Abstract
Aquaculture, the world’s fastest growing food sector, produces over half of all fish for human consumption. Aquaculture feeds include fishmeal and fish oil, extracted from wild-caught fish such as sardines, and poses ecological, food security, and economic drawbacks. Microalgae, yeasts, fungi, bacteria, and other alternative ingredients show promise as potential ingredients in aquafeeds that provide protein/amino acids, lipids, or omega-3 sources and sources of bioactive molecules. This review article discusses the issues that the literature often lacks data on, such as the recent development of using microorganisms, technological innovation, challenges, and opportunities to develop a low environmental footprint of aquaculture diet. The ingredients often require novel processing technology to improve digestibility and fish growth and reduce antinutritional factors. This is an important gap to fill because microalgae are the most frequently used organism in fish feed, particularly as a dietary supplement or mixed with other ingredients. The production, processing, and formulating steps can affect the nutritional qualities. Stepwise strategies are required to evaluate these ingredients for feed application, and in this article, I articulated the stepwise key approaches of evaluating nutritional and environmental response metrics to develop highly sustainable aquaculture feed using these microorganisms, which would guide a more judicious inclusion of these novel ingredients.
Keywords:
microorganisms; microalgae; yeast; bacteria; fungi; fishmeal; fish oil; aquaculture; environment; sustainability; feed; digestibility; nutrients; lipid; fatty acid; omega-3 1. Significance of Aquaculture and Aquafeed
Aquaculture, the fastest growing food sector globally (8% average production increase/yr. for 1970–2014), now produces about half of all fish for human consumption, as global capture fisheries have reached or exceeded their sustainable limits and plateaued at ~90 million tons/yr. [1,2,3,4]. Aquaculture is the world’s most efficient protein generator and will play a key role in solving the grand challenge of feeding more than 9 billion people by 2050 by meeting the escalating demand for water, energy, and food while conserving environmental resources and protecting livelihoods [5,6,7,8,9,10,11]. The U.S.’s demand for seafood has contributed to global aquaculture’s spectacular rise. The demand for fish and seafood has constantly increased, and aquaculture has increasingly filled this demand for human consumption, making it a fundamental part of future food production. Aquaculture is expected to continue its remarkable rise and is projected to produce 109 million metric tons of fish in 2030 for global consumers. Fed aquaculture grew 158% from 2000 to 2018 when it comprised nearly 60 million MT of production [3]. The production of aquaculture feeds for the fed aquaculture is likewise expected to increase, and 73.15 million tons of feeds are projected to be used by 2025 [12].
The environmental impacts and increasing price of aquaculture feed (aquafeeds) constrain U.S. aquaculture development [13]. Feed has the largest overall environmental impact along the supply chain of intensive fed aquaculture, wherein the yields depend on giving animals nutritionally complete formulated feeds [12]. The environmental sustainability of aquaculture food production systems is of critical concern due to its rapid expansion. Among the parameters that contribute to the overall environmental impacts, aquafeed was identified as an impact hotspot, and the production of feed ingredients contributed most to the global warming potential and other emissions [14]. The production, processing, and supply chain of feed ingredients are the major contributors of the greenhouse gas (GHG) footprint of aquaculture [15,16,17]. The use of terrestrial crops and the associated land-use change, the harvesting of wild-caught fish (e.g., anchovy, sardine, etc.) for fishmeal and fish oil, and the associated processing and transportation occur often and are commonly attributed to the greenhouse gas emissions in aquaculture [17,18,19,20,21,22]. As aquaculture is the fastest growing food production sector, we need to urgently address the greater GHG footprints associated with the production, processing, and complex supply chain of the aquafeed ingredients [3,23]. The environmental GHG emissions from aquaculture can by decreased by altering the composition of the feed and reducing eutrophication [24,25,26]. GHG emissions are also linked with the feed conversion ratio, FCR (the amount of feed required for each kilogram of fish produced), and improvements in the FCR (via lowering the FCR) can be achieved through innovation in feed composition [11,17].
High-performing novel ingredients and the associated production of microalgae feed can lead to an improved protein efficiency ratio, PER (the amount of protein required for each kilogram of fish protein produced); digestibility (how well fish can digest the nutrient in the ingredients); feed waste to the environment; and GHG emissions [14,17,27,28,29]. Aquafeed ingredients represent 40–75% of the total cost and 75–85% of the variable costs of aquaculture production, and these are key market drivers for aquaculture production [30,31]. The aquafeed market is also growing and expected to grow 8–10% per year, reaching $185 billion by 2023 [1,32]. The production of industrial aquaculture feeds is likewise expected to increase; 73.15 million tons of compound feeds are projected to be used by 2025 [12]. Approximately 51.3 million tons were produced in 2017.
1.1. Use of Fishmeal and Fish Oil in Aquafeed
Unfortunately, the fishmeal protein and fish oil ingredients in current commercial aquaculture feed (aquafeed) come from unsustainably sourced marine forage fish that are core components of marine food webs. Currently, aquafeeds use more than 70% of the world’s fishmeal and fish oil, which are rendered from small wild-caught forage fish (such as herrings, sardines, and anchovies). Globally, approximately 16.9 million of the 29 million tons of ocean-caught small fish are currently used for aquaculture feeds each year [33]. Farmed salmonids (Atlantic salmon and rainbow trout) are the largest users of fishmeal and fish oil (FMFO), and globally, these farmed species used approximately 24% of the total FM and 50% of the total FO in 2010 [34]. Higher-trophic-level marine finfish and freshwater crustaceans are the largest users of FMFO in aquafeeds, but low-trophic-level finfish species such as carp, tilapia, catfish, and milkfish are also fed with 2% to 4% of FMFO in their feeds [35,36].
Even with the diminishing inclusion of fishmeal in aquaculture feeds, an estimated shortage ranging from 0.4 to 1.32 million metric tons of fishmeal could occur by 2050, significantly impairing the aquaculture industry growth [37]. Analysts also projected that at the current rates of FM and FO consumption, aquaculture feed demands could outstrip the supply of forage fish by 2037, with disastrous consequences for the food security of billions of humans and for wild marine fish, mammals, and seabirds that forage on them [10]. Moreover, aquacultures face increased competition for FMFO from companies producing food supplements, pharmaceuticals, and feeds for other animals [35,38], causing a supply–demand squeeze: FMFO prices will keep rising steeply and supplies will be insufficient to meet growing aquafeed demands and will thus constrain aquaculture growth [1,39].
1.2. Use of Terrestrial Crops and Oils in Aquafeeds and Environmental Challenges
Aquafeed manufacturers currently over-rely on terrestrial crops (e.g., soy, corn, canola) to replace fishmeal and fish oil; these would otherwise feed livestock or people [10,40,41]. Industrial agriculture is facing a tremendous challenge due to agricultural pollution resulting in a loss of arable land. The increasing demand for these crops by aquaculture’s explosive growth could elevate the environmental problems that are caused by farming them, including high nutrient and chemical inputs, runoff that increases eutrophication, the clearing of sensitive lands (e.g., in Amazonia), high energy inputs, and greenhouse gas emissions [38,42,43]. However, typically, the cultivation of terrestrial crops such as transgenic soy requires a large volume of water, pesticides, and fertilizers, and these also cause the deforestation of areas with high biological value. The monoculture practice of growing soy destroys biodiversity, pollutes the soil, and depletes water resources [44,45,46]. The major soy-producing countries (USA, Brazil, and Argentina) account for 80% of global production. Soy is an international commodity, and huge exports further increase the carbon and environmental footprint of soy production [45,47,48].
Current aquaculture practices only exacerbate these challenges. Developing an economic and sustainably sourced aquafeed promises great relief of the pressure on foraging fish, the increase in needed farmland, the minimization of nutrient pollution, and the catalysis of a truly sustainable aquaculture. Thus, replacing FMFO ingredients with terrestrial crop ingredients will not meet the goal of having a zero-carbon footprint and could further exacerbate the deforestation and global warming potential. To reduce the carbon footprint of aquaculture feed, we should emphasize locally sourced feed ingredients and byproduct meals, which should not be transported across the world.
1.2.1. Nutritional Disadvantages of Terrestrial Crop Protein
Terrestrial plant protein and oil ingredients have several nutritional disadvantages such as a low nutrient digestibility due to high levels of antinutritional components, and a deficiency in limiting essential amino acids, for example, lysine, methionine, threonine, and tryptophan [49,50]. For example, methionine is deficient in soybean meal and lysine is deficient in cornmeal, and these are the major ingredients in current commercial aquafeeds. Replacing FM protein with terrestrial crop protein remains a significant challenge for the industry and has stimulated compensatory research such as adding single amino acids to provide missing essential amino acids. Single amino acids, compared to amino acids bound in intact protein, have the following drawbacks in aquafeed: fish cannot efficiently utilize the synthetic amino acids and excrete more N metabolic waste to the environment, and the costs and life cycle environmental impacts increase [51,52,53,54]. Other common antinutritional factors include trypsin inhibitors, haemagglutinins, phytic acid, gossypol, phytoestrogens, glucosinolates, erucic acid, alkaloids, and thiaminase [55]. However, some antinutrient components can be destroyed via a heating and drying process [56]. Another unsustainable consequence of using plant ingredients in aquafeeds is that about 70% of the phosphorus in these ingredients are phytate bound, which is a very complex form. In fish that lack sufficient endogenous phytase enzymes to make bioavailable and undigested phytate, P ends up being excreted into the environment and may cause eutrophication potential [8,55,57]. Phytate also interacts with proteins, which may affect protein digestibility in trout and increase N waste outputs [58]. Phytate P in soy and other terrestrial crops cannot be digested by fish [56,59,60]. Several studies reported that the processing of ingredients or supplementations of phytase in diets improves the digestibility of P, minerals, and protein in salmonids [54,61,62]. For example, antinutritional factors can be overcome by using soybean protein concentrates and canola protein concentrate (60–65% protein), and antinutritional factors can be reduce via other processing methods [63]. The phytase enzyme can enhance the digestibility of terrestrial plant protein; however, the instability of the enzyme during feed processing is an issue. Additionally, the possibility of more dissolved phosphorus in the solid wastes can be produced [57,64]. Additionally, the use of phytase in fish feed is still unproven if we compare it with the poultry and swine feed. Another important consideration regarding phytase activity is that it greatly relies on the gut pH and the water temperature.
1.2.2. Nutritional Disadvantages of Terrestrial Crop Oil
Long-chain polyunsaturated fatty acids (LCPUFA) such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are the major limiting fatty acids acid in terrestrial crop oils. These are the essential omega-3 fatty acids for salmonids, and the requirement is around 0.5–1.0% of the diet [65]. Some studies have shown that the full replacement of fish oil with alternative oil sources can be possible without compromising fish performance, survival, and feed efficiency over the entire production cycle [66,67,68,69]. In the fishmeal-based diets, the essential fatty acids requirement can be met from the fishmeal; thus, the complete substitution of fish oil can be possible using vegetable oils, without growth retardation or any negative health effects [70,71,72]. Salmonids have a highly efficient protein-sparing capability and lipid utilization efficacy. This specifically favors replacing fish oil with vegetable oil especially in fishmeal-based diets for salmonid aquacultures.
