Phosphate-Solubilizing Capacity of Paecilomyces lilacinus PSF7 and Optimization Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization and Identification of the Phosphate-Solubilizing Fungi
2.2. Evaluation of Phosphate-Solubilizing Capacity
2.3. Analysis of Organic Acids
2.4. Effects of Medium Composition on Phosphate-Solubilizing Capacity
2.5. Design of the Experiment
2.6. Scanning Electron Microscopy
2.7. Data Analysis
3. Results
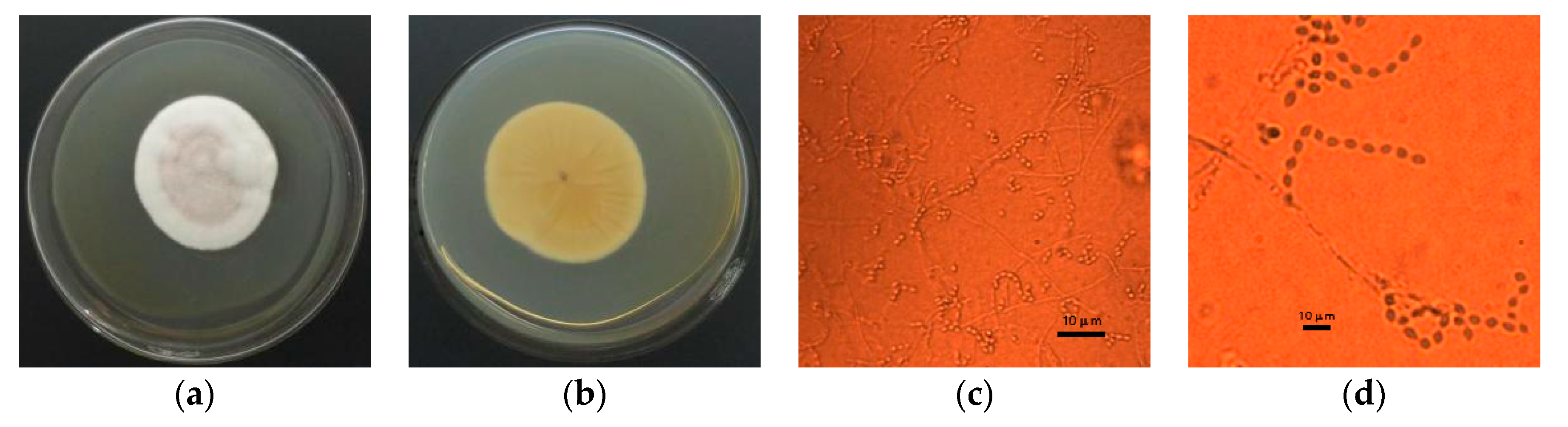
3.1. Morphological Characterization
3.2. Identification via 16S rRNA Sequence Analysis
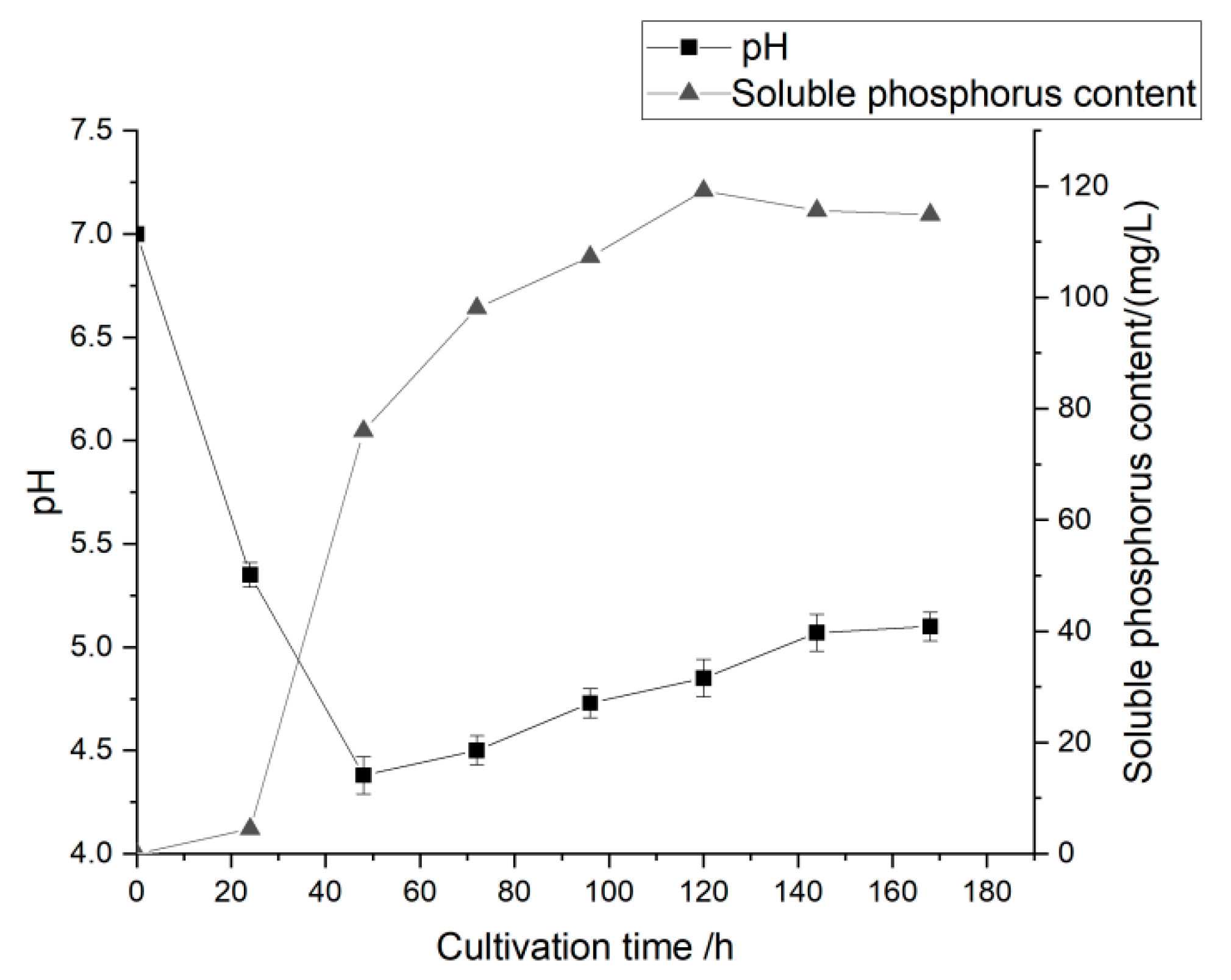
3.3. Analysis of Phosphate-Solubilizing Capacity
3.4. Analysis of Organic Acids
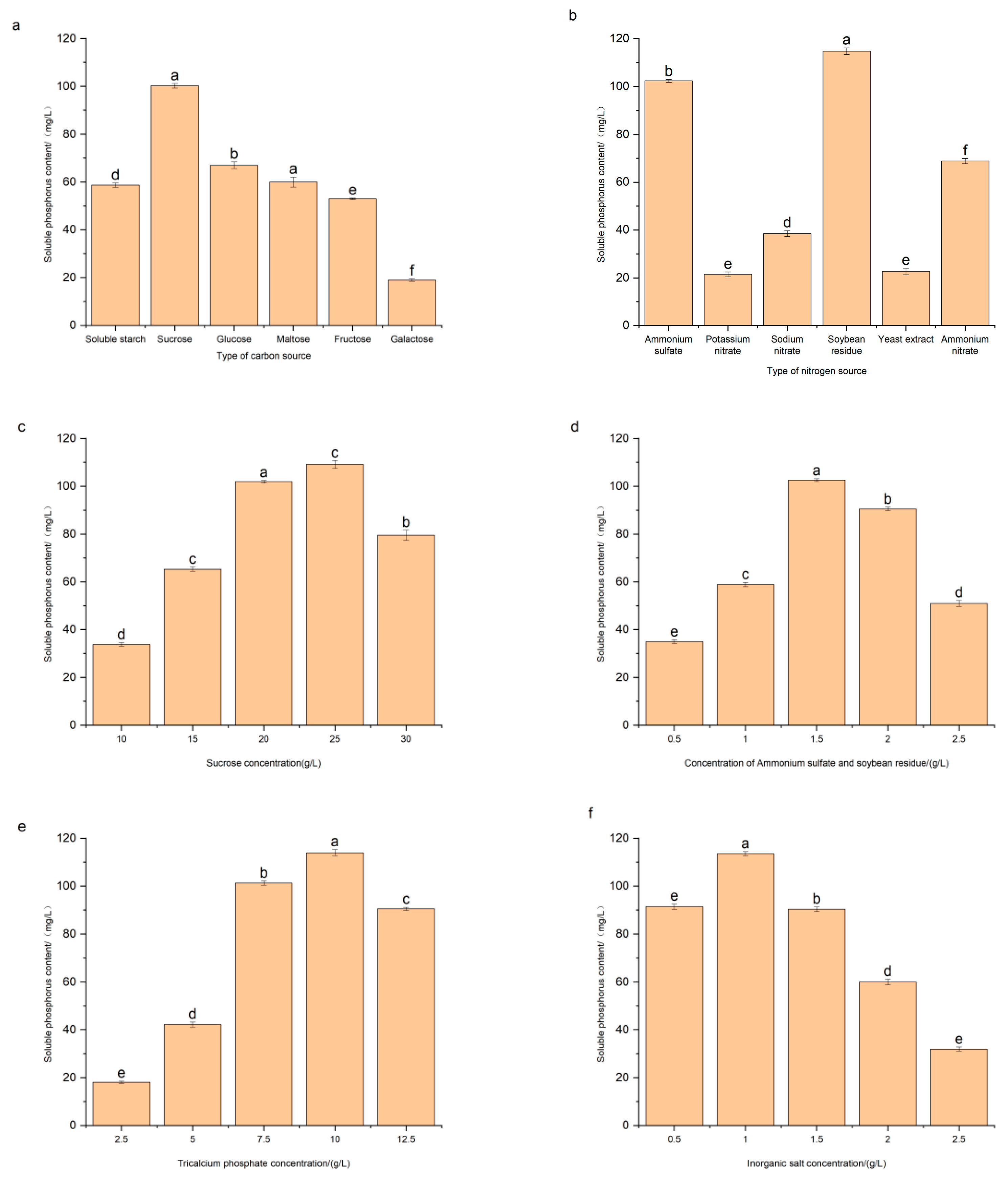
3.5. Effects of Medium Composition on Phosphate-Solubilizing Capacity
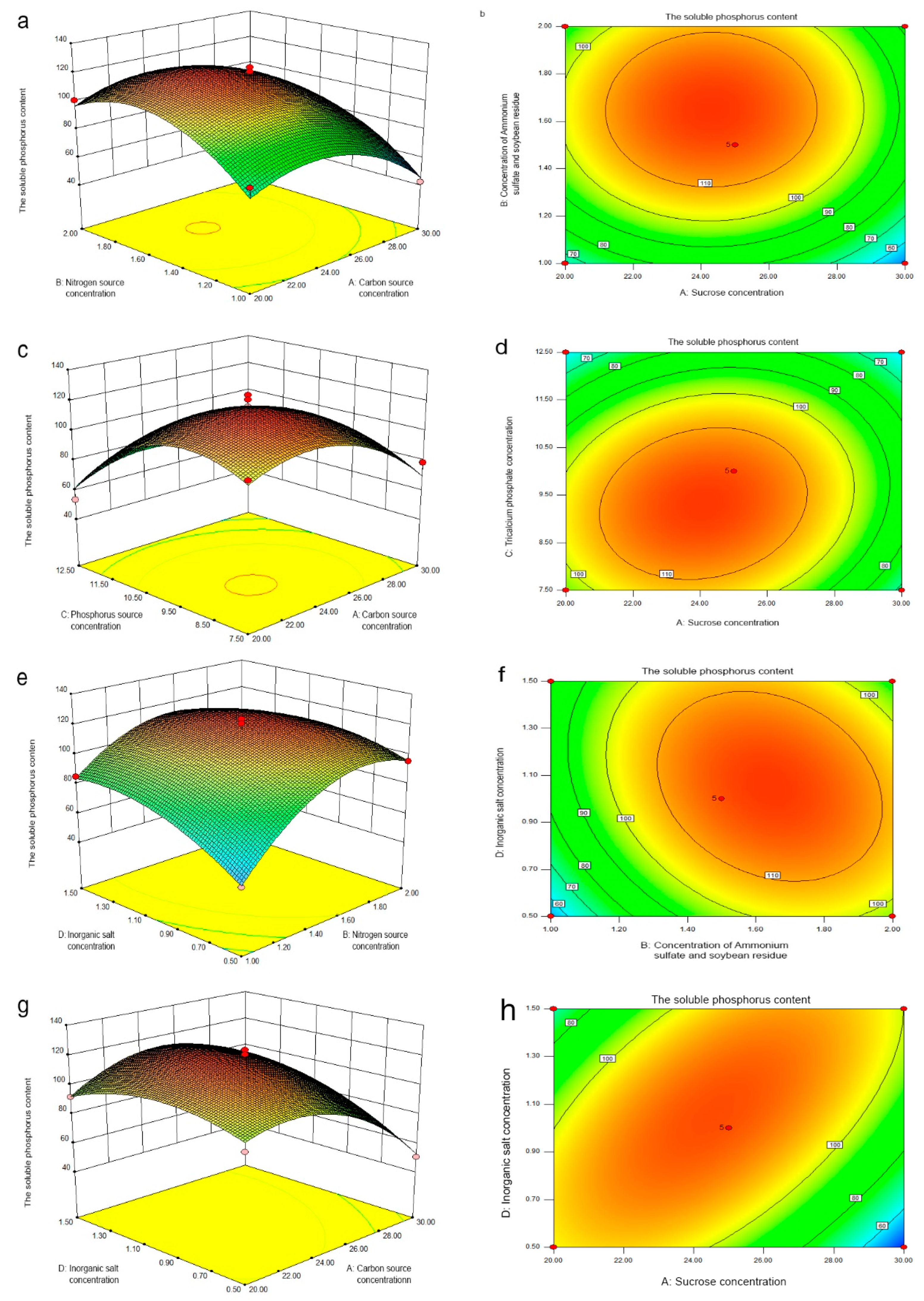
3.6. Response Surface Test Analysis
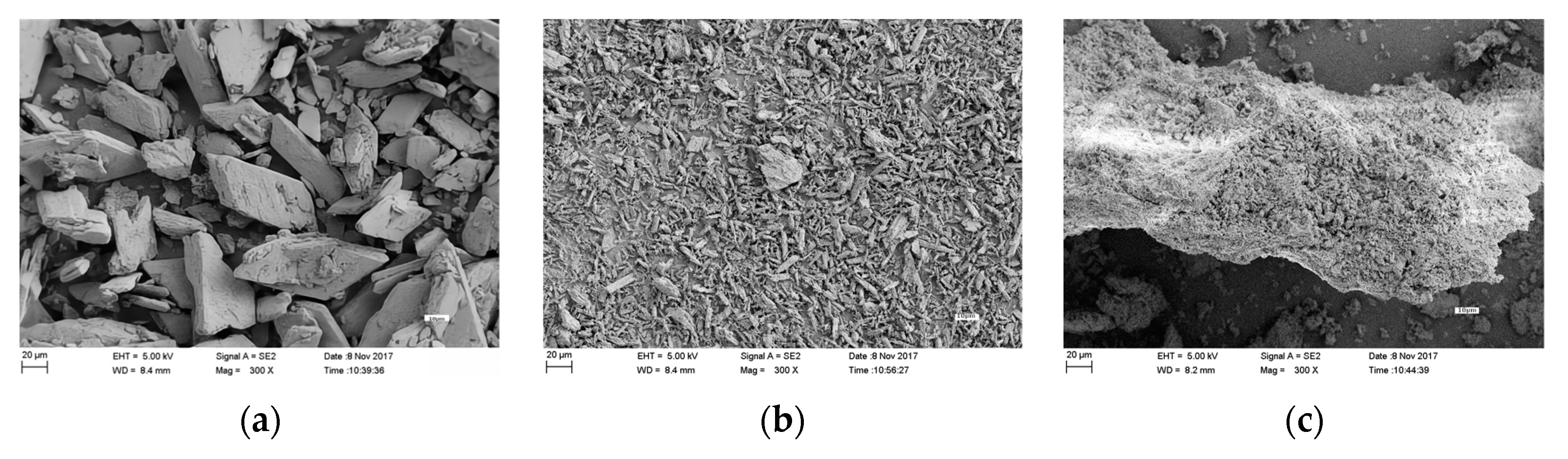
3.7. Scanning Electron Microscope Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Wang, P.C.; Fang, L.; Zhang, Q.A.; Yan, C.S.; Chen, J.C. Isolation and Characterization of Phosphate-Solubilizing Bacteria from Mushroom Residues and their Effect on Tomato Plant Growth Promotion. Pol. J. Microbiol. 2017, 66, 57–65. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi–current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Nayak, A.K.; Sharma, D.K.; Singh, C.S.; Mishra, V.K.; Singh, G.; Swarup, A. Diagnosis and Recommendation Integrated System approach for nitrogen, phosphorus, potassium, and zinc foliar diagnostic norms for aonla in central Indo-Gangetic plains. J. Plant Nutr. 2011, 34, 547–556. [Google Scholar] [CrossRef]
- Afkairin, A.; Ippolito, J.A.; Stromberger, M.; Davis, J.G. Solubilization of organic phosphorus sources by cyanobacteria and a commercially available bacterial consortium. Appl. Soil Ecol. 2021, 162, 162. [Google Scholar] [CrossRef]
- Cetner, M.D.; Kalaji, H.M.; Borucki, W.; Kowalczyk, K. Special issue in honour of Prof. Reto J. Strasser–Phosphorus deficiency affects the I-step of chlorophyll a fluorescence induction curve of radish. Photosynthetica 2020, 58, 671–681. [Google Scholar] [CrossRef]
- Harpole, W.S.; Ngaim, J.T.; Cleland, E.E.; Seabloom, E.W.; Borer, E.T.; Bracken, M.E.S.; Elser, J.J.; Gruner, D.S.; Hillebrand, H.; Shurin, J.B.; et al. Nutrient co-limitation of primary producer communities. Ecol. Lett. 2011, 14, 852–862. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Haque, E.; Paul, N.C.; Khaleque, M.A.; Al-Garni, S.M.S.; Rahman, M.; Islam, M.T. Enhancement of growth and grain yield of rice in nutrient deficient soils by rice probiotic bacteria. Rice Sci. 2017, 24, 264–273. [Google Scholar] [CrossRef]
- Havlin, J.; Tisdale, S.; Nelson, W.; Beaton, J. Soil Fertility and Fertilizers; Pearson Education India: Delhi, India, 2016. [Google Scholar]
