Acquiring Iron-Reducing Enrichment Cultures: Environments, Methods and Quality Assessments
Abstract
:1. Introduction
2. Materials and Methods
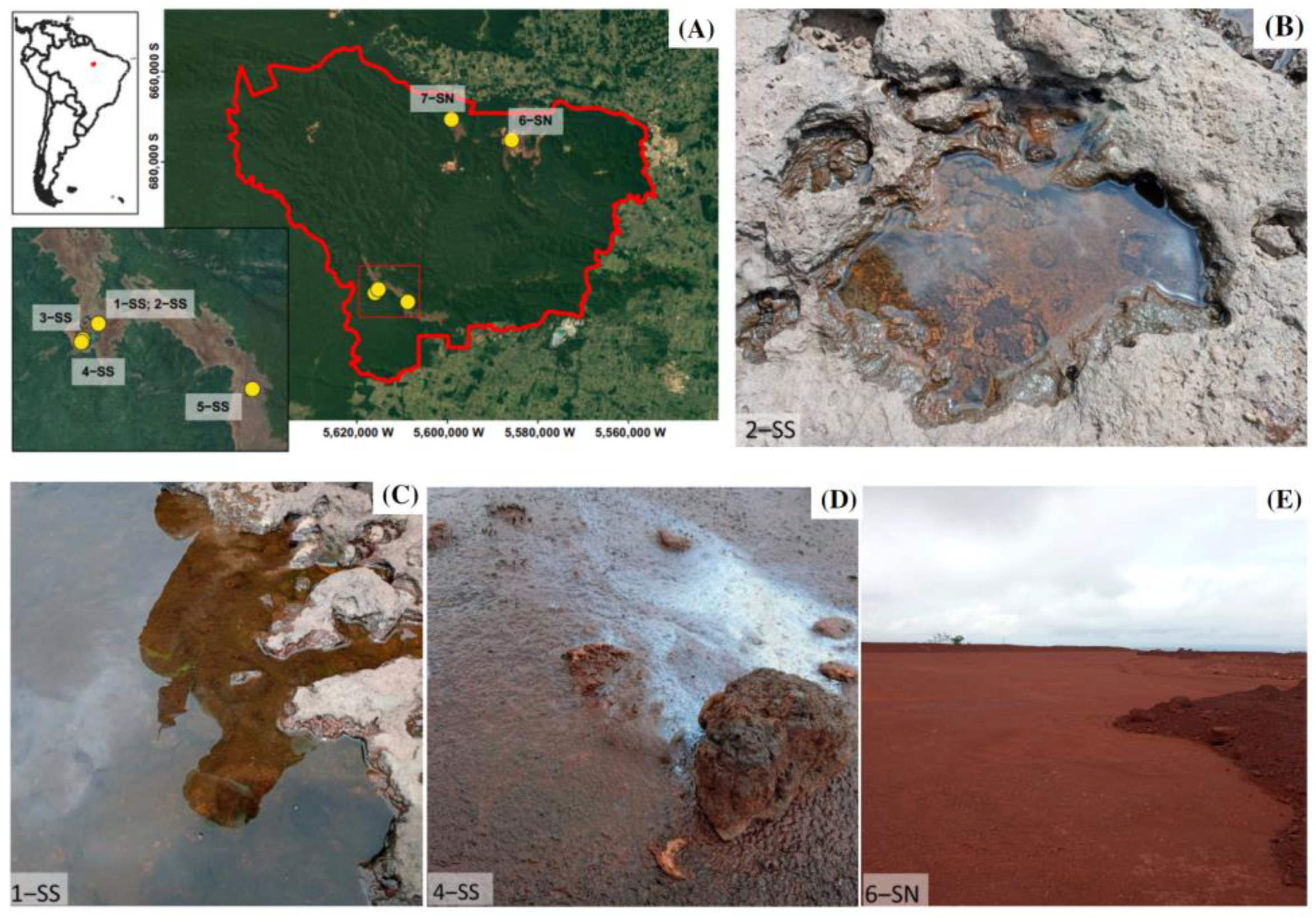
2.1. Geographical Setting and Sampling
2.2. Enrichment, Selection and Propagation of Microbial Cultures
2.3. Ferrozine Assays
2.4. Microbial Growth and Microscopy
2.5. Activity of the Electron Transport System (ETSA)
2.6. Molecular Analysis
3. Results
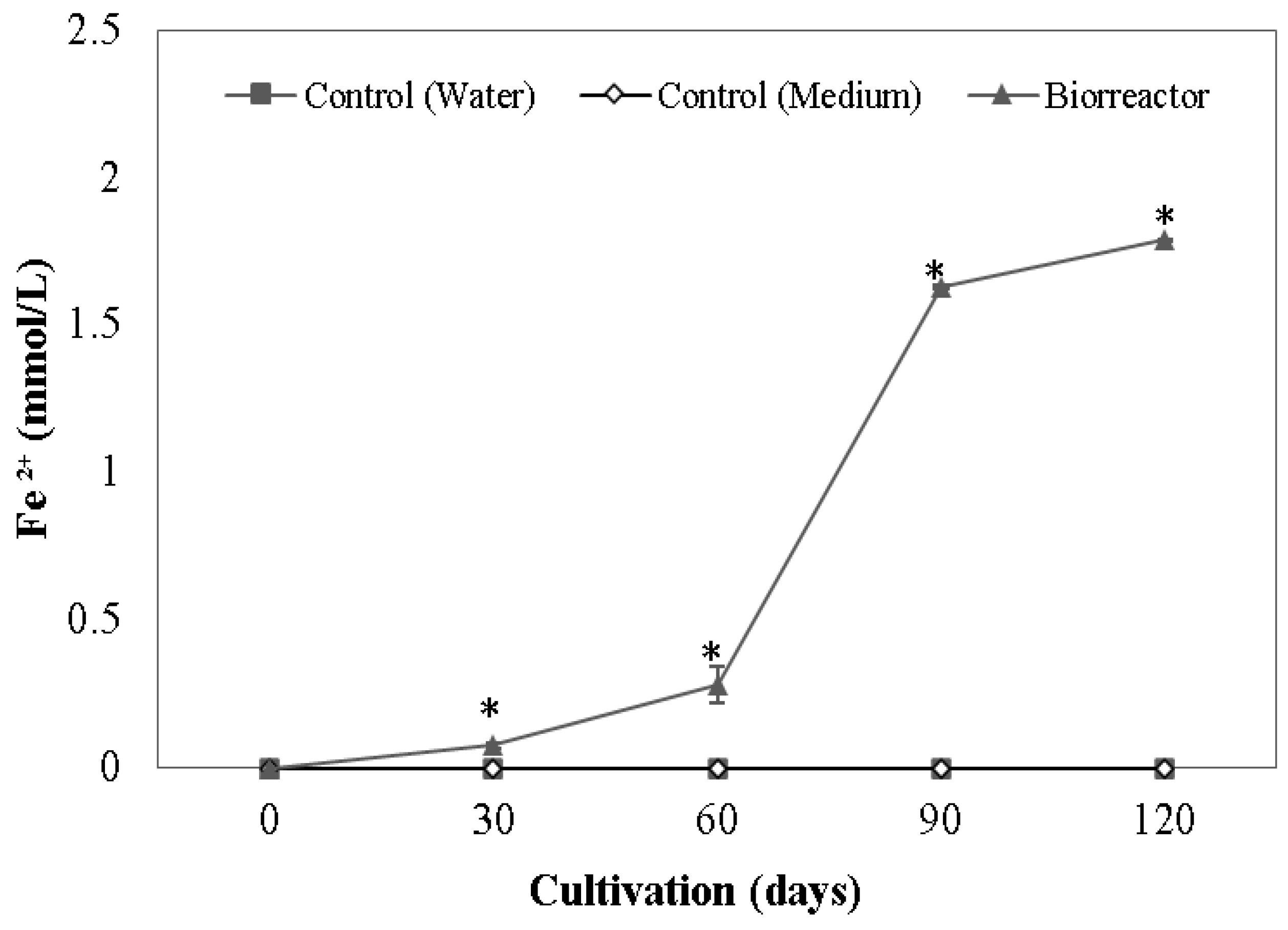
3.1. Iron Reduction of Enrichment Cultures
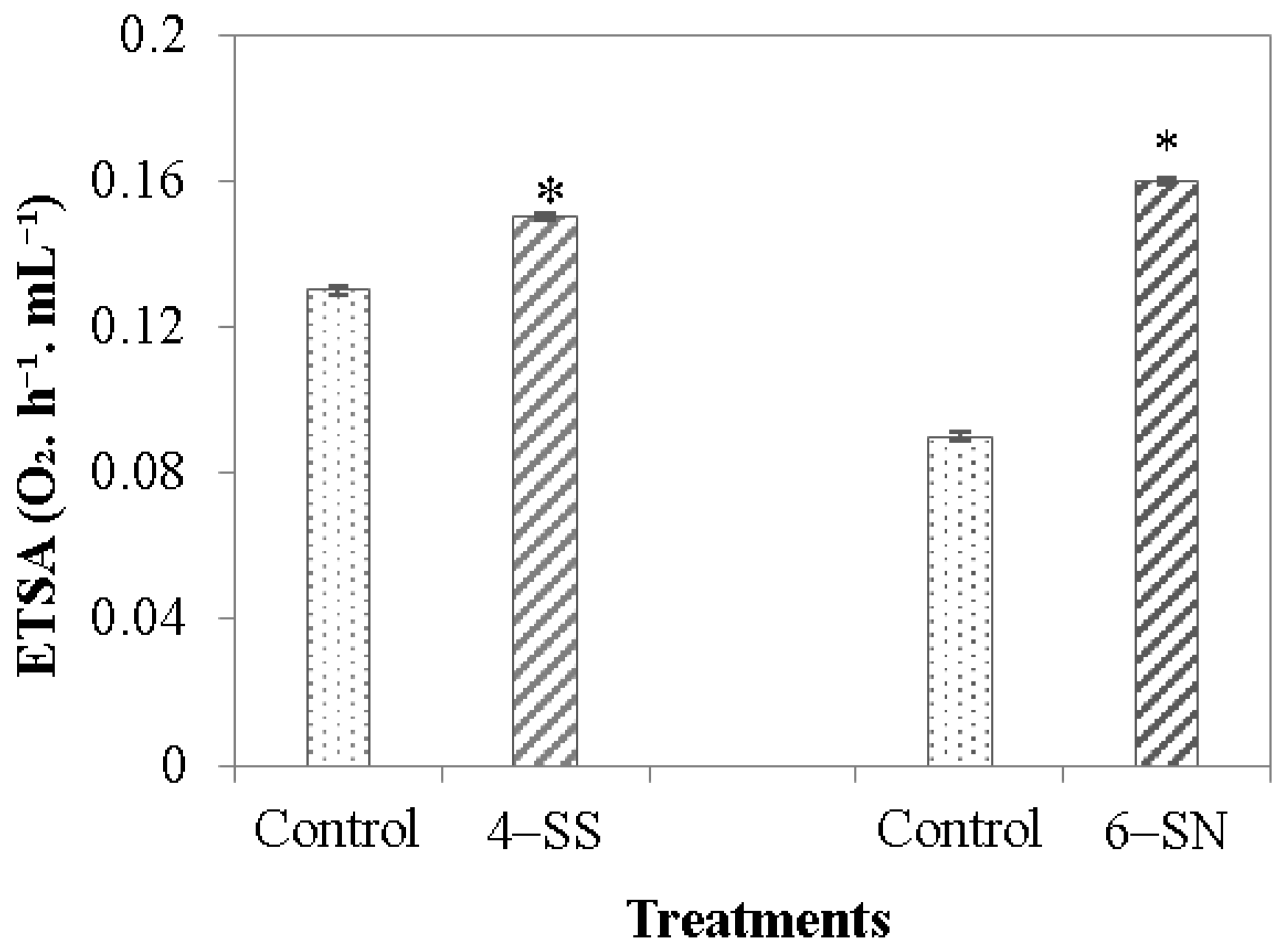
3.2. Activity of the Electron Transport System
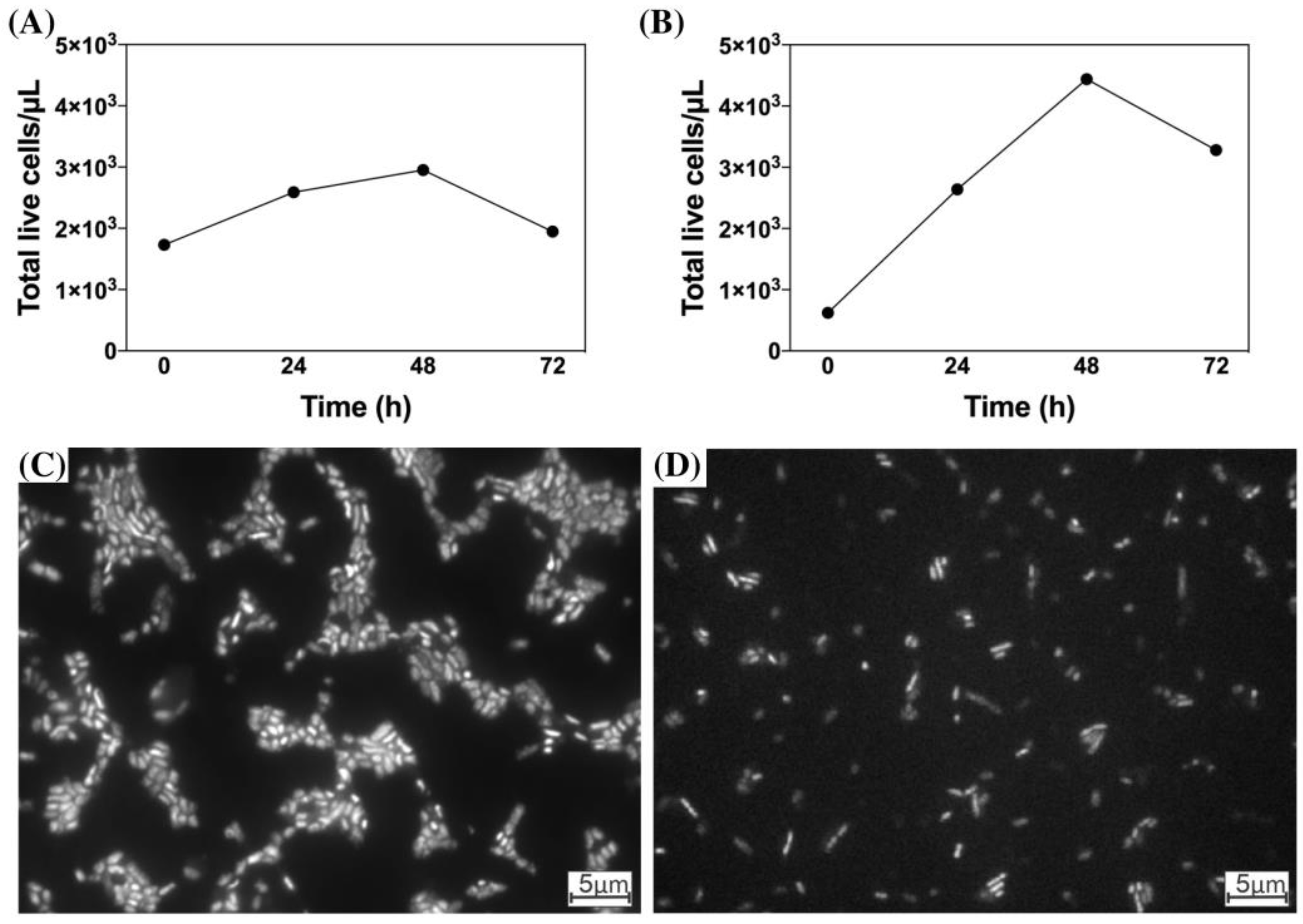
3.3. Microbial Growth and Microscopy
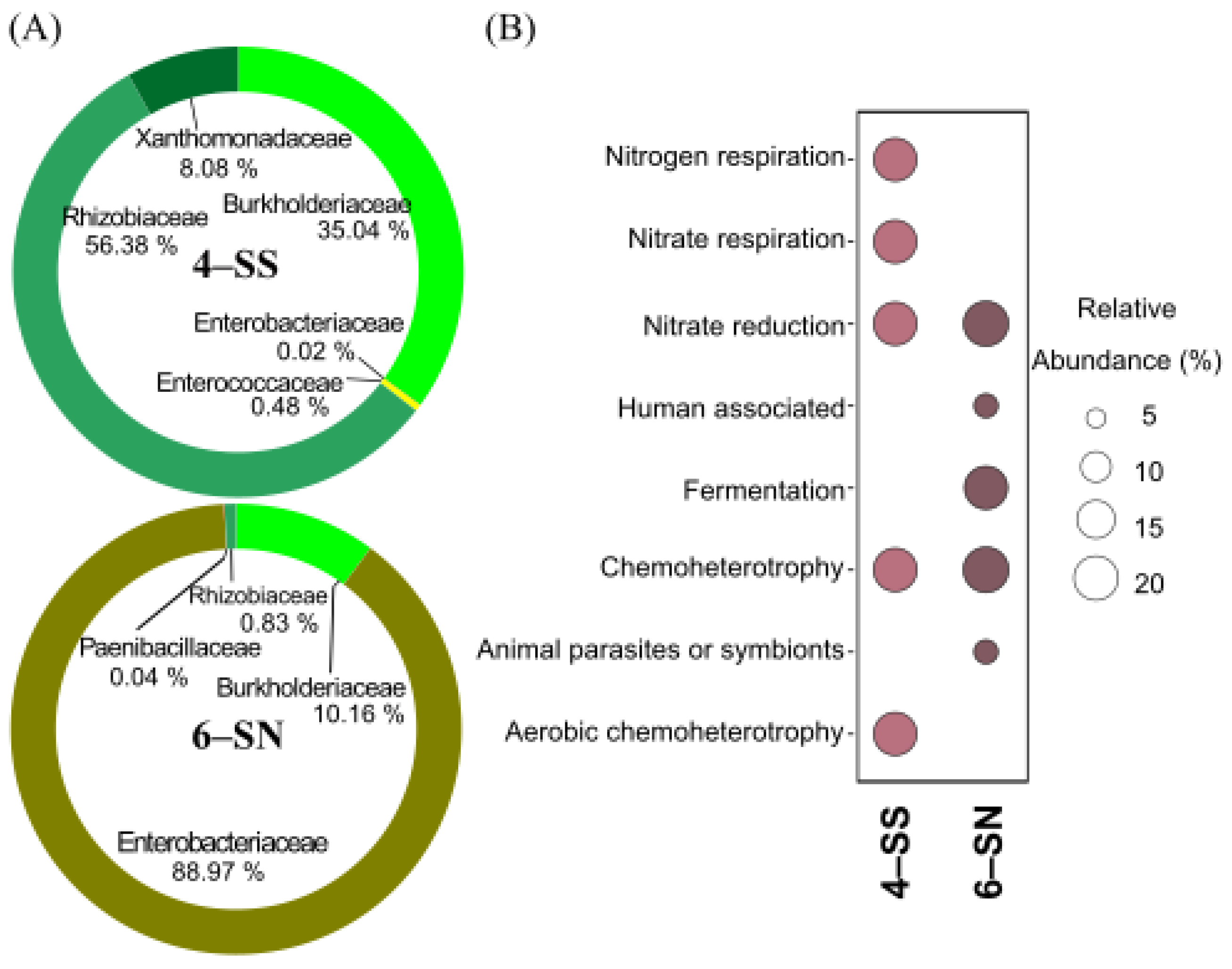
3.4. Microbial Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitre, S.K.; Mardegan, S.F.; Caldeira, C.F.; Ramos, S.J.; Neto, A.E.F.; Siqueira, J.O.; Gastauer, M. Nutrient and water dynamics of Amazonian canga vegetation differ among physiognomies and from those of other neotropical ecosystems. Plant Ecol. 2018, 219, 1341–1353. [Google Scholar] [CrossRef]
- Levett, A.; Gleeson, S.A.; Kallmeyer, J. From exploration to remediation: A microbial perspective for innovation in mining. Earth-Sci. Rev. 2021, 216, 103563. [Google Scholar] [CrossRef]
- Nunes, F.; Negreiros, D.; Fernandes, G.W. Campo rupestre: A restauração ecológica de um ecossistema ameaçado e megadiverso. Ciên. Hoje 2015, 55, 24–27. [Google Scholar]
- Gastauer, M.; Vera, M.P.O.; de Souza, K.P.; Pires, E.S.; Alves, R.; Caldeira, C.F.; Ramos, S.J.; Oliveira, G. A metagenomic survey of soil microbial communities along a rehabilitation chronosequence after iron ore mining. Sci. Data 2019, 6, 190008. [Google Scholar] [CrossRef]