1.2.3. Fillet Quality for Human Consumption
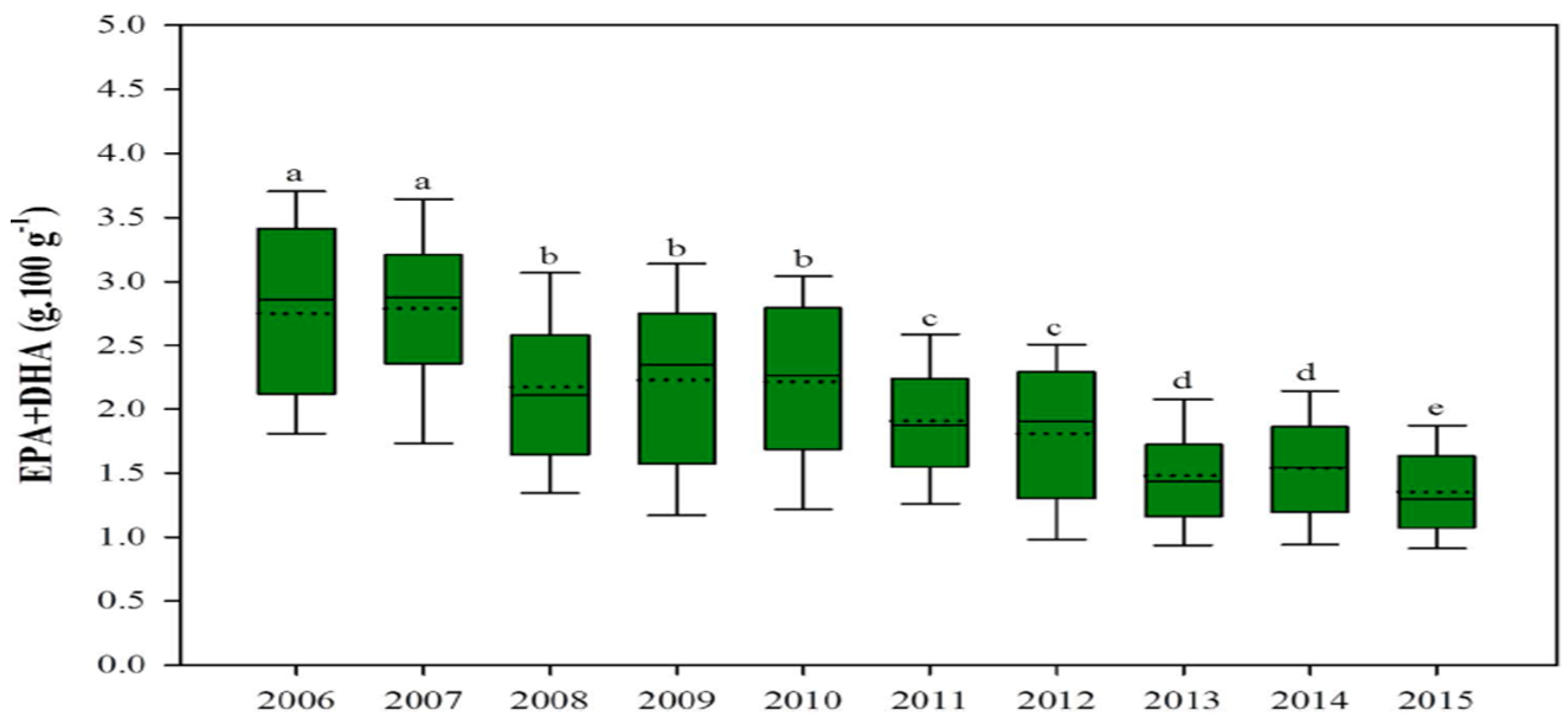
The composition of omega-3 and other nutrients in farmed fish species has been altered, and this affects human nutrition, as there are decreases in the mineral concentration of iodine, selenium, vitamin D, and most significantly, omega-3 fatty acids in fish fillet. High percentages of vegetable oils in aquafeeds alter fish muscle fatty acid composition, notably reducing omega-3 such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which aids with cardiovascular, cognitive, and neural development and provides other human health benefits [73,74,75]. Partially or totally replacing fish oil with crop oil also unfavorably changes the flesh fatty acid composition in many fish species [68,69,74]. Salmonids fillets are marketed for their human-health-benefitting properties, mainly for their high omega-3 content. This benefit of consuming farmed salmonids has decreased in recent years because current feed formulations contain higher levels of vegetable oils and lower levels of omega-3 DHA and EPA. Over the past decade, the beneficial EPA and DHA content in farmed salmonids fillets has significantly declined, mainly due to the higher inclusion of vegetable oils in aquafeeds (Figure 1) [76,77]. A recent study conducted by the University of Stirling displayed that one portion of Scottish salmon would provide 3.9 g of omega-3 (EPA and DHA); however, this was reduced to 1.9 g of EPA + DHA in 2016, because the use of terrestrial crop protein and vegetable oil increased in salmonid feed [77].
Figure 1.
Contents of EPA and DHA (omega-3) in farmed salmon have fallen significantly (source: adapted from [77] CC 4.0). For a, b, c, d in the figure caption meaning both letters appearing together means no difference.
The American Dietetic Association and Dietitians of Canada recommends a daily consumption of 500 mg of n3 LC PUFAs (EPA + DHA), which is the equivalent of two fish servings/week of approximately 100 g of cooked (130 g of raw) fatty fish [78,79]. Several studies have reported that most salmonids held in freshwater potentially have the ability to convert C18: 3n-3 (a-linolenic acid) from vegetable oil into the corresponding C20:5 n-3 (EPA) and C22:6 n-3 (DHA) in vivo via an alternating succession of desaturation and elongation [80,81,82,83,84]. However, the de novo synthesis of any nutrient including DHA can increase metabolic energy expenditure, which might be better used to support somatic growth.
It is also well known that it is easy to formulate a commercial tilapia diet with low fishmeal and no fish oil (with vegetable oil) that will not decrease growth [85,86]. This commercial formulation, however, causes an unbalanced ratio of n3:n6 in the fillet due to a reduced deposition of n3 and increased deposition of n6 [87,88]. Recently, the New York Times highlighted this issue and reported that farmed tilapia, raised largely on a commercial diet containing corn and soy, had a high n6 deposition [89]. This has led medical doctors and nutritionists to doubt the nutritional benefits of eating farmed tilapia. Several studies of current tilapia feeds show elevated levels of C18 2n-6 (linoleic acid) fatty acids from crop oils [90,91], which greatly alters the fatty acid composition of tilapia flesh [86] and can contribute to an imbalanced n-3/n-6 ratio in human consumers [88], causing an increased production of proinflammatory eicosanoids via C20:4n-6 (arachidonic acid). The desired ratio of omega-3 to omega-6 fatty acids in a person’s diet is about 1:1 for optimum health benefits [92,93,94]. Wild tilapia have n3:n6 ratios ranging from 2.6:1 to 6.4:1 and contain more n3 LC PUFAs than farmed tilapia because wild fish eat natural foods rich in n3 LC PUFA [95,96]. It is biologically possible for farmed tilapia to meet the goal of the complete replacement of fish oil using Schizochytrium-dried whole-cells, which leads to an omega-3:omega-6 ratio of 1.4:1 in tilapia fillets, which is supportive of a more favorable ratio (1:1) for human consumers [97].
Although terrestrial crop protein and vegetable oil cause significant environmental harm and have nutritional disadvantages, these are the largest alternative feed ingredients mainly due to their wide availability and the lower price of these commodities compared to FMFO. Currently, aquafeed producers are seeking more sustainable and commercially scalable substitutes for fishmeal and fish oil due to rising prices, the price volatility of these commodities, food security concerns, and environmental harm.
2. Novel Aquafeed Ingredients
2.1. Microalgae Protein and Oil
The industrial-scale production of microalgae has gained momentum for their use in aquaculture feeds [98,99,100]. Marine microalgae particularly have potential to replace fishmeal and fish oil in salmonids and other finfish feeds because of their high levels of fatty acid and protein content. Marine microalgae, Nannochloropsis oculata, Isochrysis sp., and Schizochytrium sp. showed promise in aquafeed because they are rich in EPA, DHA, protein, key amino acids (methionine and lysine), lipids, and are good sources of minerals. A recent study showed that Isochrysis sp. is a highly digestible protein, amino acid, lipid, and fatty acids source for rainbow trout (Table 1). This species could be a good candidate for fishmeal and fish oil replacement in rainbow trout diets and can be used as a health-promoting omega-3, DHA supplement in diets [100]. Research showed that lipids extracted from Desmodesmus sp. could be used (20%) in the salmon feeds without any negative effect on growth and fillet composition [101,102]. A Spirulina algal meal could be also incorporated in 10% of the rainbow trout feed without any adverse effect on fish performance [102].
Defatted N. oculata coproducts (leftover biomass oil extraction) contain approximately 20% to 45% crude protein, with good amino acid profiles. Including defatted N. oculata as a protein source into diets up to 33% for tilapia [103] and up to 10% for Atlantic salmon [104] did not affect their performance or health status. Another unique benefit of the defatted microalgae is their function in serving not only as an excellent protein source but also as a source of polyunsaturated fatty acids (PUFAs) to enrich n-3 fatty acids. Defatted N. oculata is more nutrient-dense compared to the whole cells. The digestibility of lysine (often deficient in terrestrial crop protein) was higher [103], and the EPA was also highly digestible, making it a good candidate for EPA supplementation in tilapia feed [103]. However, the defatted N. oculata caused a significantly lower digestibility of protein and methionine than the whole cells in tilapia. The decreased digestibility and growth at a higher inclusion rate of N. oculata indicated higher levels of antinutrients in the defatted N. oculata coproducts than the whole cells.

Table 1.
Summary of microalgae (Schizochytrium sp. and Isochrysis sp.) whole cells used as an oil source in research.
Table 1.
Summary of microalgae (Schizochytrium sp. and Isochrysis sp.) whole cells used as an oil source in research.
| Microalgae | Fish Species | FCR | Inclusion % of Diet | Reference |
|---|---|---|---|---|
| Schizochytrium sp. | Atlantic salmon | 1.40 and 1.42 | 5.5 and 11 | [105] |
| Schizochytrium sp. | Tilapia | 1.0, 1.0, 0.9 and 0.9 | 4, 8, 12.5, and 16.1 | [97] |
| Schizochytrium sp. | Tilapia | 1.57, 1.60 and 1.4 | 6.20 | [29] |
| Schizochytrium sp. | Rainbow trout | 1.0 and 0.98 | 9 and 6.5 | [41] |
| Schizochytrium sp. | Pacific white shrimp | Not reported | 0.6, 1.2, 1.8, 2.3 and 3.5 | [106] |
| Schizochytrium sp. | Atlantic salmon | 0.72 and 0.73 | 2.5 and 5.0 | [107] |
| Schizochytrium sp. | Rainbow trout | 0.98 | 2.50 | [100] |
| Isochrysis sp | Rainbow trout | 1.0 | 2.40 | [100] |
| Isochrysis sp | European sea bass | 1.69 and 1.76 | 7 and 14 | [108] |
Predominantly heterotrophic DHA-rich Schizochytrium sp. have been investigated for oil and have been found to be a highly digestible source of nutrients for salmonids, shrimp, tilapia, and other species and a potential substitute for fish oil in aquafeed [1]. A recent study showed a significantly improved gain in weight, feed conversion ratio, and protein efficiency ratio when fish oil was fully replaced using Schizochytrium sp. biomass in a tilapia diet [97]. It has been reported to develop a high-performing fish-free feed (full substitution of FM and FO) for tilapia by combining two marine microalgae (one is an N. oculata coproduct and another is Schizochytrium sp. biomass [97].
