Ultrastructure and Physiological Characterization of Morchella Mitospores and Their Relevance in the Understanding of the Morel Life Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Material
2.2. Induction Conditions of Mitospore Formation
2.3. Mitospore Germination
2.4. Microscopic Analysis
2.5. Determination of Growth Rate and Longevity of Mitospore-Germinated Strains
2.6. Mating Genes Detection of the Mitospore-Germinating Strains
3. Results
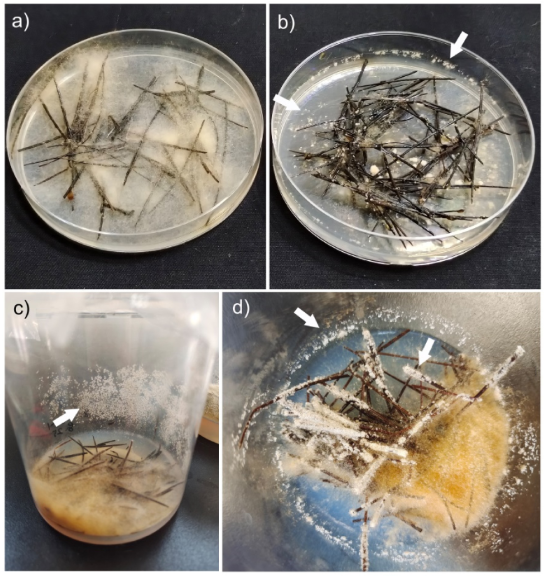
3.1. Induction of Mitospore Formation
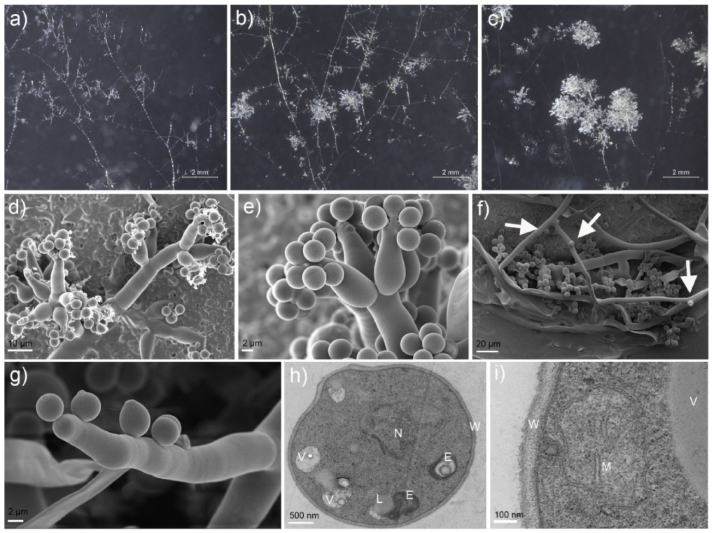
3.2. Morphological Characteristics of Mitospores
3.3. Germination Characteristics of Mitospores
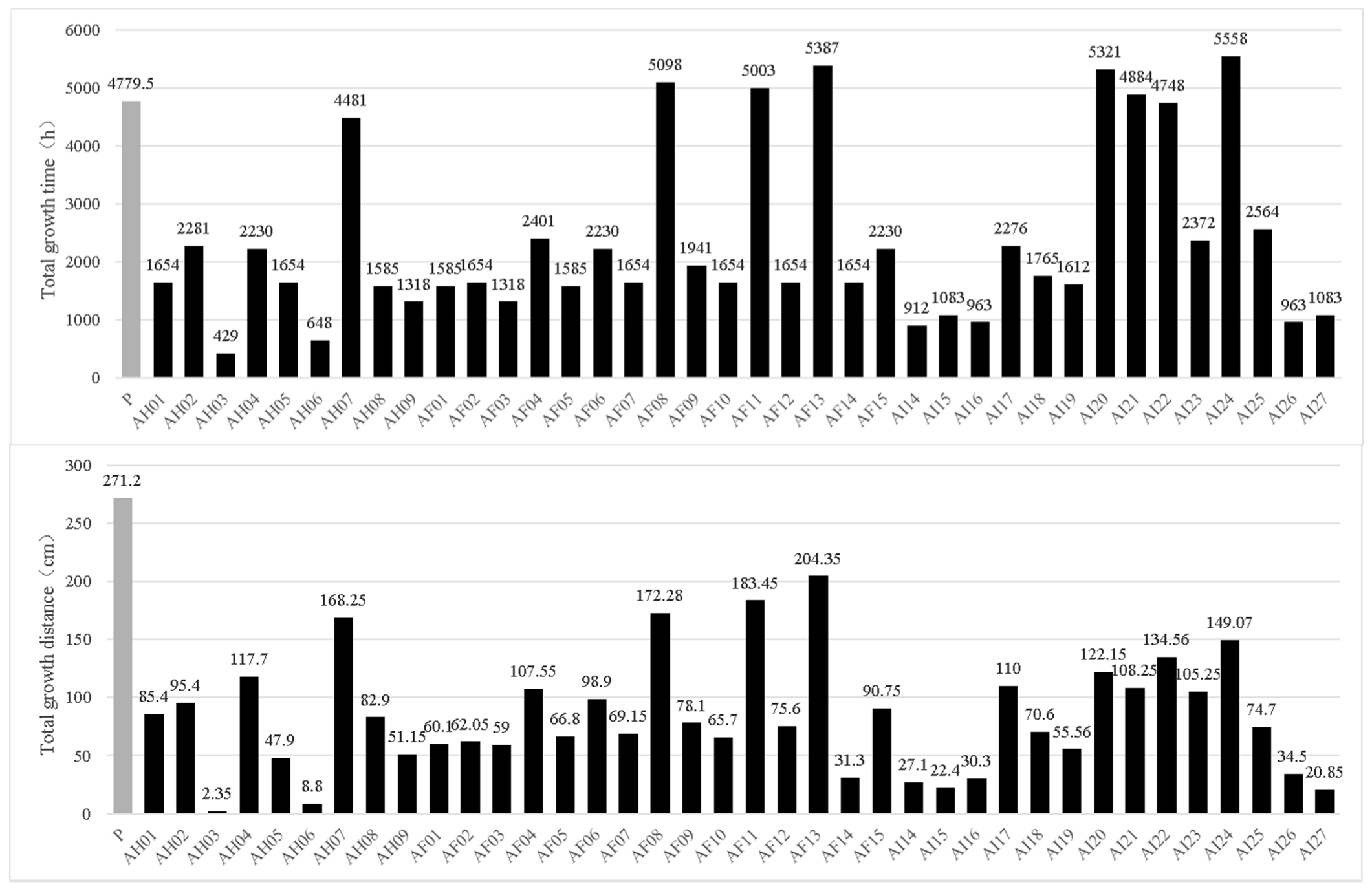
3.4. Growth Rate and Longevity of Mitospore-Germinated Strains of M. sextelata
3.5. Mating Type Genotyping of Mitospore-Germinated Strains
4. Discussion
4.1. Influence of Mitospore Formation by Nutrition, Aeration and Humidity
4.2. Mitospore Germination Conditions and Rapid Aging
4.3. The Mitospore of M. sextelata Should Be a Gamete
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pilz, D.; Rebecca, M.L.; Susan, A.; Luis, V.R.; Shannon, B.; Tricia, W.; Parks, C.G.; Erika, M.F.; Blaze, B. Ecology and Management of Morels Harvested from the Forests of Western North America; General Technical Report PNW-GTR-710 Portland. 2007; p. 161. Available online: https://www.fs.usda.gov/pnw/pubs/pnw_gtr710.pdf (accessed on 22 December 2022).
