Staphylococcus aureus Host Spectrum Correlates with Methicillin Resistance in a Multi-Species Ecosystem
Abstract
:1. Introduction
2. Material and Methods
2.1. Predictions
2.2. Host Community Studied and Sampling
2.3. Ethics Statement
2.4. Isolation and Identification of S. aureus
2.5. Spa Typing Analysis
2.6. Amplification of the mecA Gene
2.7. Statistical Analyses
3. Results
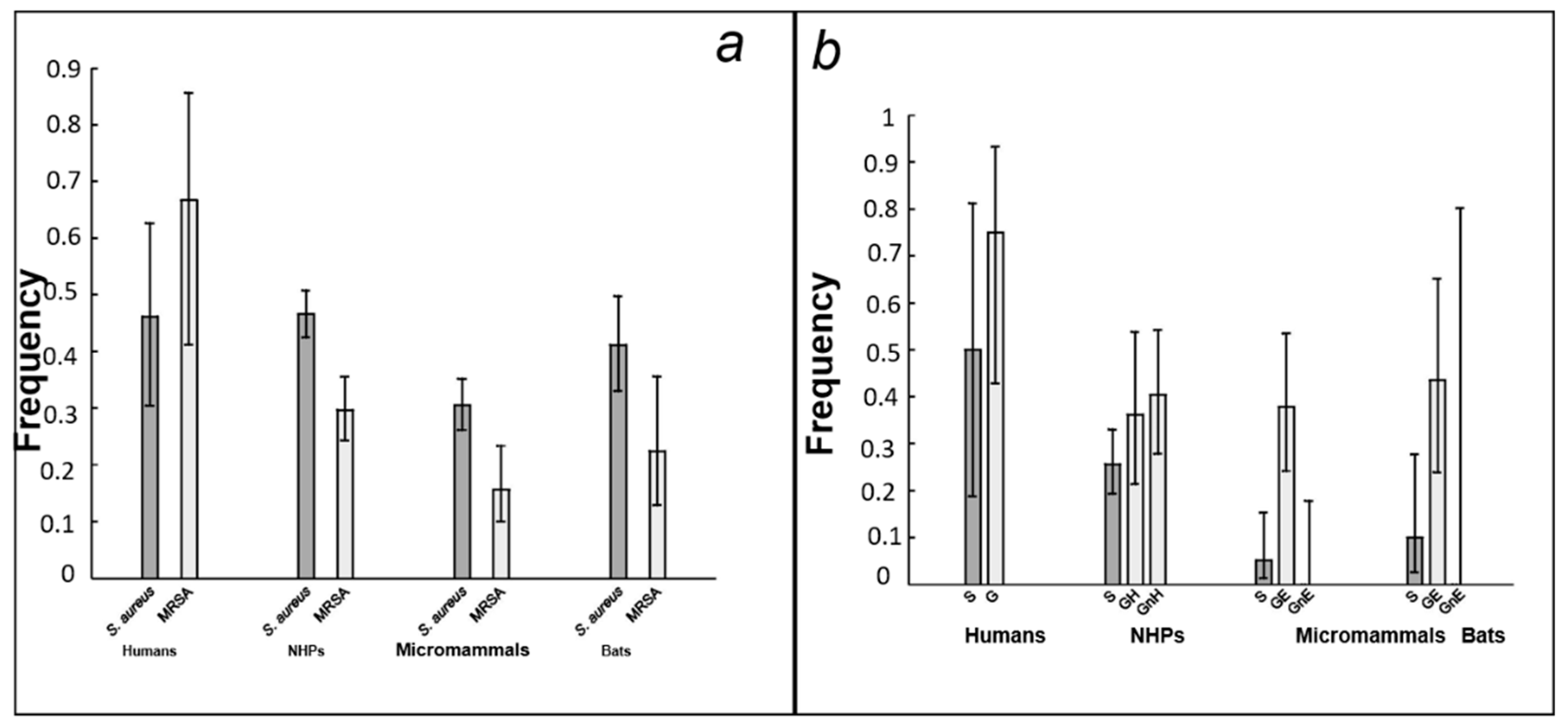
3.1. Prevalence of S. aureus in the Host Community
3.2. Spa Typing
3.3. S. aureus Population Structure and Distribution among Hosts
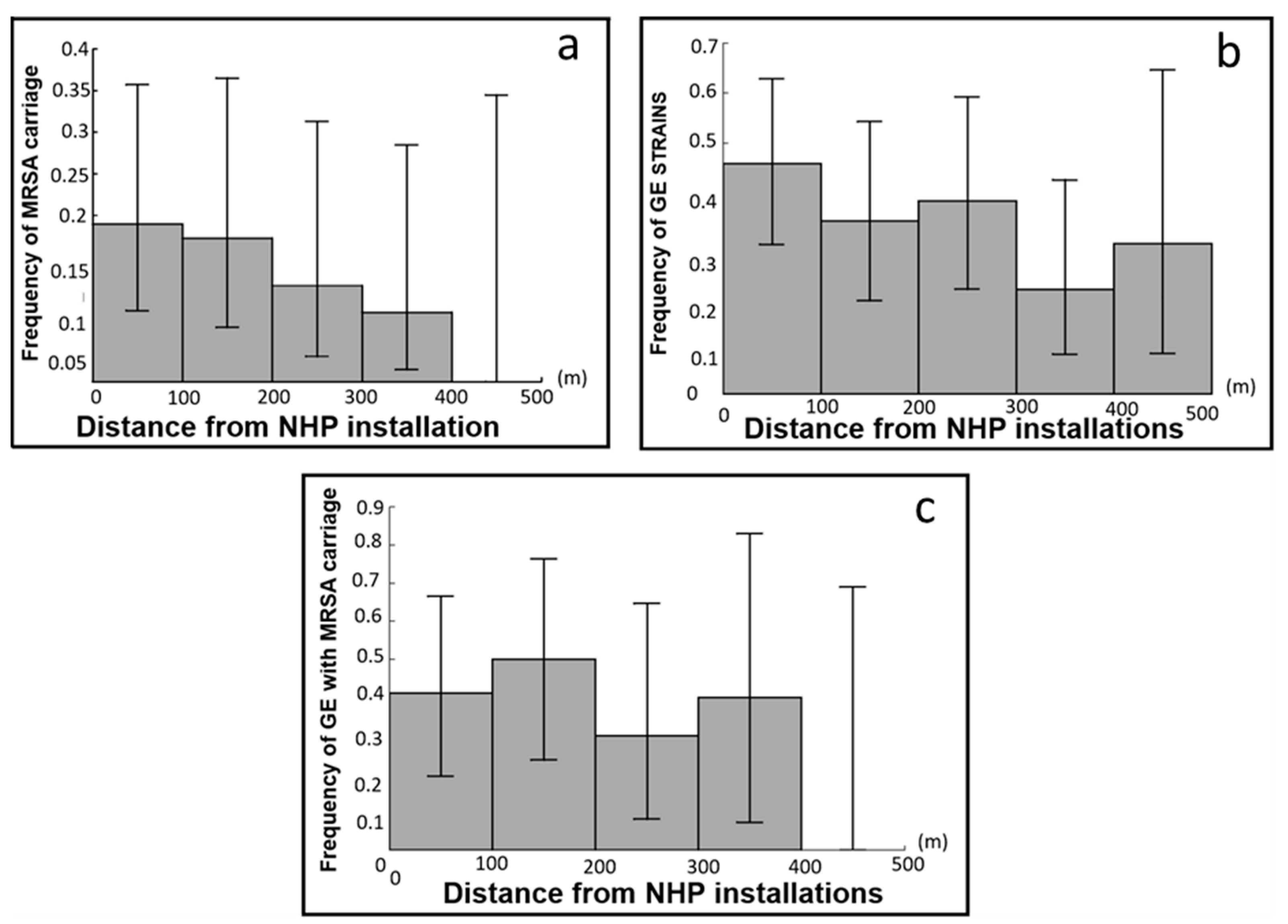
3.4. Testing the Four Predictions for MRSA Carriage
4. Discussion
4.1. MRSA Strain Prevalence
4.2. Antibacterial Drug Pressure and the Importance of the Host Spectrum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grall, N.; Barraud, O.; Wieder, I.; Hua, A.; Perrier, M.; Babosan, A.; Gaschet, M.; Clermont, O.; Denamur, E.; Catzeflis, F. Lack of dissemination of acquired resistance to β-lactams in small wild mammals around an isolated village in the A mazonian forest. Environ. Microbiol. Rep. 2015, 7, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Tristan, A.; Bes, M.; Meugnier, H.; Lina, G.; Bozdogan, B.; Courvalin, P.; Reverdy, M.-E.; Enright, M.C.; Vandenesch, F.; Etienne, J. Global distribution of Panton-Valentine leukocidin–positive methicillin-resistant Staphylococcus aureus, 2006. Emerg. Infect. Dis. 2007, 13, 594. [Google Scholar] [CrossRef]
- Sjölund, M.; Bonnedahl, J.; Hernandez, J.; Bengtsson, S.; Cederbrant, G.; Pinhassi, J.; Kahlmeter, G.; Olsen, B. Dissemination of multidrug-resistant bacteria into the Arctic. Emerg. Infect. Dis. 2008, 14, 70. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.A.; Mediannikov, O.; Abdallah, R.; Kuete Yimagou, E.; Medkour, H.; Dubourg, G.; Elamire, Y.; Afouda, P.; Ngom, I.I.; Angelakis, E. Multidrug-resistant Klebsiella pneumoniae clones from wild chimpanzees and termites in senegal. Antimicrob. Agents Chemother. 2021, 65, e02557-20. [Google Scholar] [CrossRef] [PubMed]