- Viana, P.L.; Mota, N.F.D.O.; Gil, A.D.S.B.; Salino, A.; Zappi, D.C.; Harley, R.M.; Ilkiu-Borges, A.L.; Secco, R.D.S.; Almeida, T.E.; Watanabe, M.T.C.; et al. Flora das cangas da Serra dos Carajás, Pará, Brasil: História, área de estudos e metodologia. Rodriguésia 2016, 67, 1107–1124. [Google Scholar] [CrossRef]
- Mota, N.F.D.O.; Watanabe, M.T.C.; Zappi, D.; Hiura, A.L.; Pallos, J.; Viveros, R.S.; Giulietti, A.M.; Viana, P.L. Cangas da Amazônia: A vegetação única de Carajás evidenciada pela lista de fanerógamas. Rodriguésia 2018, 69, 1435–1488. [Google Scholar] [CrossRef]
- Giulietti, A.M.; Giannini, T.C.; Mota, N.F.O.; Watanabe, M.T.C.; Viana, P.L.; Pastore, M.; Silva, U.C.S.; Siqueira, M.F.; Pirani, J.R.; Lima, H.C.; et al. Edaphic Endemism in the Amazon: Vascular Plants of the canga of Carajás, Brazil. Bot. Rev. 2019, 85, 357–383. [Google Scholar] [CrossRef]
- Skirycz, A.; Castilho, A.; Chaparro, C.; Carvalho, N.; Tzotzos, G.; Siqueira, J.O. Canga biodiversity, a matter of mining. Front. Plant Sci. 2014, 5, 653. [Google Scholar] [CrossRef]
- Gagen, E.J.; Levett, A.; Paz, A.; Bostelmann, H.; Valadares, R.B.D.S.; Bitencourt, J.A.P.; Gastauer, M.; Nunes, G.L.; Oliveira, G.; Vasconcelos, P.M.; et al. Accelerating microbial iron cycling promotes re-cementation of surface crusts in iron ore regions. Microb. Biotechnol. 2020, 13, 1960–1971. [Google Scholar] [CrossRef]