- Liu, W.; Zhang, Y.; He, P.X. Morel Biology and Cultivation; Jilin science and Technology Press: Changchun, China, 2017. [Google Scholar]
- Dissanayake, A.A.; Mills, G.L.; Bonito, G.; Rennick, B.; Nair, M.G. Chemical composition and anti-inflammatory and antiox-idant activities of extracts from cultivated morel mushrooms, species of genus Morchella (Ascomycota). Int. J. Med. Mushrooms 2021, 23, 73–83. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Geng, L.; Mao, D.; Xu, C. Production, characterization and antioxidant activity of exopolysaccharides from submerged culture of Morchella crassipes. Bioprocess Biosyst. Eng. 2012, 35, 1325–1332. [Google Scholar] [CrossRef]
- Su, C.-A.; Xu, X.-Y.; Liu, D.-Y.; Wu, M.; Zeng, F.-Q.; Zeng, M.-Y.; Wei, W.; Jiang, N.; Luo, X. Isolation and characterization of exopolysaccharide with immunomodulatory activity from fermentation broth of Morchella conica. DARU J. Pharm. Sci. 2013, 21, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlstrom, J.L.; Smith, J.E.; Weber, N.S. Mycorrhiza-like interaction by Morchella with species of the Pinaceae in pure culture synthesis. Mycorrhiza 2000, 9, 279–285. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, H.; Zhang, Y.; Dong, C. Artificial cultivation of true morels: Current state, issues and perspectives. Crit. Rev. Biotechnol. 2017, 38, 259–271. [Google Scholar] [CrossRef]
- Ower, R.D. Cultural Studies of Morels; San Francisco State University: San Francisco, CA, USA, 1980. [Google Scholar]
- Du, X.-H.; Yang, Z.L. Mating Systems in True Morels (Morchella). Microbiol. Mol. Biol. Rev. 2021, 85, e0022020. [Google Scholar] [CrossRef]
- He, P.; Cai, Y.; Yu, F.; Liu, W. Spatial and temporal disequilibrium of nuclear distribution in heterothallic Morchella importuna. J. Agric. Food Res. 2021, 6, 100240. [Google Scholar] [CrossRef]
- Shi, X.; Liu, D.; He, X.; Liu, W.; Yu, F. Epidemic Identification of Fungal Diseases in Morchella Cultivation across China. J. Fungi 2022, 8, 1107. [Google Scholar] [CrossRef]
- He, P.; Chen, Z.; Men, Y.; Wang, M.; Wang, W.; Liu, W. Activity Assay of Amylase and Xylanase Is Available for Quantitative Assessment of Strain Aging in Cultivated Culinary-Medicinal Morchella Mushrooms (Ascomycotina). Int. J. Med. Mushrooms 2023, 25, 57–64. [Google Scholar] [CrossRef]
- He, P.; Liu, W.; Cai, Y.; He, X. Strain identification and phylogenetic analysis of cultivated and wild strains of Morchella be-longing to elata clade in China. J. Zhengzhou Univ. Light Ind. (Nat. Sci.) 2015, 30, 26–29. [Google Scholar] [CrossRef]
- He, P.; Yu, M.; Cai, Y.; Liu, W.; Wang, W.; Wang, S.; Li, J. Effect of Aging on Culture and Cultivation of the Culinary-Medicinal Mushrooms Morchella importuna and M. sextelata (Ascomycetes). Int. J. Med. Mushrooms 2019, 21, 1089–1098. [Google Scholar] [CrossRef]
- He, P.; Cai, Y.; Liu, S.; Han, L.; Huang, L.; Liu, W. Morphological and ultrastructural examination of senescence in Morchella elata. Micron 2015, 78, 79–84. [Google Scholar] [CrossRef]
- Chai, H.; Chen, L.; Chen, W.; Zhao, Q.; Zhang, X.; Su, K.; Zhao, Y. Characterization of mating-type idiomorphs suggests that Morchella importuna, Mel-20 and M. sextelata are heterothallic. Mycol. Prog. 2017, 16, 743–752. [Google Scholar] [CrossRef]
- Du, X.-H.; Zhao, Q.; Xia, E.-H.; Gao, L.-Z.; Richard, F.; Yang, Z.L. Mixed-reproductive strategies, competitive mating-type distribution and life cycle of fourteen black morel species. Sci. Rep. 2017, 7, 1493. [Google Scholar] [CrossRef]