- Tiwari, S.; Patel, A.; Pandey, N.; Raju, A.; Singh, M.; Prasad, S.M. Deficiency of Essential Elements in Crop Plants. In Sustainable Solutions for Elemental Deficiency and Excess in Crop Plants; Mishra, K., Tandon, P.K., Srivastava, S., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Shafi, M.I.; Adnanm, M.; Fahad, S.; Wahid, F.; Khan, A.; Yue, Z.; Danish, S.; Zafar-ul-Hye, M.; Brtnicky, M.; Datta, R. Application of Single Superphosphate with Humic Acid Improves the Growth, Yield and Phosphorus Uptake of Wheat (Triticum aestivum L.) in Calcareous Soil. Agronomy 2020, 9, 1224. [Google Scholar] [CrossRef]
- Tilman, D.; Fargione, J.; Wolff, B.; D’antonio, C.; Dobson, A.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef]
- Mohammed, N.; Chanai, E.; Alkhorayef, M. The impact of the extensive use of phosphate fertilizers on radioactivity levels in farm soil and vegetables in Tanzania. Radioanal. Nucl. Chem. 2016, 307, 2373–2379. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Sustain. Agric. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Ludewig, U.; Yuan, L.; Neumann, G. Improving the efficiency and effectiveness of global phosphorus use: Focus on root and rhizosphere levels in the agronomic system. Front. Agric. Sci. Eng. 2019, 6, 357–365. [Google Scholar] [CrossRef]
- Elfiati, D.; Susilowati, A.; Modes, C. Potential phosphate solubilizing bacteria from the rhizosphere of Cotylelobium melanoxylon tree. IOP Conf. Ser. Earth Environ. Sci. 2021, 713, 012018. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Zafar-ul-Hye, M.; Battaglia, M.L.; Naz, R.M.M.; Saeed, B.; et al. Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef]
- Richardson, A.E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Biol. 2001, 28, 897–906. [Google Scholar] [CrossRef]
- Umar, M.I. P-Solubilizing microorganisms performance on manure and rock phosphate and their influences on soil and plant phosphorous in calcareous soils. Univ. Baghdad-Coll. Agric. 2021, 52, 437–444. [Google Scholar] [CrossRef]
- Li, Y.; Yu, X.T.; Zheng, J.R.; Gong, Z.L.; Xu, W.L. Diversity and Phosphate Solubilizing Characteristics of Cultivable Organophosphorus-Mineralizing Bacteria in the Sediments of Sancha Lake. Int. J. Environ. Res. Public Health 2022, 19, 2320. [Google Scholar] [CrossRef]
- Briste, P.S.; Tabassum, C.N. Endophytic Pseudomonas species from coastal weeds affecting in vitro phosphate solubilization and growth of wheat (Triticum aestivum) in Bangladesh. Egypt. J. Agric. Res. 2021, 99, 197–204. [Google Scholar]
- Subhashini, D.V.; Anil, K.V. Phosphate solubilising Streptomyces spp. obtained from the rhizosphere of Ceriops decandra of Corangi mangroves. Indian J. Agric. Sci. 2014, 84, 12–16. [Google Scholar]
- Seenivasagan, R.; Babalola, O.O. Utilization of microbial consortia as biofertilizers and biopesticides for the production of feasible agricultural product. Biology 2021, 10, 1111. [Google Scholar] [CrossRef]
- Ángela, P.M.Q.; Nelson, W.O.V.; Octavio, A.G.M. In vitro dissolution of acidulated rock phosphate by phosphate solubilizing microorganisms. Acta Biol. Colomb. 2015, 20, 65–71. [Google Scholar]