- Levett, A.; Vasconcelos, P.M.; Gagen, E.J.; Rintoul, L.; Spier, C.; Guagliardo, P.; Southam, G. Microbial weathering signatures in lateritic ferruginous duricrusts. Earth Planet. Sci. Lett. 2020, 538, 116209. [Google Scholar] [CrossRef]
- Bryce, C.; Blackwell, N.; Schmidt, C.; Otte, J.M.; Huang, Y.-M.; Kleindienst, S.; Tomaszewski, E.; Schad, M.; Warter, V.; Peng, C.; et al. Microbial anaerobic Fe(II) oxidation—Ecology, mechanisms and environmental implications. Environ. Microbiol. 2018, 20, 3462–3483. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, H.S.; Vasconcelos, P.M.; Farley, K.A.; Spier, C.A.; Mello, C.L. (U–Th)/He geochronology of goethite and the origin and evolution of cangas. Geochim. Cosmochim. Acta 2014, 131, 267–289. [Google Scholar] [CrossRef]
- Paz, A.; Gagen, E.J.; Levett, A.; Zhao, Y.; Kopittke, P.; Southam, G. Biogeochemical cycling of iron oxides in the rhizosphere of plants grown on ferruginous duricrust (canga). Sci. Total Environ. 2020, 713, 136637. [Google Scholar] [CrossRef]
- Lentini, C.J.; Wankel, S.D.; Hansel, C.M. Enriched Iron(III)-Reducing Bacterial Communities are Shaped by Carbon Substrate and Iron Oxide Mineralogy. Front. Microbiol. 2012, 3, 404. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Dissimilatory metal reduction. Annu. Rev. Microbiol. 1993, 47, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Bird, L.J.; Bonnefoy, V.; Newman, D.K. Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol. 2011, 19, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Kappler, A.; Bryce, C.; Mansor, M.; Lueder, U.; Byrne, J.M.; Swanner, E.D. An evolving view on biogeochemical cycling of iron. Nat. Rev. Genet. 2021, 19, 360–374. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) Reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [CrossRef]
