Sex-Related Effects of Gut Microbiota in Metabolic Syndrome-Related Diabetic Retinopathy
Abstract
:1. Introduction
2. Overview of Altered Molecular Mechanisms in Metabolic Syndrome
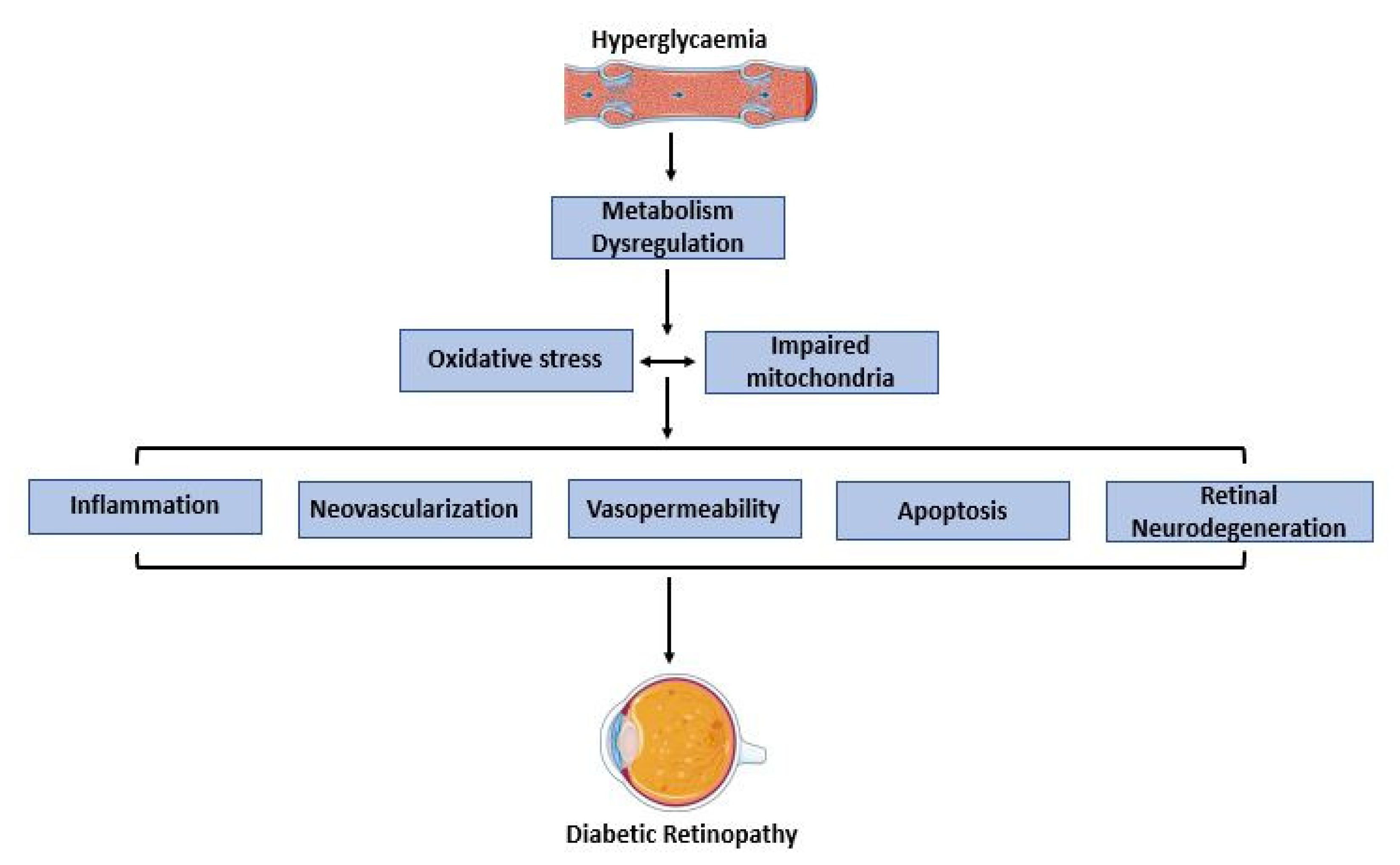
3. Diabetic Retinopathy
4. Gut Microbiota
5. Effects of Biological Sex
6. Biomarkers
7. Sex-Biased Effects of Gut Microbiota in Metabolic Syndrome-Related Diabetic Retinopathy
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Saely, C.H.; Aczel, S.; Marte, T.; Langer, P.; Hoefle, G.; Drexel, H. The metabolic syndrome, insulin resistance, and cardiovascular risk in diabetic and nondiabetic patients. J. Clin. Endocrinol. Metab. 2005, 90, 5698–5703. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Lemieux, I.; Després, J.-P. Metabolic syndrome: Past, present and future. Nutrients 2020, 12, 3501. [Google Scholar] [CrossRef]
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. Novel Insights into the Pathogenesis and Management of the Metabolic Syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef]
- Beltrán-Sánchez, H.; Harhay, M.O.; Harhay, M.M.; McElligott, S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J. Am. Coll. Cardiol. 2013, 62, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Khambaty, T.; Schneiderman, N.; Llabre, M.M.; Elfassy, T.; Moncrieft, A.E.; Daviglus, M.; Talavera, G.A.; Isasi, C.R.; Gallo, L.C.; Reina, S.A.; et al. Elucidating the multidimensionality of socioeconomic status in relation to metabolic syndrome in the hispanic community health study/study of latinos (HCHS/SOL). Int. J. Behav. Med. 2020, 27, 188–199. [Google Scholar] [CrossRef]
- Santos, A.C.; Ebrahim, S.; Barros, H. Gender, socio-economic status and metabolic syndrome in middle-aged and old adults. BMC Public Health 2008, 8, 62–68. [Google Scholar] [CrossRef]
- Yang, Y.C.; Schorpp, K.; Boen, C.; Johnson, M.; Harris, K.M. Socioeconomic status and biological risks for health and illness across the life course. J. Gerontol. Ser. B 2018, 75, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Abbate, M.; Pericas, J.; Yañez, A.M.; López-González, A.A.; De Pedro-Gómez, J.; Aguilo, A.; Morales-Asencio, J.M.; Bennasar-Veny, M. Socioeconomic inequalities in metabolic syndrome by age and gender in a spanish working population. Int. J. Environ. Res. Public Health 2021, 18, 10333. [Google Scholar] [CrossRef]
- Krieger, N. Genders, sexes, and health: What are the connections—And why does it matter? Leuk. Res. 2003, 32, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Manson, J.E.; Stevenson, J.C.; Fonseca, V.A. Menopausal hormone therapy and type 2 diabetes prevention: Evidence, mechanisms, and clinical implications. Endocr. Rev. 2017, 38, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F. Physiology and pharmacology of dpp-4 in glucose homeostasis and the treatment of type 2 diabetes. Front. Endocrinol. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef]
- Chawla, R.; Chawla, A.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef]
- Kyriazis, I.D.; Hoffman, M.; Gaignebet, L.; Lucchese, A.M.; Markopoulou, E.; Palioura, D.; Wang, C.; Bannister, T.D.; Christofidou-Solomidou, M.; Oka, S.-I.; et al. KLF5 is induced by FOXO1 and causes oxidative stress and diabetic cardiomyopathy. Circ. Res. 2021, 128, 335–357. [Google Scholar] [CrossRef]
- Lima, V.C.; Cavalieri, G.C.; Lima, M.C.; Nazario, N.O.; Lima, G.C. Risk factors for diabetic retinopathy: A case–control study. Int. J. Retin. Vitr. 2016, 2, 21. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Li, S.; Kararigas, G. Role of biological sex in the cardiovascular-gut microbiome axis. Front. Cardiovasc. Med. 2022, 8, 759735. [Google Scholar] [CrossRef] [PubMed]
- Agnoletti, D.; Piani, F.; Cicero, A.F.G.; Borghi, C. The Gut Microbiota and Vascular Aging: A State-of-the-Art and Systematic Review of the Literature. J. Clin. Med. 2022, 11, 3557. [Google Scholar] [CrossRef]
- Ruan, H.; Lodish, H.F. Insulin resistance in adipose tissue: Direct and indirect effects of tumor necrosis factor-α. Cytokine Growth Factor Rev. 2003, 14, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Tsigos, C.; Kyrou, I.; Chala, E.; Tsapogas, P.; Stavridis, J.C.; Raptis, S.A.; Katsilambros, N. Circulating tumor necrosis factor alpha concentrations are higher in abdominal versus peripheral obesity. Metabolism 1999, 48, 1332–1335. [Google Scholar] [CrossRef]
- Bastard, J.-P.; Hainque, B. Relationship between plasma plasminogen activator inhibitor 1 and insulin resistance. Diabetes/Metab. Res. Rev. 2000, 16, 192–201. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Kurz, S.; Münzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef]