- Katakweba, A.S.; Muhairwa, A.P.; Espinosa-Gongora, C.; Guardabassi, L.; Mtambo, M.M.; Olsen, J.E. spa typing and antimicrobial resistance of Staphylococcus aureus from healthy humans, pigs and dogs in Tanzania. J. Infect. Dev. Ctries. 2016, 10, 143–148. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low-and middle-income countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Ho, C.-F.; Chen, C.-J.; Su, L.-H.; Lin, T.-Y. Comparative molecular analysis of community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus isolates from children in northern Taiwan. Clin. Microbiol. Infect. 2008, 14, 1167–1172. [Google Scholar] [CrossRef]
- Klevens, R.M.; Morrison, M.A.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; Townes, J.M. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007, 298, 1763–1771. [Google Scholar] [CrossRef] [Green Version]
- Hassell, J.M.; Ward, M.J.; Muloi, D.; Bettridge, J.M.; Robinson, T.P.; Kariuki, S.; Ogendo, A.; Kiiru, J.; Imboma, T.; Kang’ethe, E.K. Clinically relevant antimicrobial resistance at the wildlife–livestock–human interface in Nairobi: An epidemiological study. Lancet Planet. Health 2019, 3, e259–e269. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.M.-L.; Lloyd, D.H.; Lindsay, J.A. Staphylococcus aureus host specificity: Comparative genomics of human versus animal isolates by multi-strain microarray. Microbiology 2008, 154, 1949–1959. [Google Scholar] [CrossRef]
- Arnold, K.E.; Williams, N.J.; Bennett, M. ‘Disperse abroad in the land’: The role of wildlife in the dissemination of antimicrobial resistance. Biol. Lett. 2016, 12, 20160137. [Google Scholar] [CrossRef] [PubMed]