- Su, C.; Zhang, M.; Lin, L.; Yu, G.; Zhong, H.; Chong, Y. Reduction of iron oxides and microbial community composition in iron-rich soils with different organic carbon as electron donors. Int. Biodeterior. Biodegrad. 2019, 148, 104881. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Levett, A.; Gagen, E.J.; Zhao, Y.; Vasconcelos, P.M.; Southam, G. Biocement stabilization of an experimental-scale artificial slope and the reformation of iron-rich crusts. Proc. Natl. Acad. Sci. USA 2020, 117, 18347–18354. [Google Scholar] [CrossRef]
- Gagen, E.J.; Levett, A.; Paz, A.; Gastauer, M.; Caldeira, C.F.; da Silva, B. Biogeochemical processes in canga ecosystems: Armoring of iron ore against erosion and importance in iron duricrust restoration in Brazil. Ore Geol. Rev. 2019, 107, 573–586. [Google Scholar] [CrossRef]
- Coupland, K.; Johnson, D.B. Evidence that the potential for dissimilatory ferric iron reduction is widespread among acidophilic heterotrophic bacteria. FEMS Microbiol. Lett. 2008, 279, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Rosche, B.; Li, X.Z.; Hauer, B.; Schmid, A.; Buehler, K. Microbial biofilms: A concept for industrial catalysis? Trends Biotechnol. 2009, 27, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Kirk, M.F. pH as a Primary Control in Environmental Microbiology: 2. Kinetic Perspective. Front. Environ. Sci. 2018, 6, 21. [Google Scholar] [CrossRef]
