi.org/10.3390/microorganisms11020330