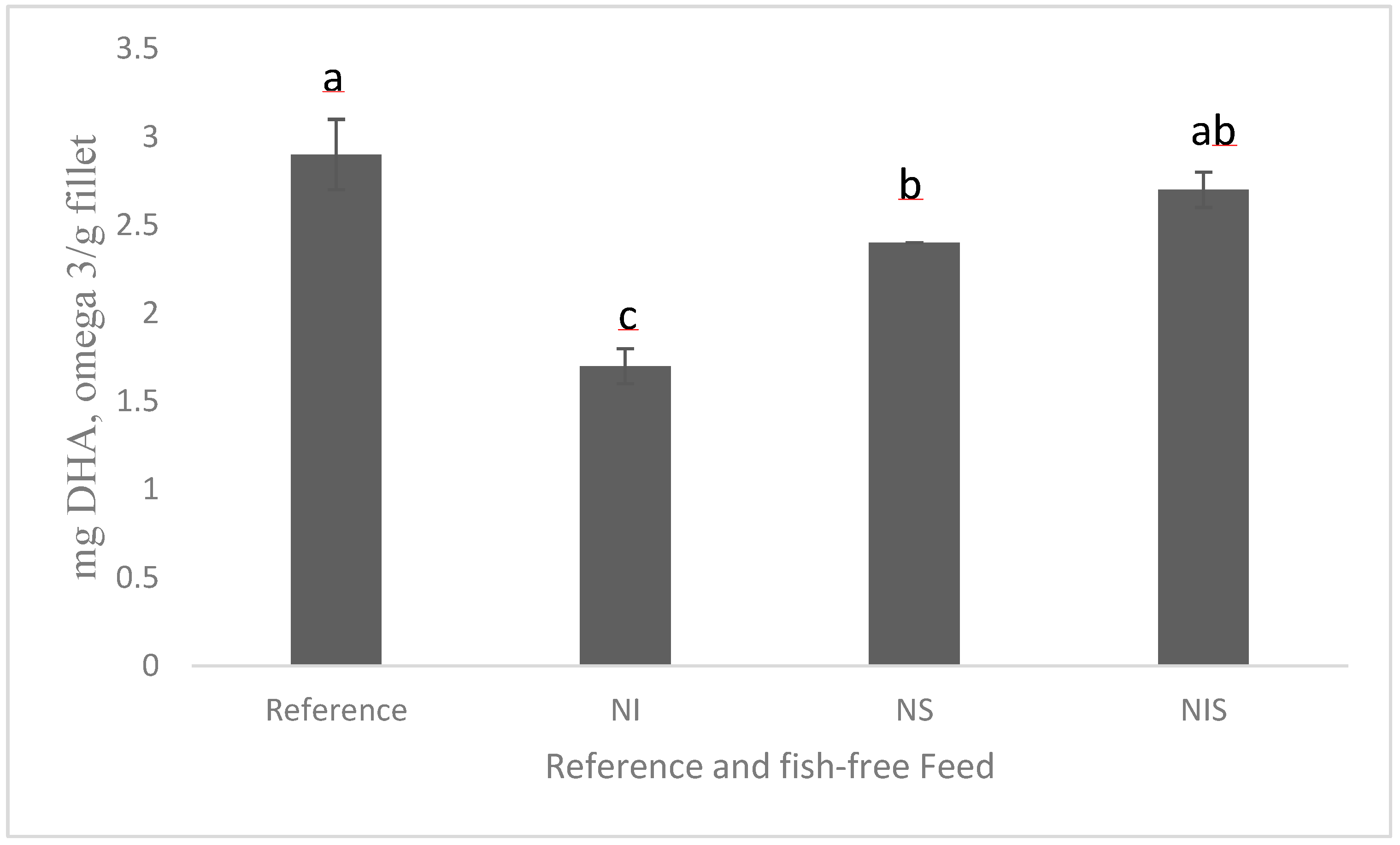
A recent study showed promise in developing a fish-free feed for rainbow trout by combining marine microalgae Nannochloropsis sp., Isochrysis sp., and Schizochytrium sp.; the detected fillet DHA levels were similar (Figure 2), although the fish-free feed exhibited a minor, but significant, lower growth (due to lower feed intake) than the fish fed a conventional feed, and this could be improved by adding feeding stimulants [100]. Schizochytrium sp. still holds promise as an alternative to fish oil because the production of heterotrophic microalgae is much easier and quicker (a few days) than phototrophic microalgal production via fermentation, which utilizes carbon sources for energy (e.g., corn, sugar cane byproduct). In addition, the cultivation conditions of microalgae, for example, light, temperature, and nutrient source, can also impact the phototrophically grown microalgal composition, including the fatty acid composition. It ultimately affects the consistent quality of the final product. There has been extensive research on the capabilities of heterotrophic algae oils and their success in commercial trials. A number of aquafeed companies have recently begun commercially producing DHA-rich oil from Schizochytrium sp. for salmon feeds [109]. Many agribusiness and animal nutrition companies started producing heterotrophic Schizochytrium sp. as fish oil substitutes (Corbion; BioMar; Archer Daniels Midland, ADM; and Veramaris). However, extracting oil from a microalgal biomass is a cost-prohibitive process compared to extracting fish oil; for example, pure microalgal oil costs twice as much as fish oil in the market. It is important to see the more commercial scale production, which will in turn reduce the cost of ingredient production. Moreover, performing life cycle environmental analyses of microalgae when producing aquafeed feed is also important to detect the effects on global warming potential, other environmental footprints, and land and freshwater use. Our recent article on the life cycle analysis of Schizochytrium sp. in aquafeeds showed that Schizochytrium sp. could be a better sustainable fish oil substitute for aquaculture feed due to its lower biotic resource depletion and global warming potential [14]. This outcome helps strengthen sustainable environmental stewardship of aquaculture feeds with Schizochytrium sp.
Figure 2.
Amounts of DHA in the fillet of rainbow trout fed experimental diets for 84 days (adapted from [100] CC 4.0). NI = Nannochloropsis sp. + Isochrysis sp.; NS = Nannochloropsis sp. + Schizochytrium sp.; NIS = Nannochloropsis sp. + Isochrysis sp. + Schizochytrium sp. For a, b, c, d in the figure caption meaning both letters appearing together means no difference.
2.2. Yeast, Fungi, and Bacteria
Saccharomyces cerevisiae, various Aspergillus, and Fusarium venenatum are widely known, but other strains such as Candida utilis, Candida, Hansenula, Pichia, Torulopsis, and Kluyveromyces marxianus are also stimulating interest as protein ingredients for aquaculture feed [37,110,111]. Several yeast meals, for example, S. cerevisiae, Candida utilis, and K. marxianus have been used in salmon feeds [110,112]. Candida utilis and K. marxianus are good sources of protein and could substitute up to 40% of the fishmeal without compromising growth [110]. However, S. cerevisiae was reported as a poor protein ingredient for fish feed.
Some bacterial strains can play an important role in producing very high crude protein contents (approximately 60 to 82% of dry cell weight) and essential amino acid levels, along with vitamins, phospholipids, and other functional compounds [113,114]. For example, several methanotrophically grown bacterial proteins have been investigated in Atlantic salmon feeds. A recent study revealed that salmon fed a 36% bacterial protein meal had a better growth rate and feed efficiency ratio than when fed the reference diet [115]. KnipBio Meal (Methylobacterium extorquens) could replace 55% of fishmeal in salmon diets with no adverse side effects upon growth [116]. Bacterial protein also showed good promise in shrimp. KnipBio Meal could replace 100% of fishmeal in shrimp diets [116]. Combining two purple nonsulfur bacteria at 1% of a diet resulted in modest growth improvements [117]. Corynebacterium ammoniagenes could replace 10–20% fishmeal in shrimp [118]. Combining bacteria and microalgae (Novacq™, CSIRO Canberra, Australia) has been tested on black tiger shrimp and prawn [119,120]. There is great potential to produce bacterial protein, but more organisms need to be identified for commercial scale production. It is interesting to note that the bacteria can be cultured on agricultural wastes (rice straw, rice hulls, manure, and starchy residue), as the substrates attain a high level of protein [121]. Additionally, bacterial proteins contain other nutrients including lipids, vitamins, and minerals [37,122]. Although bacterial proteins are very attractive for future aquafeed, the challenges associated with processing, the economy of scale, and their adoption globally need to be addressed.
2.3. Genetically Modified Oil
Western consumers consumed GM foods on a regular basis for a decade, but the human health effects of eating GM foods are not well understood. Several types of currently available vegetables and processed foods in the market are nonlabeled GMOs. Recently, Cargill deregulated genetically modified transgenic foods (canola oil/camelina oil/yeast). The DHA and EPA content in transgenic oilseed camelina can avoid the production of undesirable fatty acids [123]. GM rapeseed oil and yeast displayed a high retention of EPA and DHA in Atlantic salmon fillets [124]. However, to obtain public confidence and satisfaction, it is critical that future researchers know the long-term effects of the GM ingredients in fillets made from aquaculture-feed-fed farmed fish on human health. Additionally, fish fed GM ingredients should be evaluated to determine the sensory properties of fillet due to potential biochemical changes that could affect sensory qualities. Several commercial industries (for example, Aquaterra/Nuseed and BASF/Cargill) are currently using GM yeast and oilseed crops, which are rich in EPA or DHA or a combination of both. The process involves selecting and cloning the genes involved in EPA and DHA biosynthesis from marine algae and then inserting them into terrestrial crops. However, the public perceives any GM product as still being poor to the users. Additionally, regulatory hurdles still exist, which makes it illegal to grow GM plants in some countries.
2.4. Insect Meal
The aquafeed industry is actively seeking a diverse array of alternative feed ingredients for fishmeal, including insect meal. Insects could provide a sustainable source of protein for aquacultures using food waste. As of now, the following species are the most studied for producing protein meals, and they account for the majority of the literature: black soldier fly (Hermetia illucens, L.; BSF), common housefly (Musca domestica), yellow mealworm (Tenebrio molitor), lesser mealworm (Alphitobious diaperinus), house cricket (Acheta domesticus), banded cricket (Gryllodes sigillatus), and field cricket (Gryllus assimilis). Among these species, the BSF is considered the most attractive insect meal for aquafeed for salmonids, i.e., rainbow trout (Onchorhynchus mykiss) and Atlantic salmon (Salmo salar) [125,126,127,128,129,130,131]. Several studies have shown promise with the use of insect meals in aquafeeds, and the inclusion of insect meals is still a recent development, with many uncertainties existing that could influence whether the aquafeed industry adopts insect meals on a large scale. More research is needed to formulate feeds for specific aquaculture species. The authors of [130] for example, demonstrated the sensitivity of greenhouse gas emission results to direct emissions from the insects during growth, for which very limited data were available. Moreover, the consistent quality and quantity of supply is important for commercial feed mills to fine tune feed formulations, as the raw materials have to be the same every batch. For example, if we feed our insects different waste streams as substrates, their nutritional properties will be altered and inconsistent. Additionally, cannibalism behavior may prevent the insects from being raised uniformly, as the smaller larvae are eaten by the bigger ones. We need more research on unconventional aquaculture feed ingredients to solve these problems. Additionally, data on the antinutrient compositions (for example, chitin, protease inhibitors, oxalate, tannin, etc.), palatability, and digestibility of insect meals are lacking. Brewery and distillery wastes as byproducts are currently largely used in poultry and salmon feeds. It seems like it is not worthwhile to feed the nutrient-rich wastes to insect larvae when we could feed them directly to livestock. The use of wastes to grow insects is important, as an insect grower could target certain wastes that have no other use, although more research is needed on aspects such as the biosecurity issue. Insect larvae could be raised on a large volume of industrial manure (industrial farm), but farmers should be careful about antibiotics and other contaminants.
2.5. Fish Processing Byproduct
The processing of fish for human food generates byproducts such as heads, viscera, frames, skins, and others, such as tails, fins, scales, mince, blood, etc. These byproducts are actually good sources of protein and oil, from which fishmeal and fish oil can be yielded. Fish-processing byproducts are still not fully utilized. Approximately 25–35% of fishmeal comes from the byproducts of fish processing [132]. Approximately 10% of byproducts are generated by aquacultures, 19% of byproducts are generated by capture fisheries, and 71% of byproducts are generated by whole capture fisheries.