- Tavares, A.L.; Carmo, A.M.C.D.; Júnior, R.O.D.S.; e Souza-Filho, P.W.M.; da Silva, M.S.; Ferreira, D.B.D.S.; Júnior, W.D.R.N.; Dall’Agnol, R. Climate indicators for a watershed in the eastern amazon. Rev. Bras. Clim. 2018, 23. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Guimarães, J.T.F.; Souza-Filho, P.W.M.; da Silva, M.S.; Maurity, C.W.; Powell, M.A.; Rodrigues, T.M.; da Silva, D.F.; Mardegan, S.F.; Neto, A.E.F.; et al. Geochemistry of upland lacustrine sediments from Serra dos Carajás, Southeastern Amazon, Brazil: Implications for catchment weathering, provenance, and sedimentary processes. J. S. Am. Earth Sci. 2016, 72, 178–190. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Guimarães, J.T.; Souza-Filho, P.W.M.; Bozelli, R.L.; De Araujo, L.R.; Menezes, R.D.S.; Lopes, P.M.; Silva, M.S.; Rodrigues, T.M.; Costa, M.F.; et al. Limnological characteristics and planktonic diversity of five tropical upland lakes from Brazilian Amazon. Ann. Limnol.-Int. J. Limnol. 2017, 53, 467–483. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Rapid Assay for Microbially Reducible Ferric Iron in Aquatic Sediments. Appl. Environ. Microbiol. 1987, 53, 1536–1540. [Google Scholar] [CrossRef]
- R CORE TEAM. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Anneser, B.; Pilloni, G.; Bayer, A.; Lueders, T.; Griebler, C.; Einsiedl, F.; Richters, L. High Resolution Analysis of Contaminated Aquifer Sediments and Groundwater—What Can be Learned in Terms of Natural Attenuation? Geomicrobiol. J. 2010, 27, 130–142. [Google Scholar] [CrossRef]