- Vittecoq, M.; Godreuil, S.; Prugnolle, F.; Durand, P.; Brazier, L.; Renaud, N.; Arnal, A.; Aberkane, S.; Jean-Pierre, H.; Gauthier-Clerc, M. Antimicrobial resistance in wildlife. J. Appl. Ecol. 2016, 53, 519–529. [Google Scholar] [CrossRef]
- Bacigalupe, R.; Tormo-Mas, M.Á.; Penadés, J.R.; Fitzgerald, J.R. A multihost bacterial pathogen overcomes continuous population bottlenecks to adapt to new host species. Sci. Adv. 2019, 5, eaax0063. [Google Scholar] [CrossRef]
- Laborda, P.; Sanz-García, F.; Ochoa-Sánchez, L.E.; Gil-Gil, T.; Hernando-Amado, S.; Martínez, J.L. Wildlife and Antibiotic Resistance. Front. Cell. Infect. Microbiol. 2022, 12, 873989. [Google Scholar] [CrossRef]
- Larsson, D.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Woolhouse, M.E.; Taylor, L.H.; Haydon, D.T. Population biology of multihost pathogens. Science 2001, 292, 1109–1112. [Google Scholar] [CrossRef]
- Bäumler, A.; Fang, F.C. Host specificity of bacterial pathogens. Cold Spring Harb. Perspect. Med. 2013, 3, a010041. [Google Scholar] [CrossRef]
- Horumpende, P.G.; Sonda, T.B.; van Zwetselaar, M.; Antony, M.L.; Tenu, F.F.; Mwanziva, C.E.; Shao, E.R.; Mshana, S.E.; Mmbaga, B.T.; Chilongola, J.O. Prescription and non-prescription antibiotic dispensing practices in part I and part II pharmacies in Moshi Municipality, Kilimanjaro Region in Tanzania: A simulated clients approach. PLoS ONE 2018, 13, e0207465. [Google Scholar] [CrossRef] [Green Version]
- Essack, S.; Desta, A.; Abotsi, R.; Agoba, E. Antimicrobial resistance in the WHO African region: Current status and roadmap for action. J. Public Health 2017, 39, 8–13. [Google Scholar] [CrossRef]
- Ducrot, C.; Hobeika, A.; Lienhardt, C.; Wieland, B.; Dehays, C.; Delabouglise, A.; Bordier, M.; Goutard, F.; Patel, E.; Figuié, M. Antimicrobial resistance in Africa—How to relieve the burden on family farmers. Emerg. Infect. Dis. 2021, 27, 2515. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017; Volume 2017, pp. 1–7.
