Effects of Cellulase and Lactic Acid Bacteria on Ensiling Performance and Bacterial Community of Caragana korshinskii Silage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silage Preparation
2.2. Analysis of Fermentation Parameters, Chemical Composition, and Microbial Counts
2.3. DNA Extraction, PCR and Sequencing
2.4. Bacterial Community Sequencing Analysis
2.5. Statistical Analysis
3. Results
3.1. Chemical Composition and Microbial Counts of Fresh Caragana korshinskii
3.2. Fermentation Characteristics and Chemical Compositions of Caragana korshinskii Silage
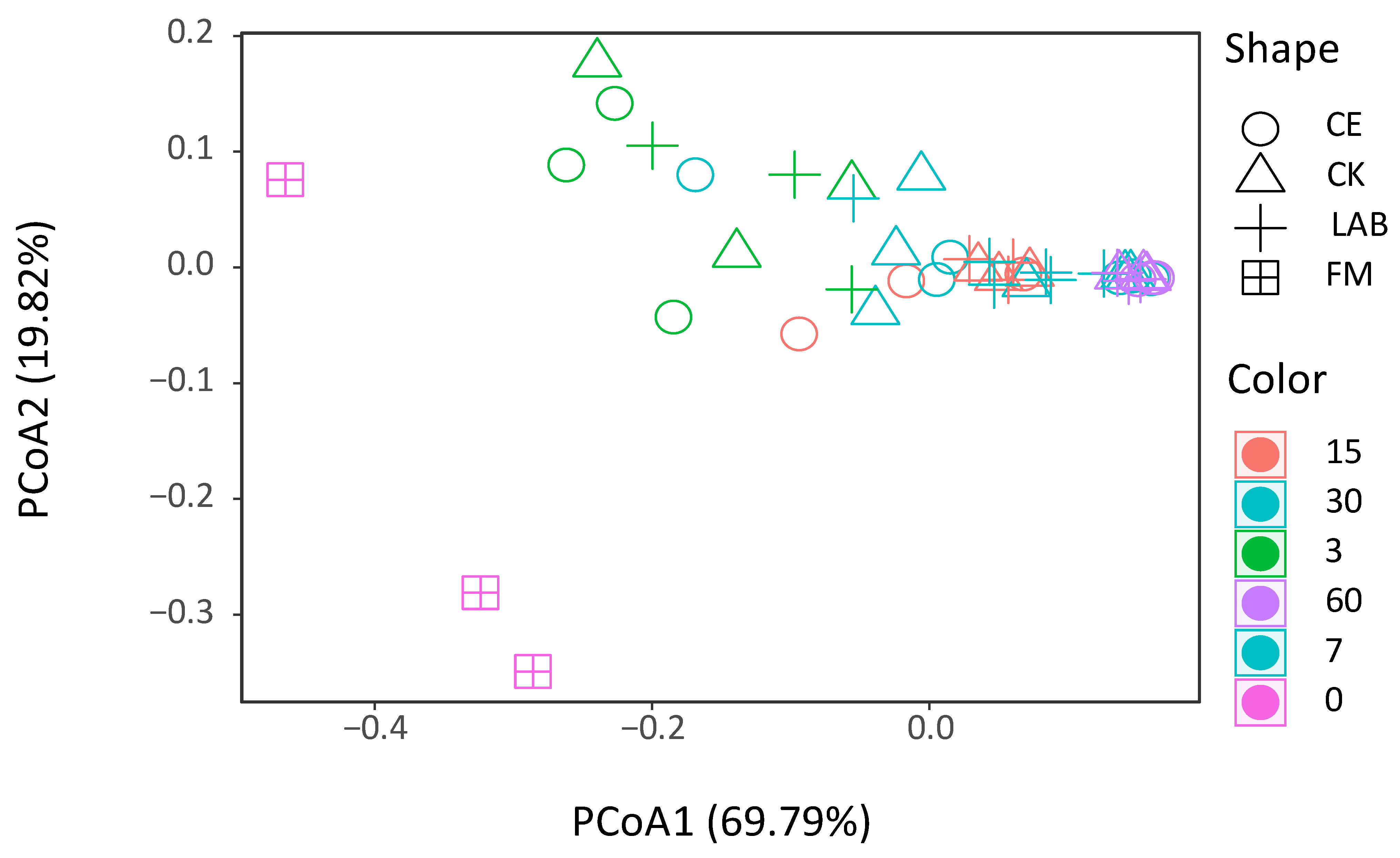
3.3. Bacterial Diversity and Community Composition in Caragana korshinskii Silage
3.4. Relationships between Fermentation Parameters and Bacterial Community
3.5. Predicted Metabolic Pathways on Three Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.N.; Zhou, Y.R.; Wang, H.M. Spatial heterogeneity of soil water content under introduced shrub (Caragana korshinskii) in desert grassland of the eastern Ningxia, China. J. Appl. Ecol. 2018, 29, 3577–3586. [Google Scholar]
- Na, X.F.; Xu, T.T.; Li, M.; Zhou, Z.N.; Ma, S.L.; Wang, J.; He, J.; Jiao, B.Z.; Ma, F. Variations of bacterial community diversity within the rhizosphere of three phylogenetically related perennial shrub plant species across environmental gradients. Front. Microbiol. 2018, 9, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.C.; Zhang, S.J.; Zhang, T.Z.; Shen, Y.Q.; Han, L.J.; Peng, Z.J. Bacterial cellulose production from ethylenediamine pretreated Caragana korshinskii Kom. Ind. Crops Prod. 2021, 164, 113340. [Google Scholar] [CrossRef]
- Zhong, C.; Sun, Z.; Zhou, Z.; Jin, M.J.; Tan, Z.L.; Jia, S.R. Chemical characterization and nutritional analysis of protein isolates from Caragana korshinskii Kom. J. Agric. Food. Chem. 2014, 62, 3217–3222. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, F. Silage and the safety and quality of dairy foods: A review. Agric. Food Sci. 2013, 22, 16–34. [Google Scholar] [CrossRef]
- Sun, Q.Z.; Gao, F.Q.; Yu, Z.; Tao, Y.; Zhao, S.F.; Cai, Y.M. Fermentation quality and chemical composition of shrub silage treated with lactic acid bacteria inoculants and cellulase additives. Anim. Sci. J. 2012, 83, 305–309. [Google Scholar] [CrossRef]
- He, L.W.; Zhou, W.; Wang, C.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Effect of cellulase and Lactobacillus casei on ensiling characteristics, chemical composition, antioxidant activity, and digestibility of mulberry leaf silage. J. Dairy Sci. 2019, 102, 9919–9931. [Google Scholar] [CrossRef]