- Huang, S.-S.; Lu, Y.-J.; Huang, J.-P.; Wu, Y.-T.; Day, Y.-J.; Hung, L.-M. The essential role of endothelial nitric oxide synthase activation in insulin-mediated neuroprotection against ischemic stroke in diabetes. J. Vasc. Surg. 2014, 59, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Zhang, Y.C.; Philips, M.I.; Sawamura, T.; Mehta, J.L. Upregulation of endothelial receptor for oxidized low-density lipoprotein (LOX-1) in cultured human coronary artery endothelial cells by angiotensin II type 1 receptor activation. Circ. Res. 1999, 84, 1043–1049. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Cheung, C.M.G.; Wong, T.Y. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef]
- Zheng, Y.; He, M.; Congdon, N. The worldwide epidemic of diabetic retinopathy. Indian J. Ophthalmol. 2012, 60, 428–431. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Joglekar, M.V.; Hardikar, A.A.; Keech, A.C.; O’Neal, D.N.; Januszewski, A.S. Biomarkers in diabetic retinopathy. Rev. Diabet. Stud. 2015, 12, 159–195. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.; O’Leary, O.E.; Stitt, A.W. The pathology associated with diabetic retinopathy. Vis. Res. 2017, 139, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.-P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Miller, W.P.; Sunilkumar, S.; Giordano, J.F.; Toro, A.L.; Barber, A.J.; Dennis, M.D. The stress response protein REDD1 promotes diabetes-induced oxidative stress in the retina by Keap1-independent Nrf2 degradation. J. Biol. Chem. 2020, 295, 7350–7361. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef]
- Safi, S.Z.; Qvist, R.; Kumar, S.; Batumalaie, K.; Ismail, I.S.B. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Bio. Med. Res. Int. 2014, 2014, 801269. [Google Scholar] [CrossRef]
- Sui, A.; Chen, X.; Demetriades, A.M.; Shen, J.; Cai, Y.; Yao, Y.; Yao, Y.; Zhu, Y.; Shen, X.; Xie, B. Inhibiting NF-κB signaling activation reduces retinal neovascularization by promoting a polarization shift in macrophages. Investig. Opthalmology Vis. Sci. 2020, 61, 4. [Google Scholar] [CrossRef]
- Madeira, M.H.; Marques, I.P.; Ferreira, S.; Tavares, D.; Santos, T.; Santos, A.R.; Figueira, J.; Lobo, C.; Cunha-Vaz, J. Retinal neurodegeneration in different risk phenotypes of diabetic retinal disease. Front. Neurosci. 2021, 15, 800004. [Google Scholar] [CrossRef]
- Carrasco, E.; Hernández, C.; Miralles, A.; Huguet, P.; Farrés, J.; Simó, R. Lower somatostatin expression is an early event in diabetic retinopathy and is associated with retinal neurodegeneration. Diabetes Care 2007, 30, 2902–2908. [Google Scholar] [CrossRef] [PubMed]
- Lieth, E.; Barber, A.J.; Xu, B.; Dice, C.; Ratz, M.J.; Tanase, D.; Strother, J.M. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes 1998, 47, 815–820. [Google Scholar] [CrossRef]
- Xie, A.; Gao, J.; Xu, L.; Meng, D. Shared mechanisms of neurodegeneration in alzheimer’s disease and parkinson’s disease. Bio. Med. Res. Int. 2014, 2014, 648740. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, J.M.; Hernández, C.; Weber, S.R.; Zhao, Y.; Dunklebarger, M.; Tiberti, N.; Laremore, T.; Simó-Servat, O.; Garcia-Ramirez, M.; Barber, A.J.; et al. Proteomic analysis of early diabetic retinopathy reveals mediators of neurodegenerative brain diseases. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Wiedemann, P. Müller glial cells in retinal disease. Ophthalmologica 2011, 227, 1–19. [Google Scholar] [CrossRef]
- Wang, L.; Liu, M.; Gao, J.; Smith, A.M.; Fujioka, H.; Liang, J.; Perry, G.; Wang, X. Mitochondrial fusion suppresses tau pathology-induced neurodegeneration and cognitive decline. J. Alzheimer’s Dis. 2021, 84, 1057–1069. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Bermon, S.; Petriz, B.; Kajėnienė, A.; Prestes, J.; Castell, L.; Franco, O.L. The microbiota: An exercise immunology perspective. Exerc. Immunol. Rev. 2015, 21, 70–79. [Google Scholar]
- McQuade, J.L.; Daniel, C.R.; Helmink, B.A.; Wargo, J.A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019, 20, e77–e91. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wang, L. Role and mechanism of gut microbiota in human disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Stewart, R.; Kim, J.-W.; Kang, H.-J.; Bae, K.-Y.; Kim, S.-W.; Shin, I.-S.; Yoon, J.-S. Changes in pro-inflammatory cytokine levels and late-life depression: A two year population based longitudinal study. Psychoneuroendocrinology 2018, 90, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. Acad. Sci. 2017, 1420, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, H.; Zhu, Q. The Impact of microbiota-gut-brain axis on diabetic cognition impairment. Front. Aging Neurosci. 2017, 9, 106. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z.; Ma, H.; Ji, S.; Chen, Z.; Cui, Z.; Chen, J.; Tang, S. Dysbiosis and implication of the gut microbiota in diabetic retinopathy. Front. Cell. Infect. Microbiol. 2021, 11, 646348. [Google Scholar] [CrossRef]
- Luca, M.; Di Mauro, M.; Di Mauro, M.; Luca, A. Gut Microbiota in alzheimer’s disease, depression, and type 2 diabetes mellitus: The role of oxidative stress. Oxidative Med. Cell. Longev. 2019, 2019, 4730539. [Google Scholar] [CrossRef]
- Kim, M.-H.; Yun, K.E.; Kim, J.; Park, E.; Chang, Y.; Ryu, S.; Kim, H.-L.; Kim, H.-N. Gut microbiota and metabolic health among overweight and obese individuals. Sci. Rep. 2020, 10, 19417. [Google Scholar] [CrossRef]