- Prasad, A.; Dixit, M.; Meena, S.K.; Kumar, A. Qualitative and quantitative estimation for phosphate solubilizing ability of Trichoderma isolates: A natural soil health enhancer. Mater. Today Proc. 2021, 3. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, S.H.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2019, 23, 101487. [Google Scholar] [CrossRef]
- Qin, L.J.; Yang, Y.Z.; Yang, X.Y. Advances in Mechanisms of Soil Phosphorus Solubilization and Dissolution by Phosphate Solubilizing Microorganisms. Life Sci. Res. 2019, 23, 59–64. [Google Scholar]
- Hussain, S.; Sharif, M.; Ahmad, W. Selection of efficient phosphorus solubilizing bacteria strains and mycorrhizea for enhanced cereal growth, root microbe status and N and P uptake in alkaline calcareous soil. Soil Sci. Plant Nutr. 2021, 2, 259–268. [Google Scholar] [CrossRef]
- Rodríguezl, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Kiml, K.Y.; McDonald, G.A.; Jordan, D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol. Fertil. Soils 1997, 24, 347–352. [Google Scholar]
- Behera, B.; Singdevsachan, S.K.; Mishra, R.; Dutta, S.; Thatoi, H. Diversity, mechanism and biotechnology of phosphate solubilising microorganism in mangrove—A review. Biocatal. Agric. Biotechnol. 2014, 3, 97–110. [Google Scholar] [CrossRef]
- Song, O.R.; Lee, S.J.; Lee, Y.S.; Lee, S.C.; Kim, K.K.; Choi, Y.L. Solubilization of insoluble inorganic phosphate by Burkholderia cepacia DA23 isolated from cultivated soil. Braz. J. Microbiol. 2008, 39, 151–156. [Google Scholar]
- Liang, J.L.; Liu, J.; Jia, P.; Yang, T.T.; Zeng, Q.W.; Zhang, S.C.; Liao, B.; Shu, W.S.; Li, J.T. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef] [PubMed]
- Archana, G.; Buch, A.; Kumar, G.N. Pivotal role of organic acid secretion by rhizobacteria in plant growth promotion. In Microorganisms in Sustainable Agriculture and Biotechnology; Springer: Dordrecht, The Netherlands, 2012; pp. 35–53. [Google Scholar]
- Zhang, H.; Han, L.; Jiang, B.; Long, C.M. Identification of a phosphate-solubilizing Tsukamurella tyrosinosolvens strain and its effect on the bacterial diversity of the rhizosphere soil of peanuts growth-promoting. World J. Microbiol Biotechnol. 2021, 37, 109. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil. 2006, 287, 15–21. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Goldstein, A.H. Recent progress in understanding the molecular genetics and biochemistry of calcium phosphate solubilization by gram negative bacteria. Biol. Agric. Hortic. 1995, 12, 185–193. [Google Scholar] [CrossRef]
- Omar, S.A. The role of rock-phosphate-solubilizing fungi and vesicular–arbusular-mycorrhiza (VAM) in growth of wheat plants fertilized with rock phosphate. World J. Microbiol. Biotechnol. 1997, 14, 211–218. [Google Scholar] [CrossRef]
- Jones, D.L. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Subha, R.N.S. Advances in Agricultural Microbiology; Butterworth-Heinemann: Oxford, UK, 1982; pp. 229–305. [Google Scholar]