- Lei, C.; Yuan, X.J.; Li, J.F.; Wang, S.R.; Dong, Z.H.; Shao, T. Effect of lactic acid bacteria and propionic acid on conservation characteristics, aerobic stability and in vitro gas production kinetics and digestibility of whole-crop corn based total mixed ration silage. J. Integr. Agric. 2017, 16, 1592–1600. [Google Scholar]
- Ogunade, I.M.; Kim, D.H.; Jiang, Y.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Control of Escherichia coli O157: H7 in contaminated alfalfa silage: Effects of silage additives. J. Dairy Sci. 2016, 99, 4427–4436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Q.M.; Li, P.; Xiao, B.X.; Yang, F.Y.; Li, D.X.; Ge, G.T. Effects of LAB inoculant and cellulase on the fermentation quality and chemical composition of forage soybean silage prepared with corn stover. Grassl. Sci. 2021, 67, 83–90. [Google Scholar] [CrossRef]
- Ke, W.C.; Ding, W.R.; Xu, D.M.; Ding, L.M.; Zhang, P.; Li, F.D. Effects of addition of malic or citric acids on fermentation quality and chemical characteristics of alfalfa silage. J. Dairy Sci. 2017, 100, 8958–8966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Wu, B.Y.L.; Nishino, N.K.; Wang, X.G.; Yu, Z. Fermentation and microbial population dynamics during the ensiling of native grass and subsequent exposure to air. Anim. Sci. J. 2016, 87, 389–397. [Google Scholar] [CrossRef]
- Sun, L.; Na, N.; Li, X.M.; Li, Z.Q.; Wang, C.; Wu, X.G. Impact of packing density on the bacterial community, fermentation, and in vitro digestibility of whole-crop barley silage. Agriculture 2021, 11, 672. [Google Scholar] [CrossRef]
- Owens, V.N.; Albrecht, K.A.; Muck, R.E.; Duke, S.H. Protein degradation and fermentation characteristics of red clover and alfalfa silage harvested with varying levels of total nonstructural carbohydrates. Crop Sci. 1999, 39, 1873–1880. [Google Scholar] [CrossRef] [Green Version]
- Van Soest, P.J.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Cota-Sánchez, J.H.; Remarchuk, K.; Ubayasena, K. Ready-to-use DNA extracted with a CTAB method adapted for herbarium specimens and mucilaginous plant tissue. Plant Mol. Biol. Rep. 2006, 24, 161–167. [Google Scholar] [CrossRef]
- Douglas, G.M.; Beiko, R.G.; Langille, M.G. Predicting the functional potential of the microbiome from marker genes using PICRUSt. In Microbiome Analysis; Humana Press: New York, NY, USA, 2018; pp. 169–177. [Google Scholar]
- Hall, M.; Beiko, R.G. 16S rRNA gene analysis with QIIME2. In Microbiome Analysis; Humana Press: New York, NY, USA, 2018; pp. 113–129. [Google Scholar]
- Li, M.; Zhou, H.L.; Zi, X.J.; Cai, Y.M. Silage fermentation and ruminal degradation of stylo prepared with lactic acid bacteria and cellulase. Anim. Sci. J. 2017, 88, 1531–1537. [Google Scholar] [CrossRef]