- Thingholm, L.B.; Rühlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hübenthal, M.; Rahnavard, G.; et al. Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe 2019, 26, 252–264.e10. [Google Scholar] [CrossRef]
- Fei, N.; Zhao, L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2012, 7, 880–884. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, H.; Zhan, L.; Lu, X.; Zhang, L. Dynamic development of fecal microbiome during the progression of diabetes mellitus in zucker diabetic fatty rats. Front. Microbiol. 2019, 10, 232. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Cani, P.D. Diabetes, obesity and gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2016, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-X.; Deng, X.-R.; Zhang, C.-H.; Yuan, H.-J. Gut microbiota and metabolic syndrome. Chin. Med. J. 2020, 133, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-glucose or-fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef]
- Copur, S.; Demiray, A.; Kanbay, M. Uric acid in metabolic syndrome: Does uric acid have a definitive role? Eur. J. Intern. Med. 2022, 103, 4–12. [Google Scholar] [CrossRef]
- Xu, D.; Lv, Q.; Wang, X.; Cui, X.; Zhao, P.; Yang, X.; Liu, X.; Yang, W.; Yang, G.; Wang, G.; et al. Hyperuricemia is associated with impaired intestinal permeability in mice. Am. J. Physiol. Liver Physiol. 2019, 317, G484–G492. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Wang, Z.; Ang, K.Y.; Huang, S.; Hou, Q.; Su, X.; Qiao, J.; Zheng, Y.; Wang, L.; et al. Intestinal microbiota distinguish gout patients from healthy humans. Sci. Rep. 2016, 6, 20602. [Google Scholar] [CrossRef]
- Beli, E.; Yan, Y.; Moldovan, L.; Vieira, C.P.; Gao, R.; Duan, Y.; Prasad, R.; Bhatwadekar, A.; White, F.A.; Townsend, S.D.; et al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes 2018, 67, 1867–1879. [Google Scholar] [CrossRef]
- Das, T.; Jayasudha, R.; Chakravarthy, S.; Prashanthi, G.S.; Bhargava, A.; Tyagi, M.; Rani, P.K.; Pappuru, R.R.; Sharma, S.; Shivaji, S. Alterations in the gut bacterial microbiome in people with type 2 diabetes mellitus and diabetic retinopathy. Sci. Rep. 2021, 11, 2738. [Google Scholar] [CrossRef]
- Layoun, A.; Santos, M.M. Bacterial cell wall constituents induce hepcidin expression in macrophages through myd88 signaling. Inflammation 2012, 35, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Moubayed, N.M.; Bhat, R.S.; Al Farraj, D.; Al Dihani, N.; El Ansary, A.; Fahmy, R.M. Screening and identification of gut anaerobes (Bacteroidetes) from human diabetic stool samples with and without retinopathy in comparison to control subjects. Microb. Pathog. 2019, 129, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Sayed, K.M.; Mahmoud, A.A. Heat shock protein-70 and hypoxia inducible factor-1αin type 2 diabetes mellitus patients complicated with retinopathy. Acta Ophthalmol. 2016, 94, e361–e366. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, K.; Li, C.; Zhang, H.; Yu, H.; Chen, K.; Yang, X.; Liu, L. Metagenomic shotgun sequencing and metabolomic profiling identify specific human gut microbiota associated with diabetic retinopathy in patients with type 2 diabetes. Front. Immunol. 2022, 13, 943325. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Hamaguchi, M.; Kaji, A.; Sakai, R.; Osaka, T.; Inoue, R.; Kashiwagi, S.; Mizushima, K.; Uchiyama, K.; Takagi, T.; et al. Intake of sucrose affects gut dysbiosis in patients with type 2 diabetes. J. Diabetes Investig. 2020, 11, 1623–1634. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, Z.; Xiong, X.; Chen, X.; Peng, J.; Yao, H.; Pu, J.; Chen, Q.; Zheng, M. Gut microbiota composition and fecal metabolic profiling in patients with diabetic retinopathy. Front. Cell Dev. Biol. 2021, 9, 2684. [Google Scholar] [CrossRef]
- Gaignebet, L.; Kararigas, G. En route to precision medicine through the integration of biological sex into pharmacogenomics. Clin. Sci. 2017, 131, 329–342. [Google Scholar] [CrossRef]
- Kararigas, G.; Seeland, U.; De Arellano, M.L.B.; Dworatzek, E.; Regitz-Zagrosek, V. Why the study of the effects of biological sex is important. Ann. Dell’istituto Super. Di Sanità. 2016, 52, 149–150. [Google Scholar] [CrossRef]
- Cui, C.; Huang, C.; Liu, K.; Xu, G.; Yang, J.; Zhou, Y.; Feng, Y.; Kararigas, G.; Geng, B.; Cui, Q. Large-scale in silico identification of drugs exerting sex-specific effects in the heart. J. Transl. Med. 2018, 16, 236. [Google Scholar] [CrossRef]
- Ruiz-Meana, M.; Boengler, K.; Garcia-Dorado, D.; Hausenloy, D.J.; Kaambre, T.; Kararigas, G.; Perrino, C.; Schulz, R.; Ytrehus, K. Ageing, sex, and cardioprotection. Br. J. Pharmacol. 2019, 177, 5270–5286. [Google Scholar] [CrossRef]