- Osei Sekyere, J.; Mensah, E. Molecular epidemiology and mechanisms of antibiotic resistance in Enterococcus spp., Staphylococcus spp., and Streptococcus spp. in Africa: A systematic review from a One Health perspective. Ann. N. Y. Acad. Sci. 2020, 1465, 29–58. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.; Van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.C.; Moritz, E.D.; Leedom Larson, K.R.; Ferguson, D.D. The environment as a factor in methicillin-resistant Staphylococcus aureus transmission. Rev. Environ. Health 2010, 25, 121–134. [Google Scholar] [CrossRef]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Moran, G.J.; Amii, R.N.; Abrahamian, F.M.; Talan, D.A. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg. Infect. Dis. 2005, 11, 928–930. [Google Scholar] [CrossRef]
- Nagel, M.; Dischinger, J.; Turck, M.; Verrier, D.; Oedenkoven, M.; Ngoubangoye, B.; Le Flohic, G.; Drexler, J.F.; Bierbaum, G.; Gonzalez, J.P. Human-associated Staphylococcus aureus strains within great ape populations in Central Africa (Gabon). Clin. Microbiol. Infect. 2013, 19, 1072–1077. [Google Scholar] [CrossRef]
- Asgeirsson, H.; Thalme, A.; Weiland, O. Staphylococcus aureus bacteraemia and endocarditis–epidemiology and outcome: A review. Infect. Dis. 2018, 50, 175–192. [Google Scholar] [CrossRef]
- Harkins, C.P.; Pichon, B.; Doumith, M.; Parkhill, J.; Westh, H.; Tomasz, A.; de Lencastre, H.; Bentley, S.D.; Kearns, A.M.; Holden, M.T. Methicillin-resistant Staphylococcus aureus emerged long before the introduction of methicillin into clinical practice. Genome Biol. 2017, 18, 130. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.; Smith, D.L.; Laxminarayan, R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg. Infect. Dis. 2007, 13, 1840. [Google Scholar] [CrossRef]
- Cosgrove, S.E.; Sakoulas, G.; Perencevich, E.N.; Schwaber, M.J.; Karchmer, A.W.; Carmeli, Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: A meta-analysis. Clin. Infect. Dis. 2003, 36, 53–59. [Google Scholar] [CrossRef]
- Schaumburg, F.; Pauly, M.; Anoh, E.; Mossoun, A.; Wiersma, L.; Schubert, G.; Flammen, A.; Alabi, A.S.; Muyembe-Tamfum, J.J.; Grobusch, M.P.; et al. Staphylococcus aureus complex from animals and humans in three remote African regions. Clin. Microbiol. Infect. 2015, 21, e341–e348. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.H.; Price, J.T. The significant but understudied impact of pathogen transmission from humans to animals. Mt. Sinai J. Med. 2009, 76, 448–455. [Google Scholar] [CrossRef]
- Richardson, E.J.; Bacigalupe, R.; Harrison, E.M.; Weinert, L.A.; Lycett, S.; Vrieling, M.; Robb, K.; Hoskisson, P.A.; Holden, M.T.; Feil, E.J. Gene exchange drives the ecological success of a multi-host bacterial pathogen. Nat. Ecol. Evol. 2018, 2, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Shepheard, M.A.; Fleming, V.M.; Connor, T.R.; Corander, J.; Feil, E.J.; Fraser, C.; Hanage, W.P. Historical zoonoses and other changes in host tropism of Staphylococcus aureus, identified by phylogenetic analysis of a population dataset. PLoS ONE 2013, 8, e62369. [Google Scholar] [CrossRef] [PubMed]
- Wichelhaus, T.A.; Hunfeld, K.-P.; Böddinghaus, B.; Kraiczy, P.; Schafer, V.; Brade, V. Rapid molecular typing of methicillin-resistant Staphylococcus aureus by PCR-RFLP. Infect. Control Hosp. Epidemiol. 2001, 22, 294–298. [Google Scholar] [CrossRef]
- Stefani, S.; Chung, D.R.; Lindsay, J.A.; Friedrich, A.W.; Kearns, A.M.; Westh, H.; MacKenzie, F.M. Meticillin-resistant Staphylococcus aureus (MRSA): Global epidemiology and harmonisation of typing methods. Int. J. Antimicrob. Agents 2012, 39, 273–282. [Google Scholar] [CrossRef]
- Miragaia, M. Factors contributing to the evolution of Meca-mediated β-lactam resistance in staphylococci: Update and new insights from whole genome sequencing (WGS). Front. Microbiol. 2018, 9, 2723. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, A.H.; Wong, A.; Kassen, R. The fitness costs of antibiotic resistance mutations. Evol. Appl. 2015, 8, 273–283. [Google Scholar] [CrossRef]