- Contreras-Govea, F.E.; Muck, R.E.; Broderick, J.A.; Weimer, P.J. Lactobacillus plantarum effects on silage fermentation and in vitro microbial yield. Anim. Feed Sci. Technol. 2013, 179, 61–68. [Google Scholar]
- Sheperd, A.C.; Maslanka, M.; Quinn, D.; Kung, L., Jr. Additives containing bacteria and enzymes for alfalfa silage. J. Dairy Sci. 1995, 78, 565–572. [Google Scholar] [CrossRef]
- Li, J.F.; Yuan, X.J.; Dong, Z.H.; Mugabe, W.Z.; Shao, T. The effects of fibrolytic enzymes, cellulolytic fungi and bacteria on the fermentation characteristics, structural carbohydrates degradation, and enzymatic conversion yields of Pennisetum sinese silage. Bioresour. Technol. 2018, 264, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Shaver, R. Interpretation and use of silage fermentation analysis reports. Focus Forage 2001, 3, 1–5. [Google Scholar]
- Bai, J.; Ding, Z.T.; Ke, W.C.; Xu, D.M.; Wang, M.S.; Huang, W.K. Different lactic acid bacteria and their combinations regulated the fermentation process of ensiled alfalfa: Ensiling characteristics, dynamics of bacterial community and their functional shifts. Microb. Biotechnol. 2021, 14, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zi, X.J.; Zhou, H.L.; Lv, R.L.; Tang, J.; Cai, Y.M. Silage fermentation and ruminal degradation of cassava foliage prepared with microbial additive. AMB Express 2019, 9, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, H.Z.; Liu, H.L.; Li, Z.P.; Nan, W.X.; Jin, C.A.; Sui, Y.T. Effect of Lactobacillus plantarum and Lactobacillus buchneri addition on fermentation, bacterial community and aerobic stability in lucerne silage. Anim. Prod. Sci. 2018, 59, 1528–1536. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Desta, S.T.; Dong, Z.; Mugabe, W.; Shao, T. Characterization of Enterococcus faecalis JF85 and Enterococcus faecium Y83 isolated from Tibetan yak (Bos grunniens) for ensiling Pennisetum sinese. Bioresour. Technol. 2018, 257, 76–83. [Google Scholar] [CrossRef]
- Desta, S.T.; Yuan, X.; Li, J.; Shao, T. Ensiling characteristics, structural and nonstructural carbohydrate composition and enzymatic digestibility of Napier grass ensiled with additives. Bioresour. Technol. 2016, 221, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Mu, L.; Xie, Z.; Hu, L.; Chen, G.; Zhang, Z. Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour. Technol. 2020, 315, 123772. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Rajion, M.A.; Goh, Y.M.; Farjam, A.S.; Sazili, A.Q.; Schonewille, J.T. The effects of adding lactic acid bacteria and cellulase in oil palm (Elais guineensis Jacq.) frond silages on fermentation quality, chemical composition and in vitro digestibility. Ital. J. Anim. Sci. 2014, 13, 3358. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, M.S.; Samaila, U.; Li, F.H.; Bai, J.; Zhang, J.Y. Lignocellulose conversion of ensiled Caragana korshinskii Kom. facilitated by Pediococcus acidilactici and cellulases. Microb. Biotechnol. 2022, 16, 432–447. [Google Scholar] [CrossRef]
- Nadeau, E.M.G.; Buxton, D.R.; Russell, J.R.; Allison, M.J.; Young, J.W. Enzyme, bacterial inoculant, and formic acid effects on silage composition of orchardgrass and alfalfa. J. Dairy Sci. 2000, 83, 1487–1502. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The biochemistry of silage, Chalcombe publications. Exp. Agric. 2008, 28, 179. [Google Scholar]
- Xiong, H.M.; Zhu, Y.C.; Wen, Z.Y.; Liu, G.B.; Guo, Y.Q.; Sun, B.L. Effects of Cellulase, Lactobacillus plantarum, and Sucrose on Fermentation Parameters, Chemical Composition, and Bacterial Community of Hybrid Pennisetum Silage. Fermentation 2022, 8, 356. [Google Scholar] [CrossRef]
- Xian, Z.Y.; Wu, J.Q.; Deng, M.; Wang, M.; Tian, H.C.; Liu, D.W. Effects of Cellulase and Lactiplantibacillus plantarum on the Fermentation Parameters, Nutrients, and Bacterial Community in Cassia alata Silage. Front. Microbiol. 2022, 13, 926065. [Google Scholar] [CrossRef]
- Zeng, T.R.; Li, X.L.; Guan, H.; Yang, W.Y.; Liu, W.G.; Liu, J. Dynamic microbial diversity and fermentation quality of the mixed silage of corn and soybean grown in strip intercropping system. Bioresour. Technol. 2020, 313, 123655. [Google Scholar] [CrossRef]
- Li, M.; Zi, X.J.; Zhou, H.L.; Lv, R.L.; Tang, J.; Cai, Y.M. Effect of lactic acid bacteria, molasses, and their combination on the fermentation quality and bacterial community of cassava foliage silage. Anim. Sci. J. 2021, 92, e13635. [Google Scholar] [CrossRef]
- Tian, H.C.; Wang, Y.; Liu, Z.C.; Hu, Z.Y.; Guo, Y.Q.; Deng, M. Effects of malic acid and sucrose on the fermentation parameters, CNCPS nitrogen fractions, and bacterial community of Moringa oleifera Leaves Silage. Microorganisms 2021, 9, 2102. [Google Scholar] [CrossRef]
- Helander, I.M.; Mattila-Sandholm, T. Fluorometric assessment of Gram-negative bacterial permeabilization. J. Appl. Microbiol. 2020, 88, 213–219. [Google Scholar] [CrossRef]
- Feng, Q.; Shi, W.; Chen, S.; Degen, A.A.; Qi, Y.; Yang, F.; Zhou, J. Addition of Organic Acids and Lactobacillus acidophilus to the Leguminous Forage Chamaecrista rotundifolia Improved the Quality and Decreased Harmful Bacteria of the Silage. Animals 2022, 12, 2260. [Google Scholar] [CrossRef]