- Altinbas, L.; Bormann, N.; Lehmann, D.; Jeuthe, S.; Wulsten, D.; Kornak, U.; Robinson, P.N.; Wildemann, B.; Kararigas, G. Assessment of bones deficient in fibrillin-1 microfibrils reveals pronounced sex differences. Int. J. Mol. Sci. 2019, 20, 6059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Ober, C.; Loisel, D.A.; Gilad, Y. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 2008, 9, 911–922. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Nugent, B.M.; Lenz, K.M. Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nat. Rev. Neurosci. 2017, 18, 471–484. [Google Scholar] [CrossRef]
- Kokras, N.; Hodes, G.E.; Bangasser, D.A.; Dalla, C. Sex differences in the hypothalamic–pituitary–adrenal axis: An obstacle to antidepressant drug development? Br. J. Pharmacol. 2019, 176, 4090–4106. [Google Scholar] [CrossRef]
- Kararigas, G.; Dworatzek, E.; Petrov, G.; Summer, H.; Schulze, T.M.; Baczko, I.; Knosalla, C.; Golz, S.; Hetzer, R.; Regitz-Zagrosek, V. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur. J. Heart Fail. 2014, 16, 1160–1167. [Google Scholar] [CrossRef]
- Gaignebet, L.; Kańduła, M.M.; Lehmann, D.; Knosalla, C.; Kreil, D.P.; Kararigas, G. Sex-specific human cardiomyocyte gene regulation in left ventricular pressure overload. Mayo Clin. Proc. 2020, 95, 688–697. [Google Scholar] [CrossRef]
- Dworatzek, E.; Baczko, I.; Kararigas, G. Effects of aging on cardiac extracellular matrix in men and women. Proteom.–Clin. Appl. 2015, 10, 84–91. [Google Scholar] [CrossRef]
- Petrov, G.; Dworatzek, E.; Schulze, T.M.; Dandel, M.; Kararigas, G.; Mahmoodzadeh, S.; Knosalla, C.; Hetzer, R.; Regitz-Zagrosek, V. Maladaptive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. JACC Cardiovasc. Imaging 2014, 7, 1073–1080. [Google Scholar] [CrossRef]
- Siokatas, G.; Papatheodorou, I.; Daiou, A.; Lazou, A.; Hatzistergos, K.E.; Kararigas, G. Sex-related effects on cardiac development and disease. J. Cardiovasc. Dev. Dis. 2022, 9, 90. [Google Scholar] [CrossRef]
- Pei, J.; Harakalova, M.; Treibel, T.; Lumbers, R.T.; Boukens, B.J.; Efimov, I.R.; Van Dinter, J.T.; González, A.; López, B.; El Azzouzi, H.; et al. H3K27ac acetylome signatures reveal the epigenomic reorganization in remodeled non-failing human hearts. Clin. Epigenetics 2020, 12, 106. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E. Estrogen signaling and cardiovascular disease. Circ. Res. 2011, 109, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Steenbergen, C. Estrogen regulation of protein expression and signaling pathways in the heart. Biol. Sex Differ. 2014, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Menazza, S.; Murphy, E. The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ. Res. 2016, 118, 994–1007. [Google Scholar] [CrossRef]
- Puglisi, R.; Mattia, G.; Carè, A.; Marano, G.; Malorni, W.; Matarrese, P. Non-genomic effects of estrogen on cell homeostasis and remodeling with special focus on cardiac ischemia/reperfusion injury. Front. Endocrinol. 2019, 10, 733. [Google Scholar] [CrossRef]
- Lowe, D.A.; Kararigas, G. Editorial: New insights into estrogen/estrogen receptor effects in the cardiac and skeletal muscle. Front. Endocrinol. 2020, 11, 141. [Google Scholar] [CrossRef]
- Kalkhoran, S.B.; Kararigas, G. Oestrogenic regulation of mitochondrial dynamics. Int. J. Mol. Sci. 2022, 23, 1118. [Google Scholar] [CrossRef]
- Ruijter, H.M.D.; Kararigas, G. Estrogen and cardiovascular health. Front. Cardiovasc. Med. 2022, 9, 886592. [Google Scholar] [CrossRef]
- Kararigas, G. Oestrogenic contribution to sex-biased left ventricular remodelling: The male implication. Int. J. Cardiol. 2021, 343, 83–84. [Google Scholar] [CrossRef]
- Schubert, C.; Raparelli, V.; Westphal, C.; Dworatzek, E.; Petrov, G.; Kararigas, G.; Regitz-Zagrosek, V. Reduction of apoptosis and preservation of mitochondrial integrity under ischemia/reperfusion injury is mediated by estrogen receptor beta. Biol. Sex Differ. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoodzadeh, S.; Dworatzek, E. The role of 17β-estradiol and estrogen receptors in regulation of Ca2+ channels and mitochondrial function in cardiomyocytes. Front. Endocrinol. 2019, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Sickinghe, A.A.; Korporaal, S.J.A.; Ruijter, H.M.D.; Kessler, E.L. Estrogen contributions to microvascular dysfunction evolving to heart failure with preserved ejection fraction. Front. Endocrinol. 2019, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Piquereau, J.; Veksler, V.; Garnier, A. Estrogens, estrogen receptors effects on cardiac and skeletal muscle mitochondria. Front. Endocrinol. 2019, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Miller, V.M.; Miller, J. Influences of sex and estrogen in arterial and valvular calcification. Front. Endocrinol. 2019, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Fliegner, D.; Forler, S.; Klein, O.; Schubert, C.; Gustafsson, J.; Klose, J.; Regitz-Zagrosek, V. Comparative proteomic analysis reveals sex and estrogen receptor β effects in the pressure overloaded heart. J. Proteome Res. 2014, 13, 5829–5836. [Google Scholar] [CrossRef]
- Kararigas, G.; Nguyen, B.T.; Jarry, H. Estrogen modulates cardiac growth through an estrogen receptor α-dependent mechanism in healthy ovariectomized mice. Mol. Cell. Endocrinol. 2014, 382, 909–914. [Google Scholar] [CrossRef]