- Ghani, A.; Rajan, S.S.S. Low cost method for producing biologically acidulated phosphate rocks: A suitable phosphorus and sulphur fertilizer for organic and conventional farming. Agro-Chem. News Brief 1995, 18, 4–7. [Google Scholar]
- Pastore, G.; Weig, A.R.; Vazquez, E.; Spohn, M. Weathering of calcareous bedrocks is strongly affected by the capacity of soil microorganisms. Geoderma 2022, 405, 115408. [Google Scholar] [CrossRef]
- Reyes, I.; Bernier, L.; Simard, R.R.; Antoun, H. Effect of nitrogen source on the solubilization of different inorganic phosphates by an isolate of Penicillium rugulosum and two UV-induced mutants. FEMS Microbiol. Ecol. 1999, 28, 281–290. [Google Scholar] [CrossRef]
- Cunningham, J.E.; Kuiack, C. Production of citric and oxalic acids and solubilization of calcium phosphate by Penicillium bilaii. Appl. Environ. Microbiol. 1992, 58, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- McRae, G.; Monreal, C.M. LC-MS/MS quantitative analysis of reducing carbohydrates in soil solutions extracted from crop rhizospheres. Anal. Bioanal. Chem. 2011, 400, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Habte, M.; Osorio, N.W. Effect of Nitrogen Form on the Effectiveness of a Phosphate-Solubilizing Fungus to Dissolve Rock Phosphate. OMICS Int. 2012, 5, 127. [Google Scholar]
- Priha, O.; Sarlin, T.; Blomberg, P.; Wendling, L.; Mäkinen, J.; Arnold, M.; Kinnunen, P. Bioleaching phosphorus from fluorapatites with acidophilic bacteria. Hydrometallurgy 2014, 150, 269–275. [Google Scholar] [CrossRef]
- Alam, S.; Khalil, S.; Ayub, N.; Rashid, M. In vitro solubilization of inorganic phosphate by phosphate solubilizing microorganism (PSM) from maize rhizosphere. Int. J. Agric. Biol. Eng. 2002, 4, 454–458. [Google Scholar]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Dai, Z.B.; Wang, X.; Hong, G. Secondary Metabolites and Their Bioactivities Produced by Paecilomyces. Molecules 2020, 25, 5077. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Anandham, R.; Krishnamoorthy, R. Paecilomyces. In Beneficial Microbes in Agro-Ecology; Academic Press: Cambridge, MA, USA, 2020; pp. 793–808. [Google Scholar]
- Bonants, P.J.; Fitters, P.F.; Thijs, H.; Belder, E.D.; Waalwijk, C.; Henfling, J.W. A basic serine protease from Paecilomyces lilacinus with biological activity against Meloidogyne hapla eggs. Microbiology 1995, 141, 775–784. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Kiewnick, S. Effect of plant species on persistence of Paecilomyces lilacinus strain 251 in soil and on root colonization by the fungus. Plant Soil 2006, 283, 25–31. [Google Scholar] [CrossRef]
- Holland, R.J.; Williams, K.L.; Nevalainen, K.M.H. Paecilomyces lilacinus strain Bioact251 is not a plant endophyte. Australas. Plant Pathol. 2003, 32, 473–478. [Google Scholar] [CrossRef]
- Kiewnick, S.; Sikora, R.A. Biological control of the root-knot nematode Meloidogyne incognita by Paecilomyces lilacinus strain 251. Biol. Control 2006, 38, 179–187. [Google Scholar] [CrossRef]