- Lang, J.; Vigouroux, A.; El Sahili, A.; Kwasiborski, A.; Aumont-Nicaise, M.; Dessaux, Y.; Shykoff, J.A.; Moréra, S.; Faure, D. Fitness costs restrict niche expansion by generalist niche-constructing pathogens. ISME J. 2017, 11, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Brakstad, O.G.; Aasbakk, K.; Maeland, J.A. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 1992, 30, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Frenay, H.; Bunschoten, A.; Schouls, L.; Van Leeuwen, W.; Vandenbroucke-Grauls, C.; Verhoef, J.; Mooi, F. Molecular typing of methicillin-resistant Staphylococcus aureus on the basis of protein A gene polymorphism. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 60–64. [Google Scholar] [CrossRef]
- Shopsin, B.; Gomez, M.; Montgomery, S.; Smith, D.; Waddington, M.; Dodge, D.; Bost, D.; Riehman, M.; Naidich, S.; Kreiswirth, B. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 1999, 37, 3556–3563. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Guss, B.; Nilsson, B.; Gatenbeck, S.; Philipson, L.; Lindberg, M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J. Biol. Chem. 1984, 259, 1695–1702. [Google Scholar] [CrossRef]
- Harmsen, D.; Claus, H.; Witte, W.; Rothgänger, J.; Claus, H.; Turnwald, D.; Vogel, U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef]
- Strommenger, B.; Kettlitz, C.; Weniger, T.; Harmsen, D.; Friedrich, A.; Witte, W. Assignment of Staphylococcus isolates to groups by spa typing, SmaI macrorestriction analysis, and multilocus sequence typing. J. Clin. Microbiol. 2006, 44, 2533–2540. [Google Scholar] [CrossRef]
- Mellmann, A.; Weniger, T.; Berssenbrügge, C.; Rothgänger, J.; Sammeth, M.; Stoye, J.; Harmsen, D. Based Upon Repeat Pattern (BURP): An algorithm to characterize the long-term evolution of Staphylococcus aureus populations based on spa polymorphisms. BMC Microbiol. 2007, 7, 98. [Google Scholar] [CrossRef]
- Predari, S.C.; Ligozzi, M.; Fontana, R. Genotypic identification of methicillin-resistant coagulase-negative staphylococci by polymerase chain reaction. Antimicrob. Agents Chemother. 1991, 35, 2568–2573. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer-Verlag: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Schaumburg, F.; Mugisha, L.; Peck, B.; Becker, K.; Gillespie, T.R.; Peters, G.; Leendertz, F.H. Drug-Resistant Human Staphylococcus aureus in Sanctuary Apes Pose a Threat to Endangered Wild Ape Populations. Am. J. Primatol. 2012, 74, 1071–1075. [Google Scholar] [CrossRef]
- Nyasinga, J.; Omuse, G.; Njenga, J.; Nyerere, A.; Abdulgader, S.; Newton, M.; Whitelaw, A.; Revathi, G. Epidemiology of Staphylococcus aureus Infections in Kenya: Current State, Gaps and Opportunities. Open J. Med. Microbiol. 2020, 10, 204. [Google Scholar] [CrossRef]
- Garoy, E.Y.; Gebreab, Y.B.; Achila, O.O.; Tekeste, D.G.; Kesete, R.; Ghirmay, R.; Kiflay, R.; Tesfu, T. Methicillin-resistant Staphylococcus aureus (MRSA): Prevalence and antimicrobial sensitivity pattern among patients—A multicenter study in Asmara, Eritrea. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 8321834. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Karageorgopoulos, D.E.; Leptidis, J.; Korbila, I.P. MRSA in Africa: Filling the global map of antimicrobial resistance. PLoS ONE 2013, 8, e68024. [Google Scholar] [CrossRef] [PubMed]
- Wangai, F.K.; Masika, M.M.; Maritim, M.C.; Seaton, R.A. Methicillin-resistant Staphylococcus aureus (MRSA) in East Africa: Red alert or red herring? BMC Infect. Dis. 2019, 19, 596. [Google Scholar] [CrossRef] [PubMed]
- WHO. Worldwide Country Situation Analysis: Response to Antimicrobial Resistance: Summary; WHO: Geneva, Switzerland, 2015.