- Kararigas, G.; Nguyen, B.T.; Zelarayán, L.C.; Hassenpflug, M.; Toischer, K.; Sanchez-Ruderisch, H.; Hasenfuss, G.; Bergmann, M.W.; Jarry, H.; Regitz-Zagrosek, V. Genetic background defines the regulation of postnatal cardiac growth by 17β-Estradiol through a β-catenin mechanism. Endocrinology 2014, 155, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Fliegner, D.; Gustafsson, J.; Regitz-Zagrosek, V. Role of the estrogen/estrogen-receptor-beta axis in the genomic response to pressure overload-induced hypertrophy. Physiol. Genom. 2011, 43, 438–446. [Google Scholar] [CrossRef]
- Sanchez-Ruderisch, H.; Queirós, A.M.; Fliegner, D.; Eschen, C.; Kararigas, G.; Regitz-Zagrosek, V. Sex-specific regulation of cardiac microRNAs targeting mitochondrial proteins in pressure overload. Biol. Sex Differ. 2019, 10, 8. [Google Scholar] [CrossRef]
- Duft, K.; Schanz, M.; Pham, H.; Abdelwahab, A.; Schriever, C.; Kararigas, G.; Dworatzek, E.; Davidson, M.M.; Regitz-Zagrosek, V.; Morano, I.; et al. 17beta-Estradiol-induced interaction of estrogen receptor alpha and human atrial essential myosin light chain modulates cardiac contractile function. Basic Res. Cardiol. 2017, 112, 1. [Google Scholar] [CrossRef]
- Lai, S.; Collins, B.C.; Colson, B.A.; Kararigas, G.; Lowe, D.A. Estradiol modulates myosin regulatory light chain phosphorylation and contractility in skeletal muscle of female mice. Am. J. Physiol. Metab. 2016, 310, E724–E733. [Google Scholar] [CrossRef]
- Mahmoodzadeh, S.; Pham, T.H.; Kuehne, A.; Fielitz, B.; Dworatzek, E.; Kararigas, G.; Petrov, G.; Davidson, M.M.; Regitz-Zagrosek, V. 17β-Estradiol-induced interaction of ERα with NPPA regulates gene expression in cardiomyocytes. Cardiovasc. Res. 2012, 96, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Kararigas, G.; Jarry, H. Dose-dependent effects of a genistein-enriched diet in the heart of ovariectomized mice. Genes Nutr. 2012, 8, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Kararigas, G.; Wuttke, W.; Jarry, H. Long-term treatment of ovariectomized mice with estradiol or phytoestrogens as a new model to study the role of estrogenic substances in the heart. Planta Med. 2011, 78, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Bito, V.; Tinel, H.; Becher, E.; Baczko, I.; Knosalla, C.; Albrecht-Küpper, B.; Sipido, K.R.; Regitz-Zagrosek, V. Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. J. Am. Coll. Cardiol. 2012, 59, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Becher, E.; Mahmoodzadeh, S.; Knosalla, C.; Hetzer, R.; Regitz-Zagrosek, V. Sex-specific modification of progesterone receptor expression by 17β-oestradiol in human cardiac tissues. Biol. Sex Differ. 2010, 1, 2. [Google Scholar] [CrossRef]
- Hein, S.; Hassel, D.; Kararigas, G. The zebrafish (danio rerio) is a relevant model for studying sex-specific effects of 17β-estradiol in the adult heart. Int. J. Mol. Sci. 2019, 20, 6287. [Google Scholar] [CrossRef]
- Fliegner, D.; Schubert, C.; Penkalla, A.; Witt, H.; Kararigas, G.; Dworatzek, E.; Staub, E.; Martus, P.; Noppinger, P.R.; Kintscher, U.; et al. Female sex and estrogen receptor-β attenuate cardiac remodeling and apoptosis in pressure overload. Am. J. Physiol. Integr. Comp. Physiol. 2010, 298, R1597–R1606. [Google Scholar] [CrossRef]
- Queirós, A.M.; Eschen, C.; Fliegner, D.; Kararigas, G.; Dworatzek, E.; Westphal, C.; Ruderisch, H.S.; Regitz-Zagrosek, V. Sex-and estrogen-dependent regulation of a miRNA network in the healthy and hypertrophied heart. Int. J. Cardiol. 2013, 169, 331–338. [Google Scholar] [CrossRef]
- Kararigas, G. Sex-biased mechanisms of cardiovascular complications in COVID-19. Physiol. Rev. 2022, 102, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Ritter, O.; Kararigas, G. Sex-Biased Vulnerability of the Heart to COVID-19. Mayo Clin. Proc. 2020, 95, 2332–2335. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Carson, P.E.; Anand, I.; Rector, T.S.; Kuskowski, M.; Komajda, M.; McKelvie, R.S.; Mcmurray, J.; Zile, M.R.; Massie, B.M.; et al. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: The irbesartan in heart failure with preserved ejection fraction (I-PRESERVE) trial. Circ. Heart Fail. 2012, 5, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.L.; Meyer, P.; Marwick, T.H.; Lam, C.S.; Kaye, D.M. Sex differences in cardiovascular pathophysiology: Why women are overrepresented in heart failure with preserved ejection fraction. Circulation 2018, 138, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, A.R.; Kararigas, G. Menopause-related estrogen decrease and the pathogenesis of HFpEF: JACC review topic of the week. J. Am. Coll. Cardiol. 2020, 75, 1074–1082. [Google Scholar] [CrossRef]
- Sabbatini, A.R.; Kararigas, G. Estrogen-related mechanisms in sex differences of hypertension and target organ damage. Biol. Sex Differ. 2020, 11, 31. [Google Scholar] [CrossRef]