- Wang, M.S.; Wang, L.A.; Yu, Z. Fermentation dynamics and bacterial diversity of mixed lucerne and sweet corn stalk silage ensiled at six ratios. Grass Forage Sci. 2019, 74, 264–273. [Google Scholar] [CrossRef]
- Alhaag, H.; Yuan, X.J.; Mala, A.; Bai, J.F.; Shao, T. Fermentation characteristics of Lactobacillus plantarum and Pediococcus species isolated from sweet sorghum silage and their application as silage inoculants. Appl. Sci. 2019, 9, 1247. [Google Scholar] [CrossRef] [Green Version]
- Sethi, S.; Datta, A.; Gupta, B.L.; Gupta, S. Optimization of cellulase production from bacteria isolated from soil. Int. Sch. Res. Not. 2013, 2013, 985685. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Zhi, D.; Wong, Y.; Tam, N.F. Biodegradation ability and dioxgenase genes of pah-degrading sphingomonas and mycobacterium strains isolated from mangrove sediments. Int. Biodeterior. Biodegr. 2010, 64, 419–426. [Google Scholar] [CrossRef]
- Ostling, C.; Lindgren, S. Influences of enterobacteria on the fermentation and aerobic stability of grass silages. Grass Forage Sci. 1995, 50, 41–47. [Google Scholar] [CrossRef]
- Sands, D.C.; Hankin, L. Selecting lysine-excreting mutants of lactobacilli for use in food and feed enrichment. Appl. Microbiol. 1974, 28, 523–524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ke, C.; Bai, J.; Li, H.; Xu, M.; Ding, T. The effect of Pediococcus acidilactici J17 with high-antioxidant activity on antioxidant, α-tocopherol, β-carotene, fatty acids, and fermentation profiles of alfalfa silage ensiled at two different dry matter contents. Anim. Feed Sci. Technol. 2020, 268, 114614. [Google Scholar] [CrossRef]
- Arenahalli, N.M.; Prashant, G.; Mandayam, S.N.; Siddalingaiya, G.P. Probiotic lactic acid bacterium from kanjika as a potential source of vitamin B12: Evidence from LC-MS, immunological and microbiological techniques. Biotechnol. Lett. 2010, 32, 503–506. [Google Scholar]




| Items | Sample |
|---|---|
| DM (g/kg FM) | 536.2 ± 7.31 |
| CP (g/kg DM) | 102.53 ± 2.75 |
| WSC (g/kg DM) | 27.69 ± 0.69 |
| NDF (g/kg DM) | 706.92 ± 2.03 |
| ADF (g/kg DM) | 594.99 ± 2.11 |
| ADL (g/kg DM) | 155.71 ± 2.22 |
| Lactic acid bacteria (Log10 cfu/g FM) | 6.11 ± 1.06 |
| Aerobic bacteria (Log10 cfu/g FM) | 8.15 ± 0.80 |
| Coliform bacteria (Log10 cfu/g FM) | 7.35 ± 2.13 |
| Yeasts (Log10 cfu/g FM) | 8.61 ± 0.33 |
| Molds (Log10 cfu/g FM) | 2.82 ± 0.28 |
| Items | Treatments | Ensiling Days | SEM | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 15 | 30 | 60 | T | D | T × D | |||
| pH value | CK | 4.24 Aa | 4.02 Ab | 3.98 Ab | 4.13 Aab | 4.12 Aab | 0.02 | <0.0001 | 0.048 | 0.4532 |
| CE | 4.04 Ba | 3.89 Cb | 3.89 Bb | 3.81 Bc | 3.74 Bd | |||||