- Ngoa, U.A.; Schaumburg, F.; Adegnika, A.A.; Kösters, K.; Möller, T.; Fernandes, J.F.; Alabi, A.; Issifou, S.; Becker, K.; Grobusch, M.P. Epidemiology and population structure of Staphylococcus aureus in various population groups from a rural and semi urban area in Gabon, Central Africa. Acta Trop. 2012, 124, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Schaumburg, F.; Alabi, A.; Mombo-Ngoma, G.; Kaba, H.; Zoleko, R.; Diop, D.; Mackanga, J.-R.; Basra, A.; Gonzalez, R.; Menendez, C. Transmission of Staphylococcus aureus between mothers and infants in an African setting. Clin. Microbiol. Infect. 2014, 20, O390–O396. [Google Scholar] [CrossRef]
- Schaumburg, F.; Alabi, A.; Köck, R.; Mellmann, A.; Kremsner, P.; Boesch, C.; Becker, K.; Leendertz, F.H.; Peters, G. Highly divergent Staphylococcus aureus isolates from African non-human primates. Environ. Microbiol. Rep. 2012, 4, 141–146. [Google Scholar] [CrossRef]
- Abdulgader, S.M.; Shittu, A.O.; Nicol, M.P.; Kaba, M. Molecular epidemiology of Methicillin-resistant Staphylococcus aureus in Africa: A systematic review. Front. Microbiol. 2015, 6, 348. [Google Scholar] [CrossRef]
- Ramdani-Bouguessa, N.; Bes, M.; Meugnier, H.; Forey, F.; Reverdy, M.-E.; Lina, G.; Vandenesch, F.; Tazir, M.; Etienne, J. Detection of methicillin-resistant Staphylococcus aureus strains resistant to multiple antibiotics and carrying the Panton-Valentine leukocidin genes in an Algiers hospital. Antimicrob. Agents Chemother. 2006, 50, 1083–1085. [Google Scholar] [CrossRef]
- Breurec, S.; Zriouil, S.; Fall, C.; Boisier, P.; Brisse, S.; Djibo, S.; Etienne, J.; Fonkoua, M.; Perrier-Gros-Claude, J.; Pouillot, R. Epidemiology of methicillin-resistant Staphylococcus aureus lineages in five major African towns: Emergence and spread of atypical clones. Clin. Microbiol. Infect. 2011, 17, 160–165. [Google Scholar] [CrossRef]
- Okuda, K.; Toepfner, N.; Alabi, A.; Arnold, B.; Bélard, S.; Falke, U.; Menschner, L.; Monecke, S.; Ruppelt-Lorz, A.; Berner, R. Molecular epidemiology of Staphylococcus aureus from Lambarene, Gabon. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Schaumburg, F.; Köck, R.; Friedrich, A.W.; Soulanoudjingar, S.; Ngoa, U.A.; von Eiff, C.; Issifou, S.; Kremsner, P.G.; Herrmann, M.; Peters, G. Population structure of Staphylococcus aureus from remote African Babongo Pygmies. PLoS Negl. Trop. Dis. 2011, 5, e1150. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, T.; Virta, M.; Laine, A.-L. Antibiotic resistance in the wild: An eco-evolutionary perspective. Philos. Trans. R. Soc. B: Biol. Sci. 2017, 372, 20160039. [Google Scholar] [CrossRef] [PubMed]
- Alighardashi, A.; Pons, M.-N.; Potier, O. Présence et devenir des médicaments dans les eaux usées urbaines, une analyse bibliographique. Rev. Sci. L’eau/J. Water Sci. 2008, 21, 413–426. [Google Scholar]
- Rather, I.A.; Kim, B.-C.; Bajpai, V.K.; Park, Y.-H. Self-medication and antibiotic resistance: Crisis, current challenges, and prevention. Saudi J. Biol. Sci. 2017, 24, 808–812. [Google Scholar] [CrossRef]
- Moran, N.A. Microbial minimalism: Genome reduction in bacterial pathogens. Cell 2002, 108, 583–586. [Google Scholar] [CrossRef]
- Iramiot, J.S.; Kajumbula, H.; Bazira, J.; Kansiime, C.; Asiimwe, B.B. Antimicrobial resistance at the human–animal interface in the Pastoralist Communities of Kasese District, South Western Uganda. Sci. Rep. 2020, 10, 14737. [Google Scholar] [CrossRef]