Currently, the byproducts of herring and tuna fisheries provide about 5 million metric tons, which accounts for around 25 percent of global fishmeal production. For example, the processing of Atlantic salmon (Salmo salar) fillet yields about 60% of byproducts that contain high levels of omega-3 DHA and EPA. Approximately 11.7 million tons of additional byproducts are generated in fish processing plants that are not certified for the production of fishmeal and fish oil [132]. The largest volume of fish processing waste is produced in North America and Oceania. Fish wastes are mainly generated in the global south, mainly due to a lack of high-quality ice, cold storages, and refrigerated transportation [3]. Capturing fish processing byproducts is often not economically viable due to logistic and technical constraints.
3. Key Strategies to Measure Nutritional and Environmental Sustainability
3.1. Ingredients Digestibility
The digestibility of aquafeed ingredients is key information for formulating economically viable and environmentally responsible feeds, but limited digestibility data are publicly available for alternative ingredients [100]. This lack of information often leads aquafeed manufacturers to extrapolate the nutritive value of ingredients from their chemical composition. Because a simple biochemical analysis and the presence of protein and amino acids in the ingredients does not ensure the level of digestible proteins and amino acids required for particular fish species, it is important to conduct digestibility experiments to identify the digestibility of ingredients and formulate the diet based on the digestible nutrient content, which can reduce feed costs, nutrient pollution (including phosphorus and nitrogen eutrophication emissions), and improve the feed conversion ratio of aquafeeds. The main reason for the improved feed conversion ratio (FCR) achieved today is due to the significantly increased digestible nutrients in and energy density of the feeds. In addition to the digestibility data, the aquafeed industry will be benefitted by using alternative ingredients in feed formulations if they have specific information on parameters such as the nutrients and antinutrient composition (for example, pectin, lectin, chitin, protease inhibitor, oxalate, tannin, etc.).
3.2. Feed Conversion Ratio (FCR)
The development of sustainable feed using alternative ingredients should be informed by the efficiency of feed with a lower FCR. A common measure of efficiency is the feed conversion ratio (FCR), calculated as the ratio of feed intake to weight gain. It reflects the environmental performance of the aquaculture because it provides an indication of the phosphorus and nitrogen waste outputs in the aquatic environment with potential negative consequences of eutrophication, greenhouse gas emissions, loss of biodiversity, and other ecosystem services. Additionally, the contribution of aquacultures to greenhouse gas emission is strongly related to the FCR and the origin of the feed components. Although, the FCRs of aquacultures have fallen over the past several decades from around 3 to around 1.35, largely because of better feed formulations, feed manufacturing methods, and on-farm feed management. Specifically, salmonids farming improved the FCR from about 2–2.25 to approximately 0.9–1.2 since 1970 due to better feed formulation using highly digestible feed ingredients, technological developments, and on-site feed management. Now it is time to make more sustainable feed formulations for other species such as carps, catfishes, tilapia, and marine shrimps. The feed conversion ratio reflects the fact that fish are the most efficient species among all commercially farm-raised animals. For example, farmed fish can convert approximately 1.35 kg of feed (dry) into 1 kg of flesh (wet); in contrast, the feed conversion of poultry is 1.8, pork is 3.5, and beef is 6.25 [9,11,37].
3.3. Life Cycle Assessment (LCA) for Ecological Impact Measures
The environmental impacts of aquafeed can be evaluated with a LCA, which is an important tool to measure the environmental impacts of food systems [53,133,134]. The development of sustainable feed using alternative ingredients should be informed by environmental impact categories including resource use (e.g., land, water, fertilizer), global warming emissions, eutrophication emissions, biodiversity loss, and negative externalities including ocean acidification [53,135]. Very limited data on life cycle environmental impacts and emissions from novel ingredients are available. The categories of the environmental footprint, determined with an LCA, should be included for novel ingredient evaluation processes [136,137]. Additionally, there is no food production system today that is truly sustainable in terms of energy use and biodiversity loss. However, this information is essential to evaluate the performance of novel ingredients and compare them with FMFO production. The alternative aquafeed industry is in its infant stages, and the broader environmental burdens of producing novel ingredients have not yet been established. A more holistic view of sustainability is therefore needed. Thus, mass producing new ingredients could depend on fossil fuels, and creating high-quality emerging protein and oil requires us to learn about the life-cycle effects of these ingredient substitutes on FMFO in diets. It is important to conduct more research on the LCAs of currently available unconventional feedstuff to better understand the more sustainable future ingredients for aquacultures.
4. Novel Technology for Improving Ingredients Quality
Improving the overall feed efficiency/feed conversion ratio and digestibility of protein ingredients will help the aquafeed industry meet the global demand for these limited ingredients. There are several factors that are responsible for enhancing feed efficiency and formulating least-cost diets: (i) the quality of the protein ingredients, which includes the protein concentration, amino acid profile, and antinutrient factors; (ii) the ability to meet protein and essential amino acid requirements for a given fish species at life stages because the relative ontogenetic stage of the fish can significantly affect the protein or essential amino acid levels required in their diet; and (iii) the ability of fish to digest and retain the ingested feed protein. New technologies have been developing to process the ingredients that can improve the feed efficiency/feed conversion ratio, digestibility, and protein efficiency. For example, microalgae are still in the infant stages of being included in aquaculture diets, and the further processing of these ingredients may enhance both the protein and energy digestibility. The nutrient digestibility of microalgae ingredients can be an issue because of the rigidity of their cell wall [97,138]. To improve the digestibility of microalgae, the cell wall can be ruptured via several methods [138,139]: enzymatic (cellulases, xylanase, glucanase), chemical (organic solvents or acids), and physical and mechanical (bead milling, extrusion, grinding, autoclaving, roasting/toasting, high-pressure homogenization, or microfluidics). Physical processing is effective at partially or completely inactivating the antinutritional factors. Physical and mechanical methods are generally preferred [140,141], as enzymatic and chemical methods can impact intracellular nutrients. Trypsin inhibitors and lectins in soybeans could be inactivated by heating or cooking [142]. A recent study showed that the whole-cells of Chlorella and the ruptured cells had the same nutrient content, but the ruptured cells had improved digestibility over the whole cells of Chlorella for essential amino acids, lipids, and carbohydrates [138].
4.1. Extrusion Processing
The novel extrusion technology offers the advantage of making use of a wider variety of ingredients. For example, twin screw extruder technology can be used to process, stabilize, and incorporate ingredients. The extruder has various capacities, along with drying, grinding, and other related equipment to conduct pilot-scale manufacturing tasks. Extrusion processing exposes ingredients to high temperatures and pressures over a short period of time, resulting in cooking and pasteurization and thus eliminating any antinutrients in the ingredients and increasing the feed intake, nutrient digestibility, and therefore the growth performance of the fish [63,104,143,144,145]. Additionally, extruded feed allows higher lipid levels to be included in the diets, and the gelatinization of starch stimulates the increase in the protein and energy digestibility of feeds. Extrusion also helps to produce more durable feed, with less fines and the ability to vary the physical characteristics of the pellet (buoyancy or sinking).
4.2. Use of Exogenous Enzymes in Aquafeed
Protease enzymes may stimulate endogenous peptidases by improving protein digestibility and hydrolyzing proteinaceous antinutrients such as lectins, trypsin inhibitors, antigenic proteins, and antinutritional allergenic proteins such as glycinin, β-conglycinin, and kafrin [146]. High-quality proteases or cocktails can be developed for specific proteins (e.g., the keratin-rich poultry feathers) based on the digestive conditions (e.g., stomach or intestinal pH), which could help enhance dietary protein digestion and utilization. The digestive tracts of monogastric animals, such as rainbow trout, lack any appreciable nonstarch polysaccharide enzyme activity [56]. Thus, treating NSP enzymes (xylanases, glucanases, cellulases) to ingredients could enhance the digestibility and utilization of nutrients supplied by alternative ingredients. Supplementation with these enzymes has shown increased nutrient digestibility, nutrient utilization, fish growth, reduced nutrient wastes, and antinutritional factors in terrestrial plant ingredients for fish including rainbow trout [147,148,149].
4.3. Use of Additives in Aquafeed to Improve Palatability
The supplementation of taurine with alternative ingredients would enhance the palatability of low fishmeal or fish-free feeds and improve digestion and lipid adsorption. Taurine is a neutral beta-amino acid derived from the metabolism of sulfur containing amino acids. In the past, taurine was not considered an essential nutrient for fish, but recent research showed that it plays a key role in aquaculture nutrition to improve growth feed intake and feed utilization [144,150,151,152]. Usually, fish-based diets are rich in taurine; therefore, salmonids or other carnivorous fish may require exogenous taurine in fish-free feed to maintain their physiological functions and increase their feed intake. The addition of lecithin to aquafeed containing low levels of FM has also been shown to improve feed intake and growth rates in trout, salmon, and flounder [153,154,155].
5. Challenges with Adopting and Opportunities to Adopt the New Aquafeeds
The typical rate (share of individual dietary components in feeds) of soy and corn combined is about 40–60% in fish feeds [97,100,103]. Replacing terrestrial crops with the other current alternatives in fish feed is likely to be too costly for broader adoption by aquafeed manufacturers and aquaculture producers. The aquafeed industry will use alternative ingredients only if it is at a cost competitive with soy and corn and at a constant quantity of supply [103,138]). Thus, researchers and aquaculture industry stakeholders need a collaborative effort and need to pursue stepwise research to identify a prime alternative that will be productive and profitable while minimizing negative environmental, social, and economic impacts. Towards this end, scientists should be pursuing stepwise research to find a sustainable solution, whether it is a novel ingredient or a combination of these ingredients or a coproduct or byproduct that can partially or fully replace crop proteins in aquafeeds, and to determine how this combination affects fish growth, flesh quality, and economic and environmental performances.
An evaluation of the sustainability of new ingredients is gaining attention. Several approaches were applied to define sustainability, but a life cycle assessment is perhaps the best approach. Our lab is now developing an open-access decision-support tool that integrates a technoeconomic and life cycle analysis and a nutritional analysis of diverse alternative ingredients, from insect meals to microalgae for fish-free aquaculture feeds.
However, we should acknowledge that the major challenge is generating consistency with the supply of microalgal ingredients to produce large quantities on an industrial scale. Another big challenge is the competitive price of unconventional ingredients with conventional ingredients. Price is the major constraint that will dictate the future course of the aquafeed sector and industry. Thus, scaling up is a real block for these novel ingredients. The big challenge is the competitive price of novel ingredients with conventional ingredients. Aquaculture feed manufacturers are willing to pay a similar price per ton for soy protein concentrate as fishmeal. As traditional fish feed ingredients make up a significantly large portion of aquafeed today, the focus has just begun to include a minor percentage of new raw ingredients because there is still not any solid guarantee of consistent supplies and because of the competitive costs. Researchers and microalgae R&D have to find ways to lower the costs of producing and processing novel ingredients. For precise feed formulations, the raw ingredients have to be the same in every batch. For example, if an insect is being produced via different waste streams, then their nutritional compositions will be inconsistent. Relatively small production volumes of novel ingredients such as microalgae, bacteria, yeast, and insect meal still supply a limited market contribution compared with traditional feed ingredients. For example, although replacing fish oil with microalgae showed promise, producing microalgae and extracting oil requires access to advanced technologies, expertise, and money. Moreover, the use of these novel ingredients is not cost effective for small-scale aquaculture producers and notably for developing aquaculture nations. Thus, at this moment, adopting novel ingredients is still challenging and may not be equitable to all producers across the world. There are multiple regulatory, economic, and environmental challenges that accompany switching from conventional aquafeeds to ones based on innovative ingredients, especially for smaller producers and producers in developing countries [33]. Despite the above limitations, if the stakeholders agreed to adopt novel ingredients for aquafeeds where it was feasible, this would be an important step towards reducing pressure on wild-caught fish in the aquaculture diet, which could secure the future sustainability of the aquaculture sector. Exploring economic incentives, investigating marketing opportunities, and promoting the ecocertification of sustainable ingredients is also needed. Consumers’ preferences for and awareness of sustainability is growing, and there is a positive public attitude towards novel ingredients, as expressed by a willingness to pay a sustainable premium for its products, which can further enhance the profitability of adopting novel ingredients.