- Constantin, M.; Raut, I.; Gurban, A.-M.; Doni, M.; Radu, N.; Alexandrescu, E.; Jecu, L. Exploring the Potential Applications of Paecilomyceslilacinus 112. Appl. Sci. 2022, 12, 7572. [Google Scholar] [CrossRef]
- Anastasiadis, I.A.; Giannakou, I.O.; Prophetou-Athanasiadou, D.A.; Gowen, S.R. The combined effect of the application of a biocontrol agent Paecilomyces lilacinus, with various practices for the control of root-knot nematodes. Crop Prot. 2008, 27, 352–361. [Google Scholar] [CrossRef]
- Zeng, Q.; Wu, X.; Wen, X. Identification and characterization of the rhizosphere phosphate-solubilizing bacterium Pseudomonas frederiksbergensis JW-SD2 and its plant growth-promoting effects on poplar seedlings. Ann. Microbiol. 2017, 67, 219–230. [Google Scholar] [CrossRef]
- Manter, D.K.; Vivanco, J.M. Use of the ITS primers, ITS1F and ITS4, to characterize fungal abundance and diversity in mixed-template samples by qPCR and length heterogeneity analysis. J. Microbiol. Methods 2007, 71, 7–14. [Google Scholar] [CrossRef]
- Sang, Y.; Jin, L.; Zhu, R.; Yu, X.Y.; Hu, S.; Wang, B.T.; Ruan, H.H.; Jin, F.J.; Lee, H.G. Phosphorus-Solubilizing Capacity of Mortierella Species Isolated from Rhizosphere Soil of a Poplar Plantation. Microorganisms 2022, 10, 2361. [Google Scholar] [CrossRef]
- Rahman, M.S.; Quadir, Q.F.; Rahman, A.; Nahar, M. Screening and characterization of Phosphorus solubilizing Bacteria and their effect on Rice seedlings. Res. Agric. Livest. Fish. 2015, 1, 27–35. [Google Scholar] [CrossRef]
- Premono, M.E.; Moawad, A.M.; Vlek, P.L.G. Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones. J. Crop Sci. 1996, 11, 13. [Google Scholar]
- Adelowo, F.E.; Agele, S.O. Spectrophotometric analysis of phosphate concentration in agricultural soil samples and water samples using molybdenum blue method. Braz. J. Biol. 2016, 3, 407–412. [Google Scholar] [CrossRef]
- Li, H.Y.; Yao, T.; Zhang, R.; Zhang, J.; Li, Z.Y.; Rong, L.Y.; Lu, X.W.; Yang, X.L.; Xia, D. Relationship between organic acids secreted from rhizosphere phosphate-solubilizing bacteria in Trifolium pratense and phosphate-solubilizing ability. Acta Pratacult. Sin. 2018, 27, 113–121. [Google Scholar]
- Javed, S.; Azeem, M.; Mahmood, S.; Al-Anazi, K.M.; Farah, M.A.; Ali, S.; Ali, B. Biotransformation of Agricultural Wastes into Lovastatin and Optimization of a Fermentation Process Using Response Surface Methodology (RSM). Agronomy 2022, 12, 2848. [Google Scholar] [CrossRef]
- Sauka, D.H.; Piccinetti, C.F.; Vallejo, D.A.; Onco, M.I.; Pérez, M.P.; Benintende, G.B. New entomopathogenic strain of Bacillus thuringiensis is able to solubilize different sources of inorganic phosphates. Appl. Soil Ecol. 2021, 160, 103839. [Google Scholar] [CrossRef]
- Cavello, I.A.; Crespo, J.M.; García, S.S.; Zapiola, J.M.; Luna, M.F.; Cavalitto, S.F. Plant growth promotion activity of keratinolytic fungi growing on a recalcitrant waste known as Hair Waste. Biotechnol. Res. Intern. 2015, 6, 952921. [Google Scholar] [CrossRef]