- He, P.; Wang, K.; Cai, Y.; Liu, W. Live cell confocal laser imaging studies on the nuclear behavior during meiosis and ascosporogenesis in Morchella importuna under artificial cultivation. Micron 2017, 101, 108–113. [Google Scholar] [CrossRef]
- Liu, W.; Chen, L.; Cai, Y.; Zhang, Q.; Bian, Y. Opposite Polarity Monospore Genome De Novo Sequencing and Comparative Analysis Reveal the Possible Heterothallic Life Cycle of Morchella importuna. Int. J. Mol. Sci. 2018, 19, 2525. [Google Scholar] [CrossRef]
- Liu, W.; Cai, Y.; He, P.; Bian, Y. Cultivation of monosporic and hybrid populations and polarity analysis of Morchella importuna. J. Fungal Res. 2019, 17, 7. [Google Scholar] [CrossRef]
- Zhang, Q.; Shu, F.; Chen, X.; Liu, W.; Bian, Y.; Kang, H. Construction of nucleus-directed fluorescent reporter systems and its application to verification of heterokaryon formation in Morchella importuna. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, J.; Geng, F.; Li, X.; Yu, J.; Zhang, Y.; Chen, Y.; Liu, D. Metabolic and proteomic analysis of morel fruiting body (Morchella importuna). J. Food Compos. Anal. 2019, 76, 51–57. [Google Scholar] [CrossRef]
- Cai, Y.; Ma, X.; Zhang, Q.; Yu, F.; Zhao, Q.; Huang, W.; Song, J.; Liu, W. Physiological Characteristics and Comparative Secretome Analysis of Morchella importuna Grown on Glucose, Rice Straw, Sawdust, Wheat Grain, and MIX Substrates. Front. Microbiol. 2021, 12, 636344. [Google Scholar] [CrossRef]
- Benucci, G.M.N.; Longley, R.; Zhang, P.; Zhao, Q.; Bonito, G.; Yu, F. Microbial communities associated with the black morel Morchella sextelata cultivated in greenhouses. Peerj 2019, 7, e7744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longley, R.; Benucci, G.M.N.; Mills, G.; Bonito, G. Fungal and bacterial community dynamics in substrates during the cultiva-tion of morels (Morchella rufobrunnea) indoors. FEMS Microbiol. Lett. 2019, 366, fnz215. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Zhang, J.; Wang, H.; Wang, Q.; Chen, M.; Juan, J.; Feng, Z.; Chen, H. Comparative transcriptome analysis reveals potential fruiting body formation mechanisms in Morchella importuna. AMB Express 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Cai, Y.; Zhang, Q.; Shu, F.; Chen, L.; Ma, X.; Bian, Y. Subchromosome-Scale Nuclear and Complete Mitochondrial Genome Characteristics of Morchella crassipes. Int. J. Mol. Sci. 2020, 21, 483. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Wang, Q.; Baiyintala; Wuhanqimuge. The whole-genome sequence analysis of Morchella sextelata. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Fu, R.; Wang, J.; Li, X.; Chen, X.; Li, Q.; Lu, D. Genome sequence and transcriptome profiles of pathogenic fungus Paecilomyces penicillatus reveal its interactions with edible fungus Morchella importuna. Comput. Struct. Biotechnol. J. 2021, 19, 2607–2617. [Google Scholar] [CrossRef]
- Fischer, R.; Kües, U. Asexual Sporulation in Mycelial Fungi. In Growth, Differentiation and Sexuality; Kües, U., Fischer, R., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2006; pp. 263–292. [Google Scholar]
- Huang, M.; Hull, C.M. Sporulation: How to survive on planet Earth (and beyond). Curr. Genet. 2017, 63, 831–838. [Google Scholar] [CrossRef]
- Kirschner, R. Sex does not sell: The argument for using the terms “anamorph” and “teleomorph” for fungi. Mycol. Prog. 2018, 18, 305–312. [Google Scholar] [CrossRef]