6. Conclusions and Future Steps
Regarding progress towards the development of novel ingredients, it is imperative to determine the nutritional evaluations of each ingredient, which includes the determination of nutritional value, palatability and feed intake, and digestibility, and requires researchers to conduct nutritional feeding experiments in the lab and feeding/growth trials at commercial farms [156]. Some ingredients may require additional processing to improve digestibility, the FCR, and reduce the antinutritional factors. The various processing could also increase the opportunities to produce lower-cost protein-rich ingredients and might reduce the overall costs of production. In the final stage of the nutritional evaluation of alternative ingredients, a comprehensive economic analysis is also important to inform decisions about the inclusion of unconventional ingredients into diets that are cost competitive with conventional ingredients. Additionally, some recent assessments showed that it might be possible to produce yields of novel ingredients such as microalgae (per unit area) several times higher than that of terrestrial crops (soy protein) using the same land footprint using atmospheric carbon and renewable energy [157]. As mentioned, the sustainable production approach clearly provides an indication of a new emerging priority to conduct a life-cycle assessment analysis of new ingredients [136,137]. Thus, to fully develop sustainable alternative ingredients, scholars must use systems-based approaches that integrate knowledge and expertise across multiple disciplines including fish nutritionists and aquaculture scientists to conduct on-farm feeding trials with farmers, environmental scientists, technoeconomic and life-cycle practitioners, extension scientists, and economists.
Funding
P.K.S. was supported by the CA Sea Grant Award (grant no. NA18OAR4170073), Agriculture and Food Research Initiative Competitive Grant award no. 2021-67016-33394 from the USDA NIFA, and Agriculture and Food Research Initiative Competitive Grant no. 2021-69014-34501 from the USDA NIFA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated by this study are presented in the tables.
Acknowledgments
Pallab K. Sarker would like to thank the Dean of Social Sciences and Executive Vice Chancellor of the University of California Santa Cruz.
Conflicts of Interest
The author declares that he has no conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture 2016: Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; ISBN 978-92-5-109185-2. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2018: Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; ISBN 978-92-5-130562-1. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- Rockström, J.; Williams, J.; Daily, G.; Noble, A.; Matthews, N.; Gordon, L.; Wetterstrand, H.; DeClerck, F.; Shah, M.; Steduto, P.; et al. Sustainable Intensification of Agriculture for Human Prosperity and Global Sustainability. Ambio 2017, 46, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Sarker, P.K.; Bureau, D.P.; Hua, K.; Drew, M.D.; Forster, I.; Were, K.; Hicks, B.; Vandenberg, G.W. Sustainability Issues Related to Feeding Salmonids: A Canadian Perspective. Rev. Aquac. 2013, 5, 199–219. [Google Scholar] [CrossRef]
- Fry, J.P.; Mailloux, N.A.; Love, D.C.; Milli, M.C.; Cao, L. Feed Conversion Efficiency in Aquaculture: Do We Measure It Correctly? Environ. Res. Lett. 2018, 13, 024017. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Jacobsen, N.S.; Essington, T.E.; Clavelle, T.; Halpern, B.S. Avoiding the Ecological Limits of Forage Fish for Fed Aquaculture. Nat. Sustain. 2018, 1, 298–303. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Tacon, A.G.J. Trends in Global Aquaculture and Aquafeed Production: 2000–2017. Rev. Fish. Sci. Aquac. 2020, 28, 43–56. [Google Scholar] [CrossRef]
- Browdy, C.L.; Hargreaves, J.A. Overcoming Technical Barriers to the Sustainable Development of Competitive Marine Aquaculture in the United States; NOAA Technical Memo NMFS F/SPO100; U.S. Department of Commerce: Silver Spring, MD, USA, 2009; 114p.
- McKuin, B.L.; Kapuscinski, A.R.; Sarker, P.K.; Cheek, N.; Colwell, A.; Schoffstall, B.; Greenwood, C. Comparative Life Cycle Assessment of Heterotrophic Microalgae Schizochytrium and Fish Oil in Sustainable Aquaculture Feeds. Elem. Sci. Anthr. 2022, 10, 00098. [Google Scholar] [CrossRef]
- Hilborn, R.; Banobi, J.; Hall, S.J.; Pucylowski, T.; Walsworth, T.E. The Environmental Cost of Animal Source Foods. Front. Ecol. Environ. 2018, 16, 329–335. [Google Scholar] [CrossRef]
- Parodi, A.; Leip, A.; De Boer, I.J.M.; Slegers, P.M.; Ziegler, F.; Temme, E.H.M.; Herrero, M.; Tuomisto, H.; Valin, H.; Van Middelaar, C.E.; et al. The Potential of Future Foods for Sustainable and Healthy Diets. Nat. Sustain. 2018, 1, 782–789. [Google Scholar] [CrossRef]
- MacLeod, M.J.; Hasan, M.R.; Robb, D.H.F.; Mamun-Ur-Rashid, M. Quantifying Greenhouse Gas Emissions from Global Aquaculture. Sci. Rep. 2020, 10, 11679. [Google Scholar] [CrossRef]
- Ellingsen, H.; Aanondsen, S.A. Environmental Impacts of Wild Caught Cod and Farmed Salmon—A Comparison with Chicken (7 Pp). Int. J. Life Cycle Assess. 2006, 11, 60–65. [Google Scholar] [CrossRef]
- Pelletier, N.; Tyedmers, P. Feeding Farmed Salmon: Is Organic Better? Aquaculture 2007, 272, 399–416. [Google Scholar] [CrossRef]
- Iribarren, D.; Vázquez-Rowe, I.; Hospido, A.; Moreira, M.T.; Feijoo, G. Estimation of the Carbon Footprint of the Galician Fishing Activity (NW Spain). Sci. Total Environ. 2010, 408, 5284–5294. [Google Scholar] [CrossRef]
- Parker, R. Implications of High Animal By-Product Feed Inputs in Life Cycle Assessments of Farmed Atlantic Salmon. Int. J. Life Cycle Assess. 2018, 23, 982–994. [Google Scholar] [CrossRef]
- Jones, A.R.; Alleway, H.K.; McAfee, D.; Reis-Santos, P.; Theuerkauf, S.J.; Jones, R.C. Climate-Friendly Seafood: The Potential for Emissions Reduction and Carbon Capture in Marine Aquaculture. BioScience 2022, 72, 123–143. [Google Scholar] [CrossRef]
- Asche, F.; Cojocaru, A.L.; Roth, B. The Development of Large Scale Aquaculture Production: A Comparison of the Supply Chains for Chicken and Salmon. Aquaculture 2018, 493, 446–455. [Google Scholar] [CrossRef]
- Han, D.; Shan, X.; Zhang, W.; Chen, Y.; Wang, Q.; Li, Z.; Zhang, G.; Xu, P.; Li, J.; Xie, S.; et al. A Revisit to Fishmeal Usage and Associated Consequences in Chinese Aquaculture. Rev. Aquac. 2018, 10, 493–507. [Google Scholar] [CrossRef]
- Little, D.C.; Young, J.A.; Zhang, W.; Newton, R.W.; Al Mamun, A.; Murray, F.J. Sustainable Intensification of Aquaculture Value Chains between Asia and Europe: A Framework for Understanding Impacts and Challenges. Aquaculture 2018, 493, 338–354. [Google Scholar] [CrossRef]
- Xu, C.; Su, G.; Zhao, K.; Xu, X.; Li, Z.; Hu, Q.; Xue, Y.; Xu, J. Current Status of Greenhouse Gas Emissions from Aquaculture in China. Water Biol. Secur. 2022, 1, 100041. [Google Scholar] [CrossRef]
- Ballester-Moltó, M.; Sanchez-Jerez, P.; Cerezo-Valverde, J.; Aguado-Giménez, F. Particulate Waste Outflow from Fish-Farming Cages. How Much Is Uneaten Feed? Mar. Pollut. Bull. 2017, 119, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Maiolo, S.; Parisi, G.; Biondi, N.; Lunelli, F.; Tibaldi, E.; Pastres, R. Fishmeal Partial Substitution within Aquafeed Formulations: Life Cycle Assessment of Four Alternative Protein Sources. Int. J. Life Cycle Assess. 2020, 25, 1455–1471. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kapuscinski, A.R.; McKuin, B.; Fitzgerald, D.S.; Nash, H.M.; Greenwood, C. Microalgae-Blend Tilapia Feed Eliminates Fishmeal and Fish Oil, Improves Growth, and Is Cost Viable. Sci. Rep. 2020, 10, 19328. [Google Scholar] [CrossRef]
- Arru, B.; Furesi, R.; Gasco, L.; Madau, F.; Pulina, P. The Introduction of Insect Meal into Fish Diet: The First Economic Analysis on European Sea Bass Farming. Sustainability 2019, 11, 1697. [Google Scholar] [CrossRef]
- Ekmekci, H.; Gül, M. Economic Structure and Problems of Trout Enterprises: A Case of Fethiye. Turk. J. Agric. Food Sci. Technol. 2017, 5, 33–42. [Google Scholar] [CrossRef]
- Global Market Insights Inc. Aquafeed Market Size Worth USD 185 Billion by 2023: Global Market Insights Inc. Available online: http://www.globenewswire.com/news-release/2016/07/06/853958/0/en/Aquafeed-Market-size-worth-USD-185-Billion-by-2023-Global-Market-Insights-Inc.html (accessed on 23 September 2020).