- Gilardi, K.V.; Gillespie, T.R.; Leendertz, F.H.; Macfie, E.J.; Travis, D.A.; Whittier, C.A.; Williamson, E.A. Best Practice Guidelines for Health Monitoring and Disease Control in Great Ape Populations; Occasional Papers of the IUCN Species Survival Commission; IUCN SSC Primate Specialist Group: Gland, Switzerland, 2015; Volume 56. [Google Scholar]

| Host | N° Isolates | p | ||||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | N | Human | NHPs | Bats | Microm. | ||
| S. aureus carriage | Humans | 18 | 21 | 39 | − | 1 | 0.705 | 0.067 |
| NHPs | 270 | 310 | 580 | − | − | 0.287 | 0.02 × 10−10 | |
| Bats | 58 | 83 | 141 | − | − | − | 0.15 | |
| Microm. | 128 | 292 | 420 | − | − | − | − | |
| Global comparison | Chi-square = 26.89 | df = 3 | p-value = 6.2 × 10−6 | |||||
| MRSA carriage | Humans | 12 | 6 | 18 | − | 0.016 | 0.008 | 0.008 |
| NHPs | 80 | 190 | 270 | − | − | 0.340 | 0.02 | |
| Bats | 13 | 45 | 58 | − | − | − | 0.35 | |
| Microm. | 20 | 108 | 128 | − | − | − | − | |
| Global comparison | Chi-square = 24.61 | df = 3 | p-value = 1.8 × 10−5 | |||||
| Spa CC | Spa Type | Associated ST | SSR Profile | Hosts | Total | |||
|---|---|---|---|---|---|---|---|---|
| Exposed Species | Unexposed Species | |||||||
| NHPs | Human | Microm. | Bats | |||||
| 2546 | t2546 | None | 04-12-17-20-17-12-17-17 | 1(0) | 4(1) | 5(1) | ||
| t056 | ST101 | 04-20-12-17-20-17-12-17-17 | 6(3) | 9(5) | 15(8) | |||
| Singleton | t537 | None | 07-23-12-21-12-20-17-12-12 | 9(1) | 1(0) | 10(1) | ||
| t15942 | None | 08-13-17-20-17-25-17-13 | 17(12) | 5(3) | 22(15) | |||
| t15969 | None | 388-12-17-12-17-12-17-174-16-13 | 11(0) | 1(0) | 12(0) | |||
| t5017 | None | 08-34-02-43-34-43-43-16-02-17-83 | 13(4) | 10(6) | 3(1) | 26(11) | ||
| t189 | ST188 | 07-23-12-21-17-34 | 20(8) | 3(3) | 23(11) | |||
| t939 | None | 04-16-34-12-34-12 | 3(3) | 15(6) | 4(2) | 22(11) | ||
| t1458 | None | 121-21-17-17-23-24 | 1(0) | 10(0) | 4(1) | 15(1) | ||
| No founder | t084 | ST15-ST18 | 07-23-12-34-34-12-12-23-02-12-23 | 18(3) | 1(0) | 19(3) | ||
| t355 | ST152/377/1633 | 07-56-12-17-16-16-33-31-57-12 | 7(4) | 4(3) | 2(1) | 13(8) | ||
| Not analysed | t1781 | None | 26-16-16 | 12(0) | 1(0) | 13(0) | ||
| t5725 | ST72 | 121 | 2(1) | 1(1) | 3(2) | |||
| Total | 13 | 93(36) | 12(9) | 68(17) | 25(10) | 198(72) | ||
| Exposure to Antibiotics | Characteristic of spa Type | Number | Host | |||
|---|---|---|---|---|---|---|
| Exposed species | Spa CC | Spa type | Associated ST | SSR profile | ||
| 15941 | t13661 | None | 26-17-17-02-17-12-12-17-16-16 | 12(0) | NHP | |
| t15961 | - | 26-17-17-02-17-12-12-16 | 18(3) | NHP | ||
| t15941 | - | 26-17-17-02-17-12-12-16-16 | 17(7) | NHP | ||
| Singletons | t122 | - | 08-16-02-16-02-25-17-24-24 | 1(1) | Human | |
| t148 | - | 07-23-12-21-12-17-20-17-12-12-17 | 10(4) | NHP | ||
| t186 | ST88 | 07-12-21-17-13-13-34-34-33-34 | 3(2) | Human | ||
| t359 | - | 07-23-12-21-17-34-34-33-34 | 10(1) | NHP | ||
| t5132 | - | 08-16-02-16-13-13-17-34-16-13 | 1(0) | Human | ||
| t7368 | - | 03-22-31-34-17 | 13(5) | NHP | ||
| t6980 | - | 26-16-34-33-13 | 1(0) | NHP | ||
| t15940 | - | 121-12-12-34-22 | 30(3) | NHP | ||
| No founder | t318 | ST30 | 15-12-16-16-02-16-02-25-17-24 | 13(2) | NHP | |
| t1848 | - | 15-12-17-16-02-16-02-25-17 | 1(0) | Human | ||
| t16031 | - | 121-34-22-31-34-17-34-17 | 2(1) | NHP | ||
| t6940 | - | 11-10-17-34-22-25 | 1(0) | NHP | ||
| t1476 | - | 11-10-17-34-24-34-22-25 | 6(1) | NHP | ||
| t15943 | - | 121-34-34-22-31-34-17-34-17 | 34(16) | NHP | ||
| Not analysed | t605 | - | 07-23 | 1(0) | NHP | |
| Total Exposed | 174(46) | |||||
| Unexposed species | 2546 | t11341 | - | 26-30-17-34-17-17-16-12-17-16 | 1(1) | bat |
| Singletons | t094 | - | 07-23-12-34-34-12-12-23 | 6(0) | microm | |