- Maheshwari, R. Microconidia ofNeurospora crassa. Fungal Genet. Biol. 1999, 26, 1–18. [Google Scholar] [CrossRef]
- Fones, H.N.; Mardon, C.; Gurr, S.J. A role for the asexual spores in infection of Fraxinus excelsior by the ash-dieback fungus Hymenoscyphus fraxineus. Sci. Rep. 2016, 6, 34638. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Wang, C.; Li, Y.; Xu, J.-R. Germination and infectivity of microconidia in the rice blast fungus Magnaporthe oryzae. Nat. Commun. 2014, 5, 4518. [Google Scholar] [CrossRef] [Green Version]
- Chai, H.; Chen, W.; Zhang, X.; Su, K.; Zhao, Y. Structural variation and phylogenetic analysis of the mating-type locus in the genusMorchella. Mycologia 2019, 111, 551–562. [Google Scholar] [CrossRef]
- Yatsiuk, I.; Saar, I.; Kalamees, K.; Sulaymonov, S.; Gafforov, Y.; O’Donnell, K.; Iryna, Y.; Irja, S.; Kuulo, K.; Shaxob, S.; et al. Epitypification of Morchella steppicola (Morchellaceae, Pezizales), a morphologically, phylogenetically and biogeographically distinct member of the Esculenta Clade from central Eurasia. Phytotaxa 2016, 284, 31–40. [Google Scholar] [CrossRef]
- Taşkin, H.; Doğan, H.H.; Büyükalaca, S. Morchella galilaea, an autumn species from Turkey. Mycotaxon 2015, 130, 215–221. [Google Scholar] [CrossRef]
- Richard, F.; Bellanger, J.-M.; Clowez, P.; Hansen, K.; O’Donnell, K.; Urban, A.; Sauve, M.; Courtecuisse, R.; Moreau, P.-A. True morels (Morchella, Pezizales) of Europe and North America: Evolutionary relationships inferred from multilocus data and a unified taxonomy. Mycologia 2015, 107, 359–382. [Google Scholar] [CrossRef]
- Molliard, M. Mycelium et forme conidienne de la morille. CR Hebd. Seances Acad. Sci. 1904, 138, 516–517. [Google Scholar]
- Masaphy, S. Biotechnology of morel mushrooms: Successful fruiting body formation and development in a soilless system. Biotechnol. Lett. 2010, 32, 1523–1527. [Google Scholar] [CrossRef]
- Carris, L.M.; Peever, T.L.; McCotter, S.W. Mitospore stages of Disciotis, Gyromitra and Morchella in the inland Pacific Northwest USA. Mycologia 2015, 107, 729–744. [Google Scholar] [CrossRef]
- Liu, W.; CAI, Y.; He, P.; Zhang, Y.; Bian, Y. Morphological and structural analysis of mitospore of Morchella importuna. J. Fungal Res. 2016, 14, 157–161. [Google Scholar]
- Baran, J.; Boroń, P. Two species of true morels (the genus Morchella, Ascomycota) recorded in the Ojców National Park (south Poland). Acta Mycol. 2017, 52, 1094. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.-H.; Li, H.; Liu, L.; Du, X.-H. Successful induction and recognition of conidiation, conidial germination and chlamydospore formation in pure culture of Morchella. Fungal Biol. 2021, 125, 285–293. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Timberlake, W.E. Developmental gene regulation in Aspergillus nidulans. Dev. Biol. 1980, 78, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-Y.; Qi, Y.-L.; Cai, L. Induction of sporulation in plant pathogenic fungi. Mycology 2012, 3, 195–200. [Google Scholar] [CrossRef]
- Sun, X.; Yu, L.; Lan, N.; Wei, S.; Yu, Y.; Zhang, H.; Zhang, X.; Li, S. Analysis of the role of transcription factor VAD-5 in conidiation of Neurospora crassa. Fungal Genet. Biol. 2012, 49, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Yu, J.-H. Genetic control of asexual sporulation in filamentous fungi. Curr. Opin. Microbiol. 2012, 15, 669–677. [Google Scholar] [CrossRef]