- Cramariuc, D.; Rogge, B.P.; Lønnebakken, M.T.; Boman, K.; Bahlmann, E.; Gohlke-Bärwolf, C.; Chambers, J.B.; Pedersen, T.R.; Gerdts, E. Sex differences in cardiovascular outcome during progression of aortic valve stenosis. Heart 2014, 101, 209–214. [Google Scholar] [CrossRef]
- Martínez-Sellés, M.; Doughty, R.N.; Poppe, K.; Whalley, G.A.; Earle, N.; Tribouilloy, C.; McMurray, J.J.; Swedberg, K.; Køber, L.; Berry, C.; et al. Gender and survival in patients with heart failure: Interactions with diabetes and aetiology. Results from the MAGGIC individual patient meta analysis. Eur. J. Heart Fail. 2012, 14, 473–479. [Google Scholar] [CrossRef]
- Horvath, C.; Kararigas, G. Sex-dependent mechanisms of cell death modalities in cardiovascular disease. Can. J. Cardiol. 2022, 38, 1844–1853. [Google Scholar] [CrossRef]
- Chang, E.; Varghese, M.; Singer, K. Gender and sex differences in adipose tissue. Curr. Diabetes Rep. 2018, 18, 69. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and gender differences in risk, pathophysiology and complications of Type 2 diabetes mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauvais-Jarvis, F. Gender differences in glucose homeostasis and diabetes. Physiol. Behav. 2018, 187, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Kanter, R.; Caballero, B. Global gender disparities in obesity: A Review. Adv. Nutr. Int. Rev. J. 2012, 3, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Estrogen and androgen receptors: Regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol. Metab. 2011, 22, 24–33. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Epidemiology of Gender Differences in Diabetes and Obesity. In Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity; Mauvais-Jarvis, F., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 3–8. [Google Scholar] [CrossRef]
- Yassin, A.; Haider, A.; Haider, K.S.; Caliber, M.; Doros, G.; Saad, F.; Garvey, W.T. Testosterone therapy in men with hypogonadism prevents progression from prediabetes to type 2 diabetes: Eight-year data from a registry study. Diabetes Care 2019, 42, 1104–1111. [Google Scholar] [CrossRef]
- Navarro, G.; Allard, C.; Xu, W.; Mauvais-Jarvis, F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity 2015, 23, 713–719. [Google Scholar] [CrossRef]
- Power, M.L.; Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br. J. Nutr. 2008, 99, 931–940. [Google Scholar] [CrossRef]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T.J. Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. 2015, 5, 241. [Google Scholar] [CrossRef]
- Chaturvedi, N.; Sjoelie, A.-K.; Porta, M.; Aldington, S.J.; Fuller, J.H.; Songini, M.; Kohner, E.M. Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes: The eurodiab prospective complications study. Diabetes Care 2001, 24, 284–289. [Google Scholar] [CrossRef]
- Kajiwara, A.; Miyagawa, H.; Saruwatari, J.; Kita, A.; Sakata, M.; Kawata, Y.; Oniki, K.; Yoshida, A.; Jinnouchi, H.; Nakagawa, K. Gender differences in the incidence and progression of diabetic retinopathy among Japanese patients with type 2 diabetes mellitus: A clinic-based retrospective longitudinal study. Diabetes Res. Clin. Pactr. 2014, 103, e7–e10. [Google Scholar] [CrossRef]
- Schmidl, D.; Schmetterer, L.; Garhöfer, G.; Popa-Cherecheanu, A. Gender differences in ocular blood flow. Curr. Eye Res. 2014, 40, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Kazama, S.; Kazama, J.J.; Ando, N. Eye diseases in women. Fukushima J. Med Sci. 2019, 65, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Sitruk-Ware, R. Hormonal contraception and thrombosis. Fertil. Steril. 2016, 106, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Bek, T.; Lund-Andersen, H.; Hansen, A.B.; Johnsen, K.B.; Sandbaek, A.; Lauritzen, T. The prevalence of diabetic retinopathy in patients with screen-detected type 2 diabetes in Denmark: The ADDITION study. Acta Ophthalmol. 2009, 87, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Foo, V.; Quah, J.; Cheung, C.M.G.; Tan, N.-C.; Zar, K.L.M.; Chan, C.M.; Lamoureux, E.; Yin, W.T.; Tan, G.; Sabanayagam, C. HbA1c, systolic blood pressure variability and diabetic retinopathy in Asian type 2 diabetics. J. Diabetes 2016, 9, 200–207. [Google Scholar] [CrossRef]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex Differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef]
- Coviello, A.D.; Sam, S.; Legro, R.; Dunaif, A. High prevalence of metabolic syndrome in first-degree male relatives of women with polycystic ovary syndrome is related to high rates of obesity. J. Clin. Endocrinol. Metab. 2009, 94, 4361–4366. [Google Scholar] [CrossRef]
- Jayasena, C.N.; Franks, S. The management of patients with polycystic ovary syndrome. Nat. Rev. Endocrinol. 2014, 10, 624–636. [Google Scholar] [CrossRef]
- Hjorth, M.F.; Roager, H.M.; Larsen, T.M.; Poulsen, S.K.; Licht, T.R.; Bahl, M.I.; Zohar, Y.; Astrup, A. Pre-treatment microbial prevotella-to-bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int. J. Obes. 2018, 42, 580–583. [Google Scholar] [CrossRef]