- Cottrell, R.S.; Blanchard, J.L.; Halpern, B.S.; Metian, M.; Froehlich, H.E. Global Adoption of Novel Aquaculture Feeds Could Substantially Reduce Forage Fish Demand by 2030. Nat. Food 2020, 1, 301–308. [Google Scholar] [CrossRef]
- Shepherd, C.J.; Jackson, A.J. Global Fishmeal and Fish-Oil Supply: Inputs, Outputs and Markets. J. Fish Biol. 2013, 83, 1046–1066. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Hasan, M.R.; Metian, M. Demand and Supply of Feed Ingredients for Farmed Fish and Crustaceans; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; ISBN 978-92-5-106933-2. [Google Scholar]
- Hasan, M.R.; Soto, D. Improving Feed Conversion Ratio and Its Impact on Reducing Greenhouse Gas Emissions in Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B.P. Recent Advances in Single Cell Protein Use as a Feed Ingredient in Aquaculture. Curr. Opin. Biotechnol. 2020, 61, 189–197. [Google Scholar] [CrossRef]
- Klinger, D.H.; Naylor, R. Searching for Solutions in Aquaculture: Charting a Sustainable Course. Annu. Rev. Environ. Resour. 2012, 37, 247–276. [Google Scholar] [CrossRef]
- Chauton, M.S.; Reitan, K.I.; Norsker, N.H.; Tveterås, R.; Kleivdal, H.T. A Techno-Economic Analysis of Industrial Production of Marine Microalgae as a Source of EPA and DHA-Rich Raw Material for Aquafeed: Research Challenges and Possibilities. Aquaculture 2015, 436, 95–103. [Google Scholar] [CrossRef]
- Hardy, R.W. Utilization of Plant Proteins in Fish Diets: Effects of Global Demand and Supplies of Fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Bélanger-Lamonde, A.; Sarker, P.K.; Ayotte, P.; Bailey, J.L.; Bureau, D.P.; Chouinard, P.Y.; Dewailly, É.; Leblanc, A.; Weber, J.-P.; Vandenberg, G.W. Algal and Vegetable Oils as Sustainable Fish Oil Substitutes in Rainbow Trout Diets: An Approach to Reduce Contaminant Exposure. J. Food Qual. 2018, 2018, 7949782. [Google Scholar] [CrossRef]
- Boissy, J.; Aubin, J.; Drissi, A.; van der Werf, H.M.G.; Bell, G.J.; Kaushik, S.J. Environmental Impacts of Plant-Based Salmonid Diets at Feed and Farm Scales. Aquaculture 2011, 321, 61–70. [Google Scholar] [CrossRef]
- Troell, M.; Naylor, R.L.; Metian, M.; Beveridge, M.; Tyedmers, P.H.; Folke, C.; Arrow, K.J.; Barrett, S.; Crépin, A.-S.; Ehrlich, P.R.; et al. Does Aquaculture Add Resilience to the Global Food System? Proc. Natl. Acad. Sci. USA 2014, 111, 13257–13263. [Google Scholar] [CrossRef]
- Garcia, M.A.; Altieri, M.A. Transgenic Crops: Implications for Biodiversity and Sustainable Agriculture. Bull. Sci. Technol. Soc. 2005, 25, 335–353. [Google Scholar] [CrossRef]
- Hoc, B.; Tomson, T.; Malumba, P.; Blecker, C.; Jijakli, M.H.; Purcaro, G.; Francis, F.; Caparros Megido, R. Production of Rainbow Trout (Oncorhynchus mykiss) Using Black Soldier Fly (Hermetia Illucens) Prepupae-Based Formulations with Differentiated Fatty Acid Profiles. Sci. Total Environ. 2021, 794, 148647. [Google Scholar] [CrossRef]
- Stamer, A. Insect Proteins—A New Source for Animal Feed: The Use of Insect Larvae to Recycle Food Waste in High-quality Protein for Livestock and Aquaculture Feeds Is Held Back Largely Owing to Regulatory Hurdles. EMBO Rep. 2015, 16, 676–680. [Google Scholar] [CrossRef]
- Boerema, A.; Peeters, A.; Swolfs, S.; Vandevenne, F.; Jacobs, S.; Staes, J.; Meire, P. Soybean Trade: Balancing Environmental and Socio-Economic Impacts of an Intercontinental Market. PLoS ONE 2016, 11, e0155222. [Google Scholar] [CrossRef]
- Mendes dos Reis, J.G.; Sanches Amorim, P.; Sarsfield Pereira Cabral, J.A.; Toloi, R.C. The Impact of Logistics Performance on Argentina, Brazil, and the US Soybean Exports from 2012 to 2018: A Gravity Model Approach. Agriculture 2020, 10, 338. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New Developments in Fish Amino Acid Nutrition: Towards Functional and Environmentally Oriented Aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef] [PubMed]
- He, J.-Y.; Tian, L.-X.; Lemme, A.; Gao, W.; Yang, H.-J.; Niu, J.; Liang, G.-Y.; Chen, P.-F.; Liu, Y.-J. Methionine and Lysine Requirements for Maintenance and Efficiency of Utilization for Growth of Two Sizes of Tilapia (Oreochromis niloticus). Aquac. Nutr. 2013, 19, 629–640. [Google Scholar] [CrossRef]
- Sarker, P.K.; Hosokawa, H. Effect of Phytase on Growth and Phosphorus Utilization in Japanese Flounder (Paralichthys olivaceus). Int. J. Recirc. Aquac. 2009, 10, 25–41. [Google Scholar] [CrossRef]
- Sarker, P.K.; Fournier, J.; Boucher, E.; Proulx, E.; de la Noüe, J.; Vandenberg, G.W. Effects of Low Phosphorus Ingredient Combinations on Weight Gain, Apparent Digestibility Coefficients, Non-Fecal Phosphorus Excretion, Phosphorus Retention and Loading of Large Rainbow Trout (Oncorhynchus mykiss). Anim. Feed. Sci. Technol. 2011, 168, 241–249. [Google Scholar] [CrossRef]
- Pelletier, N.; Tyedmers, P. Life Cycle Assessment of Frozen Tilapia Fillets From Indonesian Lake-Based and Pond-Based Intensive Aquaculture Systems. J. Ind. Ecol. 2010, 14, 467–481. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Y.-H.; Han, Z.-Z.; Dong, H.-W.; Yang, C.-H.; Zou, Z.-Y. Effects of Phytase Pretreatment of Soybean Meal and Phytase-Sprayed in Diets on Growth, Apparent Digestibility Coefficient and Nutrient Excretion of Rainbow Trout (Oncorhynchus mykiss Walbaum). Aquac. Int. 2008, 17, 143–157. [Google Scholar] [CrossRef]
- National Research Council (U.S.). Committee on Animal Nutrition. In Nutrient Requirements of Fish; National Academy Press: Washington, DC, USA, 1993; ISBN 978-0-309-04891-0. [Google Scholar]
- Jobling, M. National Research Council (NRC): Nutrient Requirements of Fish and Shrimp. Aquac. Int. 2012, 20, 601–602. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; Boeck, G.D.; Becker, K. Phytate and Phytase in Fish Nutrition. J. Anim. Physiol. Anim. Nutr. 2012, 96, 335–364. [Google Scholar] [CrossRef]
- Storebakken, T.; Shearer, K.D.; Roem, A.J. Availability of Protein, Phosphorus and Other Elements in Fish Meal, Soy-Protein Concentrate and Phytase-Treated Soy-Protein-Concentrate-Based Diets to Atlantic Salmon, Salmo salar. Aquaculture 1998, 161, 365–379. [Google Scholar] [CrossRef]
- Baruah, K.; Sahu, N.P.; Pal, A.K.; Debnath, D. Dietary Phytase: An Ideal Approach for a Cost Effective and Low-Polluting Aquafeed. NAGA WorldFish Cent. Q. 2004, 27, 15–19. [Google Scholar]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K.; et al. Feeding Aquaculture in an Era of Finite Resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, M.; Carter, C.G. Dietary Phytase Supplementation and the Utilisation of Phosphorus by Atlantic Salmon (Salmo salar L.) Fed a Canola-Meal-Based Diet. Aquaculture 2004, 240, 417–431. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Ekmann, K.S.; Pedersen, P.B.; Verlhac, V. Effect of Supplemented Fungal Phytase on Performance and Phosphorus Availability by Phosphorus-Depleted Juvenile Rainbow Trout (Oncorhynchus mykiss), and on the Magnitude and Composition of Phosphorus Waste Output. Aquaculture 2009, 286, 105–112. [Google Scholar] [CrossRef]
- Barrows, F.T.; Bellis, D.; Krogdahl, Å.; Silverstein, J.T.; Herman, E.M.; Sealey, W.M.; Rust, M.B.; Gatlin, D.M., III. Report of the Plant Products in Aquafeed Strategic Planning Workshop: An Integrated, Interdisciplinary Research Roadmap for Increasing Utilization of Plant Feedstuffs in Diets for Carnivorous Fish. Rev. Fish. Sci. 2008, 16, 449–455. [Google Scholar] [CrossRef]
- Cho, C.Y.; Bureau, D.P. Reduction of Waste Output from Salmonid Aquaculture through Feeds and Feeding. Progress. Fish-Cult. 1997, 59, 155–160. [Google Scholar] [CrossRef]
- National Research Council; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Committee on the Nutrient Requirements of Fish and Shrimp. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-47322-4. [Google Scholar]
- Drew, M.D.; Borgeson, T.L.; Thiessen, D.L. A Review of Processing of Feed Ingredients to Enhance Diet Digestibility in Finfish. Anim. Feed. Sci. Technol. 2007, 138, 118–136. [Google Scholar] [CrossRef]
- Hixson, S.M.; Parrish, C.C.; Anderson, D.M. Full Substitution of Fish Oil with Camelina (Camelina sativa) Oil, with Partial Substitution of Fish Meal with Camelina Meal, in Diets for Farmed Atlantic Salmon (Salmo salar) and Its Effect on Tissue Lipids and Sensory Quality. Food Chem. 2014, 157, 51–61. [Google Scholar] [CrossRef]
- Torstensen, B.E.; Bell, J.G.; Rosenlund, G.; Henderson, R.J.; Graff, I.E.; Tocher, D.R.; Lie, Ø.; Sargent, J.R. Tailoring of Atlantic Salmon (Salmo salar L.) Flesh Lipid Composition and Sensory Quality by Replacing Fish Oil with a Vegetable Oil Blend. J. Agric. Food Chem. 2005, 53, 10166–10178. [Google Scholar] [CrossRef]
- Turchini, G.M.; Ng, W.-K.; Tocher, D.R.; Ng, W.-K.; Tocher, D.R. Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-0-429-15103-3. [Google Scholar]
- Bell, J.G.; McEvoy, J.; Tocher, D.R.; McGhee, F.; Campbell, P.J.; Sargent, J.R. Replacement of Fish Oil with Rapeseed Oil in Diets of Atlantic Salmon (Salmo salar) Affects Tissue Lipid Compositions and Hepatocyte Fatty Acid Metabolism. J. Nutr. 2001, 131, 1535–1543. [Google Scholar] [CrossRef]
- Drew, M.D.; Ogunkoya, A.E.; Janz, D.M.; Van Kessel, A.G. Dietary Influence of Replacing Fish Meal and Oil with Canola Protein Concentrate and Vegetable Oils on Growth Performance, Fatty Acid Composition and Organochlorine Residues in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2007, 267, 260–268. [Google Scholar] [CrossRef]
- Grisdale-Helland, B.; Ruyter, B.; Rosenlund, G.; Obach, A.; Helland, S.J.; Sandberg, M.G.; Standal, H.; Røsjø, C. Influence of High Contents of Dietary Soybean Oil on Growth, Feed Utilization, Tissue Fatty Acid Composition, Heart Histology and Standard Oxygen Consumption of Atlantic Salmon (Salmo salar) Raised at Two Temperatures. Aquaculture 2002, 207, 311–329. [Google Scholar] [CrossRef]
- Bell, J.G.; Waagbø, R. Safe and Nutritious Aquaculture Produce: Benefits and Risks of Alternative Sustainable Aquafeeds. In Aquaculture in the Ecosystem; Holmer, M., Black, K., Duarte, C.M., Marbà, N., Karakassis, I., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 185–225. ISBN 978-1-4020-6810-2. [Google Scholar]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.-K. Fish Oil Replacement in Finfish Nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Olsen, Y. Resources for Fish Feed in Future Mariculture. Aquac. Environ. Interact. 2011, 1, 187–200. [Google Scholar] [CrossRef]