- Papadaki, A.; Diamantopoulou, P.; Papanikolaou, S.; Philippoussis, A. Evaluation of biomass and chitin production of Mor-chella mushrooms grown on starch-based substrates. Foods 2019, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Healy, R.A.; Smith, M.E.; Bonito, G.M.; Pfister, D.H.; Ge, Z.-W.; Guevara, G.G.; Williams, G.; Stafford, K.; Kumar, L.; Lee, T.; et al. High diversity and widespread occurrence of mitotic spore mats in ectomycorrhizalPezizales. Mol. Ecol. 2012, 22, 1717–1732. [Google Scholar] [CrossRef] [Green Version]
- Nakano, S.; Obase, K.; Nakamura, N.; Kinoshita, A.; Kuroda, K.; Yamanaka, T. Mitospore formation on pure cultures of Tuber japonicum (Tuberaceae, Pezizales) in vitro. Mycorrhiza 2022, 32, 353–360. [Google Scholar] [CrossRef]
- He, P.; Wang, K.; Cai, Y.; Hu, X.; Zheng, Y.; Zhang, J.; Liu, W. Involvement of autophagy and apoptosis and lipid accumulation in sclerotial morphogenesis of Morchella importuna. Micron 2018, 109, 34–40. [Google Scholar] [CrossRef]
- Fukumori, Y.; Nakajima, M.; Akutsu, K. Microconidia act the role as spermatia in the sexual reproduction of Botrytis cinerea. J. Gen. Plant Pathol. 2004, 70, 256–260. [Google Scholar] [CrossRef]
- Hervey, A.; Bistis, G.; Leong, I. Cultural Studies of Single Ascospore Isolates of Morchella esculenta. Mycologia 1978, 70, 1269. [Google Scholar] [CrossRef]
- Schmidt, E.L. Spore Germination of and Carbohydrate Colonization by Morchella Esculenta at Different Soil Temperatures. Mycologia 1983, 75, 870–875. [Google Scholar] [CrossRef]
- Spiers, A.G.; Hopcroft, D.H. Ultrastructural studies of the telial, basidial, and spermatial stages of the willow rust fungusMelampsora coleosporioidesin New Zealand. New Zealand J. Bot. 1988, 26, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Florance, E.R.; Denison, W.C.; Allen, T.C., Jr. Ultrastructure of Dormant and Germinating Conidia of Aspergillus Nidulans. Mycologia 1972, 64, 115–123. [Google Scholar] [CrossRef]
- Kim, K.W.; Park, E.W. Ultrastructure of spined conidia and hyphae of the rice false smut fungus Ustilaginoidea virens. Micron 2007, 38, 626–631. [Google Scholar] [CrossRef]
- Mims, C.W.; Seabury, F.; Thurston, E.L. An ultrastructural study of spermatium formation in the rust fungus Gymnosporangium iuniperi-virginianae. Am. J. Bot. 1976, 63, 997–1002. [Google Scholar] [CrossRef]
- Lowry, R.J.; Durkee, T.L.; Sussman, A.S. Ultrastructural Studies of Microconidium Formation in Neurospora crassa. J. Bacteriol. 1967, 94, 1757–1763. [Google Scholar] [CrossRef] [Green Version]
- Bistis, G.N. Chemotropic Interactions Between Trichogynes and Conidia of Opposite Mating-Type inNeurospora Crassa. Mycologia 1981, 73, 959–975. [Google Scholar] [CrossRef]
- Jiang, L.; Qian, Y.; Ling, J.; Li, T.; Du, X. Mating types of sclerotia in Morchella. J. Fungal Res. 2021, 19, 255–262. [Google Scholar]
- Volk, T.J.; Leonard, T.J. Cytology of the life-cycle of Morchella. Mycol. Res. 1990, 94, 399–406. [Google Scholar] [CrossRef]
- Shi, J.; Guo, M.; Guo, S.; Zhou, W.; Wang, Z.; Wu, X.; Li, Y. Microscopic observation and analysis of primodiam formation of Morchella conica. J. Shanxi Agric. Sci. 2019, 47, 1709–1712. [Google Scholar]



| Mitospore Properties | Culture Medium | Initial Concentration | Number That Germinated | Germination Rate | The Earliest Germination Time | The Latest Germination Time |
|---|---|---|---|---|---|---|
| Mitospores were kept at 4 °C for 1 month | SYM | 2.65 × 105 | ||||
| PDA | 2.65 × 105 | 5 | 1.88679 × 10−5 | 11 days | 39 days | |
| CYM | 2.65 × 105 | |||||