- Nebe-Von-Caron, G.; Stephens, P.; Hewitt, C.; Powell, J.; Badley, R. Analysis of bacterial function by multi-colour fluorescence flow cytometry and single cell sorting. J. Microbiol. Methods 2000, 42, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.R.; Drew, W.L.; Kobayashi, G.S.; Thompson, J.H. Microbiologia Médica; Guanabara Koogan: Rio de Janeiro, Brazil, 1992; 513p. [Google Scholar]
- Boulos, L.; Prévost, M.; Barbeau, B.; Coallier, J.; Desjardins, R. LIVE/DEAD®BacLight™: Application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 1999, 37, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Houri-Davignon, C.; Relexans, J.C.; Etcheber, H. Measurement of actual electron transport system (ETS) activity in marine sediments by incubation with INT. Environ. Technol. Lett. 1989, 10, 91–100. [Google Scholar] [CrossRef]
- Maier, R.M.; Pepper, I.L.; Gerba, C.P. Environmental Microbiology, 2nd ed.; Elsevier Inc.: Oxford, UK, 2009. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.R.M.; Silva, R.; Nunes, G.L.; Oliveira, G. PIMBA: A PIpeline for MetaBarcoding Analysis. In Advances in Bioinformatics and Computational Biology: 14th Brazilian Symposium on Bioinformatics, BSB 2021, Virtual Event, 22–26 November 2021, Proceedings; Springer International Publishing: New York, NY, USA, 2021; pp. 106–116. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Newsome, L.; Adams, R.L.; Downie, H.F.; Moore, K.L.; Lloyd, J. NanoSIMS imaging of extracellular electron transport processes during microbial iron(III) reduction. FEMS Microbiol. Ecol. 2018, 94, fiy099. [Google Scholar] [CrossRef]