- Berntssen, M.H.G.; Maage, A.; Julshamn, K.; Oeye, B.E.; Lundebye, A.-K. Carry-over of Dietary Organochlorine Pesticides, PCDD/Fs, PCBs, and Brominated Flame Retardants to Atlantic Salmon (Salmo salar L.) Fillets. Chemosphere 2011, 83, 95–103. [Google Scholar] [CrossRef]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of Sustainable Feeds on Omega-3 Long-Chain Fatty Acid Levels in Farmed Atlantic Salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef]
- Gebauer, S.K.; Psota, T.L.; Harris, W.S.; Kris-Etherton, P.M. N−3 Fatty Acid Dietary Recommendations and Food Sources to Achieve Essentiality and Cardiovascular Benefits. Am. J. Clin. Nutr. 2006, 83, 1526S–1535S. [Google Scholar] [CrossRef]
- Lucas, M.; Asselin, G.; Plourde, M.; Cunnane, S.C.; Dewailly, É.; Dodin, S. N-3 Fatty Acid Intake from Marine Food Products among Quebecers: Comparison to Worldwide Recommendations. Public Health Nutr. 2010, 13, 63–70. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Nara, T.Y. Structure, Function, and Dietary Regulation of Δ6, Δ5, and Δ9 Desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef]
- Farrell, A.P.; Friesen, E.N.; Higgs, D.A.; Ikonomou, M.G. Toward Improved Public Confidence in Farmed Fish Quality: A Canadian Perspective on the Consequences of Diet Selection. J. World Aquac. Soc. 2010, 41, 207–224. [Google Scholar] [CrossRef]
- Moya-Falcón, C.; Thomassen, M.S.; Jakobsen, J.V.; Ruyter, B. Effects of Dietary Supplementation of Rapeseed Oil on Metabolism of [1-14C]18∶1n−9, [1-14C]20∶3n−6, and [1-14C]20∶4n−3 in Atlantic Salmon Heaptocytes. Lipids 2005, 40, 709–717. [Google Scholar] [CrossRef]
- Moya-Falcón, C.; Hvattum, E.; Tran, T.N.; Thomassen, M.S.; Skorve, J.; Ruyter, B. Phospholipid Molecular Species, β-Oxidation, Desaturation and Elongation of Fatty Acids in Atlantic Salmon Hepatocytes: Effects of Temperature and 3-Thia Fatty Acids. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 145, 68–80. [Google Scholar] [CrossRef]
- Codabaccus, M.B.; Bridle, A.R.; Nichols, P.D.; Carter, C.G. Effect of Feeding Atlantic Salmon (Salmo salar L.) a Diet Enriched with Stearidonic Acid from Parr to Smolt on Growth and n-3 Long-Chain PUFA Biosynthesis. Br. J. Nutr. 2011, 105, 1772–1782. [Google Scholar] [CrossRef]
- Ng, W.-K.; Low, S.-Y. Evaluation of Spent Bleaching Clay from Palm Oil Refining as an Ingredient for Diets of Red Hybrid Tilapia, Oreochromis Sp. J. Appl. Aquac. 2005, 17, 87–97. [Google Scholar] [CrossRef]
- Ng, W.-K.; Lim, P.-K.; Sidek, H. The Influence of a Dietary Lipid Source on Growth, Muscle Fatty Acid Composition and Erythrocyte Osmotic Fragility of Hybrid Tilapia. Fish Physiol. Biochem. 2001, 25, 301–310. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Bell, M.V.; Little, D.C.; Yakupitiyage, A. Replacement of Dietary Fish Oils by Alpha-Linolenic Acid-Rich Oils Lowers Omega 3 Content in Tilapia Flesh. Lipids 2007, 42, 547–559. [Google Scholar] [CrossRef]
- Weaver, K.L.; Ivester, P.; Chilton, J.A.; Wilson, M.D.; Pandey, P.; Chilton, F.H. The Content of Favorable and Unfavorable Polyunsaturated Fatty Acids Found in Commonly Eaten Fish. J. Am. Diet. Assoc. 2008, 108, 1178–1185. [Google Scholar] [CrossRef]
- Rosenthal, E. Another Side of Tilapia, the Perfect Factory Fish. The New York Times, 3 May 2011. [Google Scholar]
- Karapanagiotidis, I.T.; Bell, M.V.; Little, D.C.; Yakupitiyage, A.; Rakshit, S.K. Polyunsaturated Fatty Acid Content of Wild and Farmed Tilapias in Thailand: Effect of Aquaculture Practices and Implications for Human Nutrition. J. Agric. Food Chem. 2006, 54, 4304–4310. [Google Scholar] [CrossRef]
- Teoh, C.-Y.; Turchini, G.M.; Ng, W.-K. Erratum to “Genetically Improved Farmed Nile Tilapia and Red Hybrid Tilapia Showed Differences in Fatty Acid Metabolism When Fed Diets with Added Fish Oil or a Vegetable Oil Blend”. Aquaculture 2011, 316, 144–154. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary Aspects of Omega-3 Fatty Acids in the Food Supply. Prostaglandins Leukot. Essent. Fat. Acids 1999, 60, 421–429. [Google Scholar] [CrossRef]
- Burghardt, P.R.; Kemmerer, E.S.; Buck, B.J.; Osetek, A.J.; Yan, C.; Koch, L.G.; Britton, S.L.; Evans, S.J. Dietary N-3:N-6 Fatty Acid Ratios Differentially Influence Hormonal Signature in a Rodent Model of Metabolic Syndrome Relative to Healthy Controls. Nutr. Metab. 2010, 7, 53. [Google Scholar] [CrossRef]
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of N-3 and n-6 Polyunsaturated Fatty Acids in Fish and Fish Products. Lipids Health Dis. 2012, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Justi, K.C.; Hayashi, C.; Visentainer, J.V.; de Souza, N.E.; Matsushita, M. The Influence of Feed Supply Time on the Fatty Acid Profile of Nile Tilapia (Oreochromis Niloticus) Fed on a Diet Enriched with n-3 Fatty Acids. Food Chem. 2003, 80, 489–493. [Google Scholar] [CrossRef]
- Young, K. Omega-6 (n-6) and Omega-3 (n-3) Fatty Acids in Tilapia and Human Health: A Review. Int. J. Food Sci. Nutr. 2009, 60, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Sarker, P.K.; Kapuscinski, A.R.; Lanois, A.J.; Livesey, E.D.; Bernhard, K.P.; Coley, M.L. Towards Sustainable Aquafeeds: Complete Substitution of Fish Oil with Marine Microalga Schizochytrium Sp. Improves Growth and Fatty Acid Deposition in Juvenile Nile Tilapia (Oreochromis niloticus). PLoS ONE 2016, 11, e0156684. [Google Scholar] [CrossRef] [PubMed]
- Beal, C.M.; Gerber, L.N.; Thongrod, S.; Phromkunthong, W.; Kiron, V.; Granados, J.; Archibald, I.; Greene, C.H.; Huntley, M.E. Marine Microalgae Commercial Production Improves Sustainability of Global Fisheries and Aquaculture. Sci. Rep. 2018, 8, 15064. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Kabir Chowdhury, M.A.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in Aquafeeds for a Sustainable Aquaculture Industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kapuscinski, A.R.; Vandenberg, G.W.; Proulx, E.; Sitek, A.J. Towards Sustainable and Ocean-Friendly Aquafeeds: Evaluating a Fish-Free Feed for Rainbow Trout (Oncorhynchus mykiss) Using Three Marine Microalgae Species. Elem. Sci. Anthr. 2020, 8, 5. [Google Scholar] [CrossRef]
- Kiron, V.; Sørensen, M.; Huntley, M.; Vasanth, G.K.; Gong, Y.; Dahle, D.; Palihawadana, A.M. Defatted Biomass of the Microalga, Desmodesmus Sp., Can Replace Fishmeal in the Feeds for Atlantic Salmon. Front. Mar. Sci. 2016, 3, 67. [Google Scholar] [CrossRef]
- Sirakov, I.; Velichkova, K.; Nikolov, G. The Effect of Algae Meal (Spirulina) on the Growth Performance and Carcass Parameters of Rainbow Trout (Oncorhynchus mykiss). J. BioSci. Biotechnol. 2012, 151–156. [Google Scholar]
- Sarker, P.K.; Kapuscinski, A.R.; Bae, A.Y.; Donaldson, E.; Sitek, A.J.; Fitzgerald, D.S.; Edelson, O.F. Towards Sustainable Aquafeeds: Evaluating Substitution of Fishmeal with Lipid-Extracted Microalgal Co-Product (Nannochloropsis oculata) in Diets of Juvenile Nile Tilapia (Oreochromis niloticus). PLoS ONE 2018, 13, e0201315. [Google Scholar] [CrossRef]
- Sørensen, M.; Gong, Y.; Bjarnason, F.; Vasanth, G.K.; Dahle, D.; Huntley, M.; Kiron, V. Nannochloropsis Oceania-Derived Defatted Meal as an Alternative to Fishmeal in Atlantic Salmon Feeds. PLoS ONE 2017, 12, e0179907. [Google Scholar] [CrossRef]
- Sprague, M.; Walton, J.; Campbell, P.J.; Strachan, F.; Dick, J.R.; Bell, J.G. Replacement of Fish Oil with a DHA-Rich Algal Meal Derived from Schizochytrium Sp. on the Fatty Acid and Persistent Organic Pollutant Levels in Diets and Flesh of Atlantic Salmon (Salmo salar, L.) Post-Smolts. Food Chem. 2015, 185, 413–421. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Filer, K.; Xue, Y.; Ai, Q.; Mai, K. Replacement of Fish Oil with a DHA-Rich Schizochytrium Meal on Growth Performance, Activities of Digestive Enzyme and Fatty Acid Profile of Pacific White Shrimp (Litopenaeus vannamei) Larvae. Aquac. Nutr. 2017, 23, 1113–1120. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Mørkøre, T.; Nengas, I.; Berge, R.K.; Sweetman, J. Microalgae and Organic Minerals Enhance Lipid Retention Efficiency and Fillet Quality in Atlantic Salmon (Salmo salar L.). Aquaculture 2016, 451, 47–57. [Google Scholar] [CrossRef]
- Tibaldi, E.; Chini Zittelli, G.; Parisi, G.; Bruno, M.; Giorgi, G.; Tulli, F.; Venturini, S.; Tredici, M.R.; Poli, B.M. Growth Performance and Quality Traits of European Sea Bass (D. Labrax) Fed Diets Including Increasing Levels of Freeze-Dried Isochrysis Sp. (T-ISO) Biomass as a Source of Protein and n-3 Long Chain PUFA in Partial Substitution of Fish Derivatives. Aquaculture 2015, 440, 60–68. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 Long-Chain Polyunsaturated Fatty Acids, EPA and DHA: Bridging the Gap between Supply and Demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- Øverland, M.; Karlsson, A.; Mydland, L.T.; Romarheim, O.H.; Skrede, A. Evaluation of Candida Utilis, Kluyveromyces Marxianus and Saccharomyces Cerevisiae Yeasts as Protein Sources in Diets for Atlantic Salmon (Salmo salar). Aquaculture 2013, 402–403, 1–7. [Google Scholar] [CrossRef]
- Glencross, B.D.; Huyben, D.; Schrama, J.W. The Application of Single-Cell Ingredients in Aquaculture Feeds—A Review. Fishes 2020, 5, 22. [Google Scholar] [CrossRef]
- Øvrum Hansen, J.; Hofossæter, M.; Sahlmann, C.; Ånestad, R.; Reveco-Urzua, F.E.; Press, C.M.; Mydland, L.T.; Øverland, M. Effect of Candida Utilis on Growth and Intestinal Health of Atlantic Salmon (Salmo salar) Parr. Aquaculture 2019, 511, 734239. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein-State-of-the-Art, Industrial Landscape and Patents 2001-2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Suman, G.; Nupur, M.; Anuradha, S.; Pradeep, B. Single Cell Protein Production: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 251–262. [Google Scholar]
- Aas, T.S.; Grisdale-Helland, B.; Terjesen, B.F.; Helland, S.J. Improved Growth and Nutrient Utilisation in Atlantic Salmon (Salmo salar) Fed Diets Containing a Bacterial Protein Meal. Aquaculture 2006, 259, 365–376. [Google Scholar] [CrossRef]