- Mueller, S.; Saunier, K.; Hanisch, C.; Norin, E.; Alm, L.; Midtvedt, T.; Cresci, A.; Silvi, S.; Orpianesi, C.; Verdenelli, M.C.; et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: A cross-sectional study. Appl. Environ. Microbiol. 2006, 72, 1027–1033. [Google Scholar] [CrossRef] [Green Version]
- Bridgewater, L.C.; Zhang, C.; Wu, Y.; Hu, W.; Zhang, Q.; Wang, J.; Li, S.; Zhao, L. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci. Rep. 2017, 7, 10776. [Google Scholar] [CrossRef] [PubMed]
- Steegenga, W.T.; Mischke, M.; Lute, C.; Boekschoten, M.V.; Pruis, M.G.; Lendvai, A.; Verkade, H.J.; Boekhorst, J.; Timmerman, H.M.; Plösch, T.; et al. Sexually dimorphic characteristics of the small intestine and colon of prepubescent C57BL/6 mice. Biol. Sex Differ. 2014, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Park, Y.-H.; Sim, M.; Kim, S.-A.; Joung, H.; Shin, D.-M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res. Microbiol. 2019, 170, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Fransen, F.; van Beek, A.A.; Borghuis, T.; Meijer, B.; Hugenholtz, F.; van der Gaast-De Jongh, C.; Savelkoul, H.F.; De Jonge, M.I.; Faas, M.M.; Boekschoten, M.V.; et al. The Impact of gut microbiota on gender-specific differences in immunity. Front. Immunol. 2017, 8, 754. [Google Scholar] [CrossRef]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.D.; Gao, X.; Bless, E.P.; Chen, J.; Tetel, M.J. Estradiol and high fat diet associate with changes in gut microbiota in female ob/ob mice. Sci. Rep. 2019, 9, 20192. [Google Scholar] [CrossRef] [PubMed]
- Baber, R.J.; Panay, N.; Fenton, A.; the IMS Writing Group. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016, 19, 109–150. [Google Scholar] [CrossRef]
- Kaliannan, K.; Robertson, R.C.; Murphy, K.; Stanton, C.; Kang, C.; Wang, B.; Hao, L.; Bhan, A.K.; Kang, J.X. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 2018, 6, 205. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group; Atkinson, A.J.J.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Spilker, B.A.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Tamhane, M.; Cabrera-Ghayouri, S.; Abelian, G.; Viswanath, V. Review of biomarkers in ocular matrices: Challenges and opportunities. Pharm. Res. 2019, 36, 40. [Google Scholar] [CrossRef] [PubMed]
- Amitani, M.; Asakawa, A.; Amitani, H.; Inui, A. The role of leptin in the control of insulin-glucose axis. Front. Neurosci. 2013, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, G.A.; Barrett-Connor, E.; Cummins, K.M.; Daniels, L.B.; Wassel, C.L.; Ix, J.H. Sex-specific association of fetuin-a with type 2 diabetes in older community-dwelling adults: The rancho bernardo study. Diabetes Care 2013, 36, 1994–2000. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shin, H.J.; Ding, E.; van Dam, R. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2009, 302, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-C.; Qin, L.-Q.; Ye, J.-K. Leptin levels and risk of type 2 diabetes: Gender-specific meta-analysis. Obes. Rev. 2013, 15, 134–142. [Google Scholar] [CrossRef]
- Rasul, S.; Ilhan, A.; Reiter, M.H.; Baumgartner-Parzer, S.; Kautzky-Willer, A. Relations of adiponectin to levels of metabolic parameters and sexual hormones in elderly type 2 diabetic patients. Gend. Med. 2011, 8, 93–102. [Google Scholar] [CrossRef]
- Abbasi, A.; Corpeleijn, E.; Van Der Schouw, Y.T.; Stolk, R.P.; Spijkerman, A.; Van Der A, D.L.; Navis, G.; Bakker, S.J.L.; Beulens, J.W.J. Parental history of type 2 diabetes and cardiometabolic biomarkers in offspring. Eur. J. Clin. Investig. 2012, 42, 974–982. [Google Scholar] [CrossRef]
- Piani, F.; Melena, I.; Tommerdahl, K.L.; Nokoff, N.; Nelson, R.G.; Pavkov, M.E.; van Raalte, D.H.; Cherney, D.Z.; Johnson, R.J.; Nadeau, K.J.; et al. Sex-related differences in diabetic kidney disease: A review on the mechanisms and potential therapeutic implications. J. Diabetes its Complicat. 2021, 35, 107841. [Google Scholar] [CrossRef]
- Daniels, L.B.; Maisel, A.S. Cardiovascular biomarkers and sex: The case for women. Nat. Rev. Cardiol. 2015, 12, 588–596. [Google Scholar] [CrossRef]
- Melander, O.; Maisel, A.S.; Almgren, P.; Manjer, J.; Belting, M.; Hedblad, B.; Engström, G.; Kilger, U.; Nilsson, P.; Bergmann, A.; et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA 2012, 308, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Stadlmayr, A.; Aigner, E.; Huber-Schönauer, U.; Niederseer, D.; Zwerina, J.; Husar-Memmer, E.; Hohla, F.; Schett, G.; Patsch, W.; Datz, C. Relations of vitamin D status, gender and type 2 diabetes in middle-aged Caucasians. Acta Diabetol. 2014, 52, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zorena, K.; Raczyńska, D.; Raczyńska, K. Biomarkers in diabetic retinopathy and the therapeutic implications. Mediat. Inflamm. 2013, 2013, 193604. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, S.; Orešković, I.; Bišćan, F.; Kaštelan, H.; Antunica, A.G. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem. Med. 2020, 30, 385–399. [Google Scholar] [CrossRef]