- Bôto, M.L.; Magalhães, C.; Perdigão, R.; Alexandrino, D.A.M.; Fernandes, J.P.; Bernabeu, A.M.; Ramos, S.; Carvalho, M.F.; Semedo, M.; LaRoche, J.; et al. Harnessing the Potential of Native Microbial Communities for Bioremediation of Oil Spills in the Iberian Peninsula NW Coast. Front. Microbiol. 2021, 12, 633659. [Google Scholar] [CrossRef]
- Chen, R.; Liu, H.; Tong, M.; Zhao, L.; Zhang, P.; Liu, D.; Yuan, S. Impact of Fe(II) oxidation in the presence of iron-reducing bacteria on subsequent Fe(III) bio-reduction. Sci. Total Environ. 2018, 639, 1007–1014. [Google Scholar] [CrossRef]
- Ginn, B.; Meile, C.; Wilmoth, J.; Tang, Y.; Thompson, A. Rapid Iron Reduction Rates Are Stimulated by High-Amplitude Redox Fluctuations in a Tropical Forest Soil. Environ. Sci. Technol. 2017, 51, 3250–3259. [Google Scholar] [CrossRef]
- Adams, L.K.; Boothman, C.; Lloyd, J.R. Identification and characterization of a novel acidotolerant Fe(III)-reducing bacterium from a 3000-year-old acidic rock drainage site. FEMS Microbiol. Lett. 2007, 268, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Zacarías, C.; Cantú-Cárdenas, M.E.; López-Chuken, U.J.; Parra-Saldívar, R.; Garza-Gonzalez, M.T.; Rostro-Alanis, M.D.J.; Villarreal-Chiu, J.F. Dissimilatory reduction of Fe(III) by a novel Serratia marcescens strain with special insight into the influence of prodigiosin. Int. Microbiol. 2019, 23, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.W.; Auler, A.S.; Barton, M.D.; Sasowsky, I.D.; Senko, J.M.; Barton, H.A. Fe(III) Reducing Microorganisms from Iron Ore Caves Demonstrate Fermentative Fe(III) Reduction and Promote Cave Formation. Geomicrobiol. J. 2017, 35, 311–322. [Google Scholar] [CrossRef]