| MYM | 2.65 × 105 | 2 | 7.54717 × 10−6 | 12 days | 20 days | |
| CHM | 2.65 × 105 | 2 | 7.54717 × 10−6 | 14 days | 59 days | |
| Mitospores were kept at 15 °C for 2 months | SYM | 1.34 × 105 | ||||
| PDA | 1.34 × 105 | 1 | 7.46269 × 10−6 | 9 days | 9 days | |
| CYM | 1.34 × 105 | |||||
| MYM | 1.34 × 105 | 3 | 2.23881 × 10−5 | 13 days | 30 days | |
| CHM | 1.34 × 105 | 1 | 7.46269 × 10−6 | 36 days | ||
| Mitospores were kept at 4 °C for 2 months | CYM | 4.92 × 106 | 13 | 2.64228 × 10−6 | 12 days | 24 days |
| CYM | 4.92 × 105 | |||||
| CYM | 4.92 × 104 | |||||
| PDA | 4.92 × 106 | 16 | 3.25203 × 10−6 | 9 days | 25 days | |
| PDA | 4.92 × 105 | 4 | 8.13008 × 10−6 | 12 days | 33 days | |
| PDA | 4.92 × 104 | |||||
| MYG | 4.92 × 106 | 21 | 4.26829 × 10−6 | 10 days | 30 days | |
| MYG | 4.92 × 105 | 2 | 4.06504 × 10−6 | 20 days | 30 days | |
| MYG | 4.92 × 104 | |||||
| Mitospores were kept at 15 °C for 3 months | CYM | 7.58 × 105 | 2 | 2.63852 × 10−6 | 7 days | 12 days |
| CYM | 7.58 × 104 | |||||
| PDA | 7.58 × 105 | 7 | 9.23483 × 10−6 | 7 days | 21 days | |
| PDA | 7.58 × 104 | 2 | 2.63852 × 10−5 | 17 days | 23 days | |
| MYG | 7.58 × 105 | 15 | 1.97889 × 10−5 | 6 days | 59 days | |
| MYG | 7.58 × 104 | |||||
| Mitospores were kept at 4 °C for 7 months | SYM | 2.46 × 105 | 2 | 8.13008 × 10−6 | 18 days | 21 days |
| PDA | 2.46 × 105 | 2 | 8.13008 × 10−6 | 17 days | 32 days | |
| CYM | 2.46 × 105 | 2 | 8.13008 × 10−6 | 18 days | ||
| MYM | 2.46 × 105 | 3 | 1.21951 × 10−5 | 15 days | 19 days | |
| CHM | 2.46 × 105 | 0 | 0 | Nonexistant | Nonexistant | |
| Mitospores were kept at 4 °C for 7 months | SYM | 3.45 × 104 | 0 | 0 | Nonexistant | Nonexistant |
| PDA | 3.45 × 104 | 0 | 0 | Nonexistant | Nonexistant | |
| CYM | 3.45 × 104 | 0 | 0 | Nonexistant | Nonexistant | |
| MYM | 3.45 × 104 | 0 | 0 | Nonexistant | Nonexistant | |
| CHM | 3.45 × 104 | 2 | 5.7971 × 10−5 | 6 days | 13 days | |
| Mitospores were kept at 4 °C for 9 months | CYM | 9.17 × 104 | 0 | 0 | Nonexistant | Nonexistant |
| PDA | 9.17 × 104 | 0 | 0 | Nonexistant | Nonexistant | |
| MYG | 9.17 × 104 | 1 | 1.09051 × 10−5 | 30 days | 30 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; He, P.; Zhang, J.; Wu, L.; Er, L.; Shi, X.; Gu, Z.; Yu, F.; Pérez-Moreno, J. Ultrastructure and Physiological Characterization of Morchella Mitospores and Their Relevance in the Understanding of the Morel Life Cycle. Microorganisms 2023, 11, 345. https://doi.org/10.3390/microorganisms11020345
Liu W, He P, Zhang J, Wu L, Er L, Shi X, Gu Z, Yu F, Pérez-Moreno J. Ultrastructure and Physiological Characterization of Morchella Mitospores and Their Relevance in the Understanding of the Morel Life Cycle. Microorganisms. 2023; 11(2):345. https://doi.org/10.3390/microorganisms11020345
Chicago/Turabian StyleLiu, Wei, Peixin He, Jin Zhang, Liyuan Wu, Lingfang Er, Xiaofei Shi, Zhijia Gu, Fuqiang Yu, and Jesús Pérez-Moreno. 2023. "Ultrastructure and Physiological Characterization of Morchella Mitospores and Their Relevance in the Understanding of the Morel Life Cycle" Microorganisms 11, no. 2: 345. https://doi.org/10.3390/microorganisms11020345
APA StyleLiu, W., He, P., Zhang, J., Wu, L., Er, L., Shi, X., Gu, Z., Yu, F., & Pérez-Moreno, J. (2023). Ultrastructure and Physiological Characterization of Morchella Mitospores and Their Relevance in the Understanding of the Morel Life Cycle. Microorganisms, 11(2), 345. https://doi.org/10.3390/microorganisms11020345