- Ogata, N.; Matsuoka, M.; Matsuyama, K.; Shima, C.; Tajika, A.; Nishiyama, T.; Wada, M.; Jo, N.; Higuchi, A.; Minamino, K.; et al. Plasma concentration of pigment epithelium-derived factor in patients with diabetic retinopathy. J. Clin. Endocrinol. Metab. 2007, 92, 1176–1179. [Google Scholar] [CrossRef]
- Ozturk, B.T.; Bozkurt, B.; Kerimoglu, H.; Okka, M.; Kamis, U.; Gunduz, K. Effect of serum cytokines and VEGF levels on diabetic retinopathy and macular thickness. Mol. Vis. 2009, 15, 1906–1914. [Google Scholar]
- Rajab, H.A.; Baker, N.L.; Hunt, K.J.; Klein, R.; Cleary, P.A.; Lachin, J.; Virella, G.; Lopes-Virella, M.F. The predictive role of markers of Inflammation and endothelial dysfunction on the course of diabetic retinopathy in type 1 diabetes. J. Diabetes its Complicat. 2015, 29, 108–114. [Google Scholar] [CrossRef]
- Pusparajah, P.; Lee, L.-H.; Kadir, K.A. Molecular markers of diabetic retinopathy: Potential screening tool of the future? Front. Physiol. 2016, 7, 200. [Google Scholar] [CrossRef]
- Sharma, S.; Purohit, S.; Sharma, A.; Hopkins, D.; Steed, L.; Bode, B.; Anderson, S.W.; Caldwell, R.; She, J.-X. Elevated serum levels of soluble TNF receptors and adhesion molecules are associated with diabetic retinopathy in patients with type-1 diabetes. Mediat. Inflamm. 2015, 2015, 279393. [Google Scholar] [CrossRef]
- Midena, E.; Frizziero, L.; Midena, G.; Pilotto, E. Intraocular fluid biomarkers (liquid biopsy) in human diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 3549–3560. [Google Scholar] [CrossRef]
- Youngblood, H.; Robinson, R.; Sharma, A.; Sharma, S. Proteomic biomarkers of retinal inflammation in diabetic retinopathy. Int. J. Mol. Sci. 2019, 20, 4755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, T.; Leach, S.T.; Katz, T.; Day, A.S.; Ooi, C.Y. Fecal biomarkers of intestinal health and disease in children. Front. Pediatr. 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B.; Galla, S.; Chakraborty, S.; Cheng, X.; Yeo, J.; Mell, B.; Zhang, H.; et al. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Lee, C.; Ko, G. Oral microbiota: Microbial biomarkers of metabolic syndrome independent of host genetic factors. Front. Cell. Infect. Microbiol. 2017, 7, 516. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Cani, P.; Delzenne, N.; Amar, J.; Burcelin, R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol. Biol. 2008, 56, 305–309. [Google Scholar] [CrossRef]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef]
- Maric-Bilkan, C. Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin. Sci. 2017, 131, 833–846. [Google Scholar] [CrossRef]
- Krishnan, K.C.; Vergnes, L.; Acín-Pérez, R.; Stiles, L.; Shum, M.; Ma, L.; Mouisel, E.; Pan, C.; Moore, T.M.; Péterfy, M.; et al. Sex-specific genetic regulation of adipose mitochondria and metabolic syndrome by Ndufv2. Nat. Metab. 2021, 3, 1552–1568. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; Farber, C.R.; Orozco, L.; Kang, H.M.; Ghazalpour, A.; Siemers, N.; Neubauer, M.; Neuhaus, I.; Yordanova, R.; Guan, B.; et al. A high-resolution association mapping panel for the dissection of complex traits in mice. Genome Res. 2010, 20, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Nookaew, I.; Svensson, P.-A.; Jacobson, P.; Jernås, M.; Taube, M.; Larsson, I.; Andersson-Assarsson, J.; Sjöström, L.; Froguel, P.; Walley, A.; et al. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. J. Clin. Endocrinol. Metab. 2013, 98, E370–E378. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166. [Google Scholar] [CrossRef]
- Lee, C.; Chae, S.; Jo, S.; Jerng, U.; Bae, S. The Relationship between the Gut Microbiome and Metformin as a Key for Treating Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef]
- Verma, A.; Xu, K.; Du, T.; Zhu, P.; Liang, Z.; Liao, S.; Zhang, J.; Raizada, M.K.; Grant, M.B.; Li, Q. Expression of Human ACE2 in Lactobacillus and Beneficial Effects in Diabetic Retinopathy in Mice. Mol. Ther. Methods Clin. Dev. 2019, 14, 161–170. [Google Scholar] [CrossRef]
- Li, Q.; Xu, K.; Du, T.; Zhu, P.; Verma, A. Recombinant Probiotics Expressing Angio-tensin-(1-7) Improves Glucose Metabolism and Diabetes-Induced Renal and Retinal Injury. Diabetes 2018, 67 (Suppl. S1), 33-LB. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Llorca, A.; Kararigas, G. Sex-Related Effects of Gut Microbiota in Metabolic Syndrome-Related Diabetic Retinopathy. Microorganisms 2023, 11, 447. https://doi.org/10.3390/microorganisms11020447
García-Llorca A, Kararigas G. Sex-Related Effects of Gut Microbiota in Metabolic Syndrome-Related Diabetic Retinopathy. Microorganisms. 2023; 11(2):447. https://doi.org/10.3390/microorganisms11020447
Chicago/Turabian StyleGarcía-Llorca, Andrea, and Georgios Kararigas. 2023. "Sex-Related Effects of Gut Microbiota in Metabolic Syndrome-Related Diabetic Retinopathy" Microorganisms 11, no. 2: 447. https://doi.org/10.3390/microorganisms11020447