- Sirota-Madi, A.; Olender, T.; Helman, Y.; Ingham, C.; Brainis, I.; Roth, D.; Ben-Jacob, E. Genome sequence of the pattern forming Paenibacillus vortex bacterium reveals potential for thriving in complex environments. BMC Genom. 2010, 11, 710. [Google Scholar] [CrossRef]
- Raza, W.; Shen, Q. Growth, Fe3+ reductase activity, and siderophore production by Paenibacillus polymyxa SQR-21 under differential iron conditions. Curr. Microbiol. 2010, 61, 390–395. [Google Scholar] [CrossRef]
- Cutting, R.S.; Coker, V.S.; Fellowes, J.W.; Lloyd, J.R.; Vaughan, D.J. Mineralogical and morphological constraints on the reduction of Fe (III) minerals by Geobacter sulfurreducens. Geoch. Cosmoch. Acta 2009, 73, 4004–4022. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, A.F.; da Silva, R.d.S.S.; Prado, I.G.d.O.; Bitencourt, J.A.P.; Gastauer, M. Acquiring Iron-Reducing Enrichment Cultures: Environments, Methods and Quality Assessments. Microorganisms 2023, 11, 448. https://doi.org/10.3390/microorganisms11020448
Cardoso AF, da Silva RdSS, Prado IGdO, Bitencourt JAP, Gastauer M. Acquiring Iron-Reducing Enrichment Cultures: Environments, Methods and Quality Assessments. Microorganisms. 2023; 11(2):448. https://doi.org/10.3390/microorganisms11020448
Chicago/Turabian StyleCardoso, Aline Figueiredo, Rayara do Socorro Souza da Silva, Isabelle Gonçalves de Oliveira Prado, José Augusto Pires Bitencourt, and Markus Gastauer. 2023. "Acquiring Iron-Reducing Enrichment Cultures: Environments, Methods and Quality Assessments" Microorganisms 11, no. 2: 448. https://doi.org/10.3390/microorganisms11020448