- Gupta, R.; Singal, R.; Shankar, A.; Kuhad, R.; Saxena, R.K. A modified plate assay for screening phosphate solubilizing microorganisms. J. Gen. Appl. Microbiol. 1994, 40, 255–260. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, J.; Zhang, J.Q.; Xu, W.L.; Mou, Z.S. Characteristics of inorganic phosphate-solubilizing bacteria from the sediments of a eutrophic lake. Int. J. Environ. Res. Public Health 2019, 16, 2141. [Google Scholar] [CrossRef]
- Bashan, Y.; Kamnev, A.A.; De-Bashan, L.E. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: A proposal for an alternative procedure. Biol. Fertil. Soils 2013, 49, 465–479. [Google Scholar] [CrossRef]
- Pengnoo, A.; Hashidoko, Y.; Onthong, J.; Gimsanguan, S.; Sae-ong, M.; Shinano, T.; Watanabe, T.; Osaki, M. Screening of phosphate-solubilizing microorganisms in rhizosphere and rhizoplane of adverse soil-adapting plants in Southern Thailand. Tropics 2007, 16, 1–7. [Google Scholar] [CrossRef]
- Qureshi, A.S.; Dahot, U.; Panhwar, S.I. Biosynthesis of alkaline phosphatase by Escherichia coli. EFRL 13 in submerged fermentation. World Appl. Sci. J. 2010, 8, 50–56. [Google Scholar]
- Wang, J.; Zhao, Y.; Maqbool, F. Capability of Penicillium oxalicum Y2 to release phosphate from different insoluble phosphorus sources and soil. Folia Microbiol. 2021, 66, 69–77. [Google Scholar] [CrossRef]
- Doilom, M.; Guo, J.W.; Phookamsak, R.; Mortimer, P.E.; Karunarathna, S.C.; Dong, W.; Liao, C.F.; Yan, K.; Pem, D.; Suwannarach, N.; et al. Screening of phosphate-solubilizing fungi from air and soil in Yunnan, China: Four novel species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front. Microbiol. 2020, 11, 585215. [Google Scholar] [CrossRef]
- Hernández-Leal, T.I.; Carrión, G.; Heredia, G. Solubilización in vitro de fosfatos por una cepa de Paecilomyces lilacinus (Thom) Samson. Agrociencia 2011, 45, 881–892. [Google Scholar]
- Bayat, J.; Hashemi, S.H.; Khoshbakht, K.; Deihimfard, R. Interpolation of the Soil Nutrients (Nitrate and Phosphate), Organic Carbon, EC and pH in Agricultural Lands at the South of Tehran City. J. Environ. Sci. 2016, 4, 1–12. [Google Scholar]
- Mehta, P.; Walia, A.; Kakkar, N.; Shirkot, C.K. Tricalcium phosphate solubilisation by new endophyte Bacillus methylotrophicus CKAM isolated from apple root endosphere and its plant growth-promoting activities. Acta Physiol. Plant. 2014, 36, 2033–2045. [Google Scholar] [CrossRef]
- Efthymiou, A.K.; Jensen, B.; Jakobsen, I. The roles of mycorrhiza and Penicillium inoculants in phosphorus uptake by biochar-amended wheat. Soil Biol. Biochem. 2018, 127, 168–177. [Google Scholar] [CrossRef]
- Wei, Z.; Zuo, H.; Li, J.; Ding, G.C.; Zhan, Y.B.; Zhang, L.; Wu, W.L.; Su, L.H.; Wei, Y.Q. Insight into the mechanisms of insoluble phosphate transformation driven by the interactions of compound microbes during composting. Environ. Sci. Pollut. Res. 2021, 28, 32844–32855. [Google Scholar] [CrossRef] [PubMed]