- Tlusty, M.; Rhyne, A.; Szczebak, J.T.; Bourque, B.; Bowen, J.L.; Burr, G.; Marx, C.J.; Feinberg, L. A Transdisciplinary Approach to the Initial Validation of a Single Cell Protein as an Alternative Protein Source for Use in Aquafeeds. PeerJ 2017, 5, e3170. [Google Scholar] [CrossRef] [PubMed]
- Chumpol, S.; Kantachote, D.; Nitoda, T.; Kanzaki, H. Administration of Purple Nonsulfur Bacteria as Single Cell Protein by Mixing with Shrimp Feed to Enhance Growth, Immune Response and Survival in White Shrimp (Litopenaeus vannamei) Cultivation. Aquaculture 2018, 489, 85–95. [Google Scholar] [CrossRef]
- Hamidoghli, A.; Yun, H.; Won, S.; Kim, S.; Farris, N.W.; Bai, S.C. Evaluation of a Single-Cell Protein as a Dietary Fish Meal Substitute for Whiteleg Shrimp Litopenaeus vannamei. Fish Sci. 2019, 85, 147–155. [Google Scholar] [CrossRef]
- Glencross, B.; Irvin, S.; Arnold, S.; Blyth, D.; Bourne, N.; Preston, N. Effective Use of Microbial Biomass Products to Facilitate the Complete Replacement of Fishery Resources in Diets for the Black Tiger Shrimp, Penaeus Monodon. Aquaculture 2014, 431, 12–19. [Google Scholar] [CrossRef]
- Arnold, S.; Smullen, R.; Briggs, M.; West, M.; Glencross, B. The Combined Effect of Feed Frequency and Ration Size of Diets with and without Microbial Biomass on the Growth and Feed Conversion of Juvenile Penaeus Monodon. Aquac. Nutr. 2016, 22, 1340–1347. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Kim, S.-H.; Pandey, A. Microbial Strategies for Bio-Transforming Food Waste into Resources. Bioresour. Technol. 2020, 299, 122580. [Google Scholar] [CrossRef]
- Olmos Soto, J.; Paniagua-Michel, J.D.J.; Lopez, L.; Ochoa, L. Functional Feeds in Aquaculture. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1303–1319. ISBN 978-3-642-53971-8. [Google Scholar]
- Ruiz-Lopez, N.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Successful High-Level Accumulation of Fish Oil Omega-3 Long-Chain Polyunsaturated Fatty Acids in a Transgenic Oilseed Crop. Plant J. 2014, 77, 198–208. [Google Scholar] [CrossRef]
- Thomassen, M.S.; Rein, D.; Berge, G.M.; Østbye, T.-K.; Ruyter, B. High Dietary EPA Does Not Inhibit ∆5 and ∆6 Desaturases in Atlantic Salmon (Salmo salar L.) Fed Rapeseed Oil Diets. Aquaculture 2012, 360–361, 78–85. [Google Scholar] [CrossRef]
- Lock, E.-J.; Biancarosa, I.; Gasco, L. Insects as Raw Materials in Compound Feed for Aquaculture. In Edible Insects in Sustainable Food Systems; Halloran, A., Flore, R., Vantomme, P., Roos, N., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 263–276. ISBN 978-3-319-74011-9. [Google Scholar]
- Lock, E.R.; Arsiwalla, T.; Waagbø, R. Insect Larvae Meal as an Alternative Source of Nutrients in the Diet of Atlantic Salmon (Salmo salar) Postsmolt. Aquac. Nutr. 2016, 22, 1202–1213. [Google Scholar] [CrossRef]
- Biancarosa, I.; Sele, V.; Belghit, I.; Ørnsrud, R.; Lock, E.-J.; Amlund, H. Replacing Fish Meal with Insect Meal in the Diet of Atlantic Salmon (Salmo salar) Does Not Impact the Amount of Contaminants in the Feed and It Lowers Accumulation of Arsenic in the Fillet. Food Addit. Contam. Part A 2019, 36, 1191–1205. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Waagbø, R.; Biancarosa, I.; Pelusio, N.; Li, Y.; Krogdahl, Å.; Lock, E.-J. Potential of In-sect-Based Diets for Atlantic Salmon (Salmo salar). Aquaculture 2018, 491, 72–81. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.-J. Black Soldier Fly Larvae Meal Can Replace Fish Meal in Diets of Sea-Water Phase Atlantic Salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Salomone, R.; Saija, G.; Mondello, G.; Giannetto, A.; Fasulo, S.; Savastano, D. Environmental Impact of Food Waste Bioconversion by Insects: Application of Life Cycle Assessment to Process Using Hermetia Illucens. J. Clean. Prod. 2017, 140, 890–905. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the Suitability of a Partially Defatted Black Soldier Fly (Hermetia Illucens L.) Larvae Meal as Ingredient for Rainbow Trout (Oncorhynchus mykiss Walbaum) Diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Jackson and Newton Project to Model the Use of Fisheries By-Products in The Production of Marine Ingredients, with Special Reference to the Omega 3 Fatty Acids Epa and Dha. Available online: https://www.iffo.com/system/files/downloads/Report%20IoA%20IFFO%20project%20Final_0.pdf (accessed on 25 September 2020).
- Henriksson, P.J.G.; Belton, B.; Jahan, K.M.; Rico, A. Measuring the Potential for Sustainable Intensification of Aquaculture in Bangladesh Using Life Cycle Assessment. Proc. Natl. Acad. Sci. USA 2018, 115, 2958–2963. [Google Scholar] [CrossRef]
- Campos, I.; Pinheiro Valente, L.M.; Matos, E.; Marques, P.; Freire, F. Life-Cycle Assessment of Animal Feed Ingredients: Poultry Fat, Poultry by-Product Meal and Hydrolyzed Feather Meal. J. Clean. Prod. 2020, 252, 119845. [Google Scholar] [CrossRef]
- Sandhu, H.; Scialabba, N.E.-H.; Warner, C.; Behzadnejad, F.; Keohane, K.; Houston, R.; Fujiwara, D. Evaluating the Holistic Costs and Benefits of Corn Production Systems in Minnesota, US. Sci. Rep. 2020, 10, 3922. [Google Scholar] [CrossRef]
- Sillman, J.; Nygren, L.; Kahiluoto, H.; Ruuskanen, V.; Tamminen, A.; Bajamundi, C.; Nappa, M.; Wuokko, M.; Lindh, T.; Vainikka, P.; et al. Bacterial Protein for Food and Feed Generated via Renewable Energy and Direct Air Capture of CO2: Can It Reduce Land and Water Use? Glob. Food Secur. 2019, 22, 25–32. [Google Scholar] [CrossRef]
- Ketzer, F.; Skarka, J.; Rösch, C. Critical Review of Microalgae LCA Studies for Bioenergy Production. Bioenergy Res. 2018, 11, 95–105. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Yasumaru, F.; Lemos, D. In Vitro Prediction of Digestible Protein Content of Marine Microalgae (Nannochloropsis granulata) Meals for Pacific White Shrimp (Litopenaeus vannamei) and Rainbow Trout (Oncorhynchus mykiss). Algal Res. 2017, 21, 76–80. [Google Scholar] [CrossRef]
- Teuling, E.; Wierenga, P.A.; Agboola, J.O.; Gruppen, H.; Schrama, J.W. Cell Wall Disruption Increases Bioavailability of Nannochloropsis Gaditana Nutrients for Juvenile Nile Tilapia (Oreochromis niloticus). Aquaculture 2019, 499, 269–282. [Google Scholar] [CrossRef]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Disruption of Microalgal Cells for the Extraction of Lipids for Biofuels: Processes and Specific Energy Requirements. Biomass Bioenergy 2012, 46, 89–101. [Google Scholar] [CrossRef]
- Safi, C.; Charton, M.; Ursu, A.V.; Laroche, C.; Zebib, B.; Pontalier, P.-Y.; Vaca-Garcia, C. Release of Hydro-Soluble Microalgal Proteins Using Mechanical and Chemical Treatments. Algal Res. 2014, 3, 55–60. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Saldívar, S.O.S. Inactivation Methods of Trypsin Inhibitor in Legumes: A Review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [CrossRef]
- Draganovic, V.; van der Goot, A.J.; Boom, R.; Jonkers, J. Assessment of the Effects of Fish Meal, Wheat Gluten, Soy Protein Concentrate and Feed Moisture on Extruder System Parameters and the Technical Quality of Fish Feed. Anim. Feed. Sci. Technol. 2011, 165, 238–250. [Google Scholar] [CrossRef]
- Barrows, F.T.; Frost, J.B. Evaluation of the Nutritional Quality of Co-Products from the Nut Industry, Algae and an Invertebrate Meal for Rainbow Trout, Oncorhynchus mykiss. Aquaculture 2014, 434, 315–324. [Google Scholar] [CrossRef]
- Simon, C.J.; Blyth, D.; Ahmad Fatan, N.; Suri, S. Microbial Biomass (NovacqTM) Stimulates Feeding and Improves the Growth Performance on Extruded Low to Zero-Fishmeal Diets in Tilapia (GIFT Strain). Aquaculture 2019, 501, 319–324. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Ravindran, V. Effect of Exogenous Enzymes in Maize-Based Diets Varying in Nutrient Density for Young Broilers: Growth Performance and Digestibility of Energy, Minerals and Amino Acids. Br. Poult. Sci. 2008, 49, 37–44. [Google Scholar] [CrossRef]
- Sinha, A.K.; Kumar, V.; Makkar, H.P.S.; De Boeck, G.; Becker, K. Non-Starch Polysaccharides and Their Role in Fish Nutrition—A Review. Food Chem. 2011, 127, 1409–1426. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Verlhac, V.; Hjermitslev, N.H.; Ekmann, K.S.; Fischer, M.; Klausen, M.; Pedersen, P.B. Effects of Exogenous Enzymes on Apparent Nutrient Digestibility in Rainbow Trout (Oncorhynchus mykiss) Fed Diets with High Inclusion of Plant-Based Protein. Anim. Feed. Sci. Technol. 2012, 171, 181–191. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Knudsen, K.E.B.; Verlhac, V.; Ekmann, K.S.; Pedersen, P.B. Supplementing Enzymes to Extruded, Soybean-Based Diet Improves Breakdown of Non-Starch Polysaccharides in Rainbow Trout (Oncorhynchus mykiss). Aquac. Nutr. 2016, 22, 419–426. [Google Scholar] [CrossRef]
- Qi, G.; Ai, Q.; Mai, K.; Xu, W.; Liufu, Z.; Yun, B.; Zhou, H. Effects of Dietary Taurine Supplementation to a Casein-Based Diet on Growth Performance and Taurine Distribution in Two Sizes of Juvenile Turbot (Scophthalmus maximus L.). Aquaculture 2012, 358–359, 122–128. [Google Scholar] [CrossRef]
- Watson, A.M.; Barrows, F.T.; Place, A.R. Effects of Graded Taurine Levels on Juvenile Cobia. N. Am. J. Aquac. 2014, 76, 190–200. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M. Is Dietary Taurine Supplementation Beneficial for Farmed Fish and Shrimp? A Comprehensive Review. Rev. Aquac. 2014, 6, 241–255. [Google Scholar] [CrossRef]
- Poston, H.A. Effect of Body Size on Growth, Survival, and Chemical Composition of Atlantic Salmon Fed Soy Lecithin and Choline. Progress. Fish-Cult. 1990, 52, 226–230. [Google Scholar] [CrossRef]
- Poston, H.A. Response of Rainbow Trout to Soy Lecithin, Choline, and Autoclaved Isolated Soy Protein. Progress. Fish-Cult. 1991, 53, 85–90. [Google Scholar] [CrossRef]
- Trushenski, J.T.; Kanczuzewski, K.L. Traditional and Modified Soy Oils as Substitutes for Fish Oil in Feeds for Hybrid Striped Bass. N. Am. J. Aquac. 2013, 75, 295–304. [Google Scholar] [CrossRef]
- Engle, C. The Value of Alternative Feed Ingredients. J. World Aquac. Soc. 2017, 48, 377–380. [Google Scholar] [CrossRef]
- Lopes da Silva, T.; Moniz, P.; Silva, C.; Reis, A. The Dark Side of Microalgae Biotechnology: A Heterotrophic Biorefinery Platform Directed to ω-3 Rich Lipid Production. Microorganisms 2019, 7, 670. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).