| LAB | 4.02 Ba | 3.97 Bab | 3.95 ABbc | 3.9 ABc | 3.8 Bd | |||||
| LA (g/kg DM) | CK | 19.07 Aab | 23.87 Aab | 24.57 Aa | 23.44 Aab | 15.26 Bb | 0.94 | 0.0025 | 0.724 | 0.0132 |
| CE | 23.81 Ab | 24.73 Ab | 25.13 Ab | 27.92 Ab | 38.35 Aa | |||||
| LAB | 26.40 Aa | 28.06 Aa | 26.13 Aa | 24.36 Aa | 25.19 Ba | |||||
| AA (g/kg DM) | CK | 7.27 Ab | 8.24 Ab | 6.61 Ab | 12.90 Aa | 9.03 Bab | 0.63 | <0.0001 | 0.0003 | <0.0001 |
| CE | 7.57 Ab | 4.94 Ab | 5.08 Ab | 9.49 Ab | 18.37 Aa | |||||
| LAB | 3.29 Aa | 6.07 Aa | 6.12 Aa | 3.52 Ba | 4.83 Ba | |||||
| NH3-N (g/kg DM) | CK | 0.15 Bb | 0.10 Ab | 0.09 Ab | 0.34 Aa | 0.17 Ab | 0.03 | <0.0001 | <0.0001 | <0.0001 |
| CE | 0.05 Ba | ND | ND | ND | ND | |||||
| LAB | 0.62 Aa | 0.11 Ac | ND | 0.28 Ab | ND | |||||
| Items | Treatments | Ensiling Days | SEM | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 15 | 30 | 60 | T | D | T × D | |||
| DM (g/kg FM) | CK | 395.42 Aa | 391.87 Ba | 383.42 Ab | 369.63 Bc | 359.61 Bd | 1.73 | <0.0001 | <0.0001 | 0.0067 |
| CE | 397.63 Aa | 393.78 Aba | 385.35 Ab | 377.73 Abc | 371.69 Ac | |||||
| LAB | 398.43 Aa | 395.39 Aa | 388.76 Ab | 380.90 Ac | 375.77 Ad | |||||
| NDF (g/kg DM) | CK | 688.28 Aa | 677.67 Ab | 670.33 Ac | 662.62 Ad | 658.47 Ad | 2.31 | <0.0001 | <0.0001 | 0.0016 |
| CE | 677.67 Ba | 660.76 Cb | 648.02 Cc | 639.27 Bd | 635.55 Ce | |||||
| LAB | 685.18 Aa | 672.22 Bb | 666.30 Bc | 657.73 Ad | 651.08 Be | |||||
| ADF (g/kg DM) | CK | 582.67 Aa | 573.10 Ab | 565.65 Ac | 556.95 Ad | 545.80 Ae | 2.62 | <0.0001 | <0.0001 | 0.0425 |
| CE | 571.61 Ba | 556.17 Bb | 544.33 Cc | 535.94 Cd | 518.88 Be | |||||
| LAB | 580.42 Aa | 569.53 Ab | 561.29 Bb | 551.06 Bc | 540.74 Ad | |||||
| ADL (g/kg DM) | CK | 149.05 Aa | 145.65 Ab | 143.67 Abc | 141.79 Ac | 139.08 Ad | 0.78 | <0.0001 | <0.0001 | 0.9621 |
| CE | 142.31 Ba | 138.19 Bb | 135.69 Cc | 133.28 Bd | 130.90 Be | |||||
| LAB | 148.42 Aa | 144.80 Aab | 141.16 Bbc | 139.77 Abc | 138.24 Ac | |||||
| CP (g/kg DM) | CK | 101.24 Bd | 102.60 Abc | 103.51 Bab | 103.28 Cbc | 104.19 Ba | 0.27 | <0.0001 | <0.0001 | <0.0001 |
| CE | 102.55 Ae | 103.83 Ad | 104.28 Ac | 105.46 Ab | 106.35 Aa | |||||
| LAB | 99.63 Cd | 101.03 Bc | 103.59 Bb | 104.29 Bb | 105.82 Aa | |||||
| WSC (g/kg DM) | CK | 23.78 Aa | 20.07 Bb | 18.66 Cbc | 16.14 Ccd | 15.70 Bd | 0.63 | <0.0001 | 0.0006 | 0.3776 |
| CE | 28.1 Aa | 27.12 Aa | 26.53 Aa | 26.24 Aa | 25.99 Aa | |||||
| LAB | 23.95 Aa | 21.91 Ba | 21.43 Ba | 21.17 Ba | 20.06 Ba | |||||
| Items | Treatment | Ensiling Days | SEM | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 15 | 30 | 60 | T | D | T × D | |||