- Illmer, P.; Schinner, F. Solubilization of inorganic phosphates by microorganisms isolated from forest soils. Soil Biol. Biochem. 1992, 24, 389–395. [Google Scholar] [CrossRef]
- Nelofer, R.; Syed, Q.; Nadeem, M.; Bashir, F.; Mazhar, S.; Hassan, A. Isolation of phosphate-solubilizingfungus from soil to supplement biofertilizer. Arab. J. Sci. Eng. 2016, 41, 2131–2138. [Google Scholar] [CrossRef]
- Teng, Z.; Shao, W.; Zhang, K.; Huo, Y.Q.; Li, M. Characterization of phosphate solubilizing bacteria isolated from heavy metal contaminated soils and their potential for lead immobilization. J. Env. Manag. 2019, 231, 189–197. [Google Scholar] [CrossRef]
- Saber, W.; El-Naggar, E.A.; Abdal-Aziz, A.A. Bioconversion of Lignocellulosic Wastes into Organic Acids by Cellulolytic Rock Phosphate-Solubilizing Fungal Isolates Grown under Solid-State Fermentation Conditions. Res. J. Microbiol. 2010, 5, 1–20. [Google Scholar] [CrossRef]
- Perrig, D.; Boiero, M.L.; Masciarelli, O.A.; Penna, C.; Ruiz, O.A.; Cassán, F.D.; Luna, M.V. Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl. Microbiol. Biotechnol. 2007, 75, 1143–1150. [Google Scholar] [CrossRef]
- Panda, R.; Panda, S.; Panda, C. Solubilisation of Uganda low grade Rock Phosphate by Pseudomonas fluorescence. Res. J. Recent. Sci. 2013, 2, 250–254. [Google Scholar]
- Reyes, I.; Bernier, L.; Antoun, H. Rock phosphate solubilization and colonization of maize rhizosphere by wild and genetically modifified strains of Penicillium rugulosum. Microb. Ecol. 2002, 44, 39–48. [Google Scholar] [CrossRef]
- Tahir, M.A.; Ibrahim, M.; Sarwar, G.; Iftikhar, Y.; SangKeun, H.; KyungHwa, H.; YongSeon, Z. Impact of indigenous industrial compost on the growth of coarse and fine rice varieties under saline environment. Pertanika J. Trop. Agric. Sci. 2013, 36, 61–70. [Google Scholar]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Archana, G.; Buch, A.; Kumar, G.N. Pivotal role of organic aciBarley genotypes differ in capacity of soluble extracellular phosphatase and depletion of organic phosphorus in the rhizosphere soid secretion by rhizobacteria in plant Growth promotion. In Microorganisms in Sustainable Agriculture and Biotechnology, Part 1; Satyanarayana, T., Johri, B.N., Prakash, A., Eds.; Springer Science and Business Media BV: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Sulbaran, M.; Perez, E.; Ball, M.M.; Bahsas, A.; Yarzábal, L.A. Characterization of the mineral phosphate-solubilizing capacity of Pantoea aglomerans MMB051 isolated from an iron-rich soil in southeastern Venezuela (Bolivar State). Curr. Microbiol. 2009, 4, 58. [Google Scholar]
- Vassilev, N.; Mendes, G.; Costa, M.; Vassileva, M. Biotechnological Tools for Enhancing Microbial Solubilization of Insoluble Inorganic Phosphates. Geomicrobiol. J. 2014, 31, 751–763. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, P.S.; Zhang, B.X.; Wang, Y.P.; Meng, J.; Gao, Y.F.; He, X.M.; Hu, X.M. Identification of phosphate-solubilizing microorganisms and determination of their phosphate-solubilizing activity and growth-promoting capability. BioResources 2020, 15, 2560–2578. [Google Scholar] [CrossRef]