| t3464 | - | 07-23-02-12-23-02-02-34 | 9(0) | microm | ||
| t15195 | - | 388-76-76-12-687-174-16-16-17 | 1(0) | bat | ||
| t15962 | - | 121-16-25-16-12-16-13-25-17 | 1(0) | bat | ||
| t15963 | - | 388-12-174-174-16-13-16-13 | 1(0) | bat | ||
| t15964 | - | 391-76-76-12-76-174-16-337-17 | 3(0) | bat | ||
| t15967 | - | 26-349-34-23-96-58-34-82-82-82-24 | 4(0) | bat | ||
| t15973 | - | 26-17-20-17-17-16-16 | 9(0) | microm | ||
| No founder | t15968 | - | 07-56-12-17-16-16-33-31-414-12 | 6(0) | bat | |
| t002 | ST5; ST231 | 26-23-17-34-17-20-17-12-17-16 | 15(1) | microm | ||
| t2173 | - | 07-23-17-13-17-20-17-12-17-16 | 1(0) | microm | ||
| t12895 | - | 621-23-12-34-34-12-12-23-02-12-23 | 5(1) | bat | ||
| Not analysed | t586 | - | 26-16 | 7(1) | microm | |
| t693 | - | 7 | 4(0) | microm | ||
| t779 | - | 8 | 4(1) | bat | ||
| t15972 | - | 08-21-21-33 | 7(1) | microm | ||
| t15966 | - | 26-12-25-17 | 4(0) | bat | ||
| Total Unexposed | 88(6) | |||||
| Total All | 36 | 262(52) | ||||
| Investigated Effect | Base Model | Chi-Square | Df | p | Coefficient [95% CI] | Odds Ratio [95% CI] |
|---|---|---|---|---|---|---|
| Housing (enclosure vs. aviaries) | a*s+g + (1|spe) | 6.74 | 1 | 0.001 | 1.16 [0.41; 1.91] | 3.19 [1.51; 6.75] |
| Host | Base Model | Strain | Estimate | Std. Error | z | p | Odds Ratio [95% CI] |
|---|---|---|---|---|---|---|---|
| Humans | H0 | Generalist | 0.44 | 1.14 | 0.38 | 0.69 | 1.55 [0.16; 14.44] |
| NHPs | a+s+spe | GH | 0.92 | 0.46 | 2.01 | 0.04 | 2.5 [1.02; 6.17] |
| GnH | 0.85 | 0.39 | 2.19 | 0.03 | 2.33 [1.09; 5] | ||
| Generalist (any) | 0.87 | 0.33 | 2.63 | 0.08 | 2.38 [1.26; 4.53] | ||
| Bats | H0 | GE | 1.93 | 0.73 | 2. 61 | 0.009 | 6.89 [1.65; 28.78] |
| GnE | −∞ | NA | NA | 0.99 | NA | ||
| Generalist (any) | 1.79 | 0.73 | 2.45 | 0.01 | 5.98 [1.43; 25.03] | ||
| Microm. | D | GE | 2.31 | 0.68 | 3.41 | 0.0006 | 10.05 [2.67; 37.82] |
| GnE | NA | NA | NA | NA | NA | ||
| Generalist (any) | 1.717 | 0.663 | 2.59 | 0.0096 | 5.53 [1.51; 20.43] |
| Investigated Effect | Group of Infected Hosts | Response Variable | Analysis | Base Model | Coefficient [95% CI] | Odd-Ratio [95% CI] |
|---|---|---|---|---|---|---|
| Distance from human installation | Microm.: all | MRSA carriage | P4a | H0 | −0.0052 [−0.0096; −0.008] | 0.59 [0.38; 0.92] |
| Microm.: all | GE strain carriage | P4b | S | −0.0030 [−0.0061; 0.0002] | 0.74 [0.54; 1.02] | |
| Microm.: GE | MRSA carriage | P4c | H0 | −0.0033 [−0.0083; 0.0017] | 0.72 [0.44; 1.19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngoubangoye, B.; Fouchet, D.; Boundenga, L.A.; Cassan, C.; Arnathau, C.; Meugnier, H.; Tsoumbou, T.-A.; Dibakou, S.E.; Otsaghe Ekore, D.; Nguema, Y.O.; et al. Staphylococcus aureus Host Spectrum Correlates with Methicillin Resistance in a Multi-Species Ecosystem. Microorganisms 2023, 11, 393. https://doi.org/10.3390/microorganisms11020393
Ngoubangoye B, Fouchet D, Boundenga LA, Cassan C, Arnathau C, Meugnier H, Tsoumbou T-A, Dibakou SE, Otsaghe Ekore D, Nguema YO, et al. Staphylococcus aureus Host Spectrum Correlates with Methicillin Resistance in a Multi-Species Ecosystem. Microorganisms. 2023; 11(2):393. https://doi.org/10.3390/microorganisms11020393
Chicago/Turabian StyleNgoubangoye, Barthelémy, David Fouchet, Larson Amédée Boundenga, Cécile Cassan, Céline Arnathau, Helene Meugnier, Thierry-Audrey Tsoumbou, Serge Ely Dibakou, Désiré Otsaghe Ekore, Yasmine Okomo Nguema, and et al. 2023. "Staphylococcus aureus Host Spectrum Correlates with Methicillin Resistance in a Multi-Species Ecosystem" Microorganisms 11, no. 2: 393. https://doi.org/10.3390/microorganisms11020393