| OTUs | CK | 250.67 Abc | 222 Abc | 318.33 Aa | 276.67 Aab | 215.33 Ac | 6.35 | 0.513 | <0.0001 | 0.1206 |
| CE | 268.33 Aab | 241 Abc | 310.33 ABa | 222 Abc | 188.67 Ac | |||||
| LAB | 268 Aa | 214.67 Ab | 284.67 Ba | 265.33 Aa | 214.33 Ab | |||||
| Chao1 | CK | 251.21 Abc | 222.07 Ac | 318.68 Aa | 277.17 Aab | 215.85 Ac | 6.33 | 0.5146 | <0.0001 | 0.119 |
| CE | 268.48 Aab | 241.56 Abc | 310.72 ABa | 222.61 Abc | 189.32 Ac | |||||
| LAB | 268.47 Aa | 214.77 Ab | 284.67 Ba | 265.35 Aa | 215.14 Ab | |||||
| Simpson | CK | 0.88 ABa | 0.83 Aab | 0.86 Aab | 0.72 Ab | 0.76 Aab | 0.03 | 0.0032 | <0.0001 | 0.0044 |
| CE | 0.91 Aa | 0.75 Aa | 0.86 Aa | 0.51 Ab | 0.52 Bb | |||||
| LAB | 0.86 Ba | 0.74 Aab | 0.88 Aa | 0.63 Ab | 0.29 Cc | |||||
| Shannon | CK | 4.45 Aa | 3.98 Aab | 4.29 Aab | 3.72 Aab | 3.41 Ab | 0.16 | 0.0166 | <0.0001 | 0.0067 |
| CE | 4.98 Aa | 3.76 Ab | 4.47 Aab | 2.27 Ac | 2.21 Bc | |||||
| LAB | 4.45 Aab | 3.49 Abc | 4.51 Aa | 3.15 Ac | 1.53 Bd | |||||
| Coverage | CK | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0 | NS | NS | NS |
| CE | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |||||
| LAB | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |||||
| Pielou-e | CK | 0.56 Aa | 0.51 ABa | 0.52 ABa | 0.46 ABa | 0.44 Ba | 0.02 | 0.0097 | <0.0001 | 0.0046 |
| CE | 0.62 Aa | 0.47 Ba | 0.54 ABa | 0.29 Ca | 0.29 Cb | |||||
| LAB | 0.55 Aa | 0.45 ABa | 0.55 Aa | 0.39 Ba | 0.2 Cb | ☐ | ☐ | ☐ | ☐ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, B.; Qiu, R.; Wang, Z.; Liu, Y.; Bao, J.; Sun, L.; Liu, T.; Ge, G.; Jia, Y. Effects of Cellulase and Lactic Acid Bacteria on Ensiling Performance and Bacterial Community of Caragana korshinskii Silage. Microorganisms 2023, 11, 337. https://doi.org/10.3390/microorganisms11020337
Bai B, Qiu R, Wang Z, Liu Y, Bao J, Sun L, Liu T, Ge G, Jia Y. Effects of Cellulase and Lactic Acid Bacteria on Ensiling Performance and Bacterial Community of Caragana korshinskii Silage. Microorganisms. 2023; 11(2):337. https://doi.org/10.3390/microorganisms11020337
Chicago/Turabian StyleBai, Baochao, Rui Qiu, Zhijun Wang, Yichao Liu, Jian Bao, Lin Sun, Tingyu Liu, Gentu Ge, and Yushan Jia. 2023. "Effects of Cellulase and Lactic Acid Bacteria on Ensiling Performance and Bacterial Community of Caragana korshinskii Silage" Microorganisms 11, no. 2: 337. https://doi.org/10.3390/microorganisms11020337
APA StyleBai, B., Qiu, R., Wang, Z., Liu, Y., Bao, J., Sun, L., Liu, T., Ge, G., & Jia, Y. (2023). Effects of Cellulase and Lactic Acid Bacteria on Ensiling Performance and Bacterial Community of Caragana korshinskii Silage. Microorganisms, 11(2), 337. https://doi.org/10.3390/microorganisms11020337