- Fatima, F.; Ahmad, M.M.; Verma, S.R.; Pathak, N. Relevance of phosphate solubilizing microbes in sustainable crop production: A review. Int. J. Environ. Sci. Technol. 2022, 19, 9283–9296. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, F.; Ma, L. Phosphate-solubilizing bacteria from safflower rhizosphere and their effect on seedling growth. Open Life Sci. 2019, 14, 246–254. [Google Scholar] [CrossRef]
- Osorno, L.; Osorio, N.W. Effect of carbon and nitrogen source and concentration on rock phosphate dissolution induced by fungi. J. Appl. Biotechnol. 2014, 2, 32. [Google Scholar] [CrossRef]
- Chai, B.; Wang, S.; Li, S.; Lei, X.H.; Li, M. Roles of bacterial biomass, physiology and community in sediment phosphorus solubilizing at varying hydrostatic pressures. J. Clean. Prod. 2021, 282, 124531. [Google Scholar] [CrossRef]
- Qiu, S.; Lian, B. Weathering of phosphorus-bearing mineral powder and calcium phosphate by Aspergillus niger. Chin. J. Geochem. 2012, 31, 390–397. [Google Scholar] [CrossRef]
- Xu, J.; Huang, L.; Chen, C.; Wang, J.; Long, X.X. Effective lead immobilization by phosphate rock solubilization mediated by phosphate rock amendment and phosphate solubilizing bacteria. Chemosphere 2019, 237, 124540. [Google Scholar] [CrossRef] [PubMed]


| Run | Concentration of Sucrose | Concentrations of Ammonium Sulfate and Soybean Residue | Concentration of Tricalcium Phosphate | Concentration of Inorganic Salt | Phosphate-Solubilizing Capacity (mg/L) |
|---|---|---|---|---|---|
| 0 | −1 | 0 | 1 | 85.47 | |
| 2 | 0 | 0 | 1 | 1 | 65.77 |
| 3 | 0 | 1 | 1 | 0 | 65.99 |
| 4 | 1 | 0 | −1 | 0 | 79.00 |
| 5 | 0 | 1 | −1 | 0 | 96.67 |
| 6 | 1 | −1 | 0 | 0 | 42.09 |
| 7 | 0 | 0 | −1 | −1 | 90.00 |
| 8 | 0 | −1 | 0 | −1 | 50.95 |
| 9 | 0 | 0 | 0 | 0 | 120.67 |
| 10 | −1 | 0 | 0 | 1 | 92.16 |
| 11 | 1 | 0 | 0 | −1 | 50.44 |
| 12 | 1 | 0 | 0 | 1 | 93.00 |
| 13 | 0 | −1 | −1 | 0 | 56.57 |
| 14 | 1 | 1 | 0 | 0 | 68.76 |
| 15 | −1 | −1 | 0 | 0 | 76.91 |
| 16 | 0 | −1 | 1 | 0 | 46.42 |
| 17 | 0 | 1 | 0 | 1 | 98.05 |
| 18 | 0 | 0 | 1 | −1 | 60.09 |
| 19 | −1 | 0 | 1 | 0 | 53.62 |
| 20 | 0 | 0 | 0 | 0 | 123.72 |
| 21 | 0 | 0 | 0 | 0 | 116.12 |
| 22 | −1 | 0 | 0 | −1 | 90.93 |
| 23 | 1 | 0 | 1 | 0 | 42.17 |
| 24 | 0 | 0 | −1 | 1 | 101.72 |
| 25 | 0 | 1 | 0 | −1 | 96.00 |
| 26 | 0 | 0 | 0 | 0 | 108.23 |
| 27 | −1 | 1 | 0 | 0 | 100.98 |
| 28 | −1 | 0 | −1 | 0 | 102.13 |
| 29 | 0 | 0 | 0 | 0 | 115.55 |
| Strain | d (mm) | D (mm) | PSI |
|---|---|---|---|
| PSF7 | 1.99 ± 0.09 b | 2.29 ± 0.10 b | 1.15 ± 0.003 a |
| Oxalate Acid | Tartaric Acid | Formic Acid | Malic Acid | Lactic Acid | Acetic Acid | Fumaric Acid | Citric Acid | |
|---|---|---|---|---|---|---|---|---|
| Organic acid concentration (mg/L) | 168.66 ± 4.07 | 243.08 ± 15.36 | 239.88 ± 8.97 | 201.50 ± 7.44 | 56.29 ± 16.17 | 28.63 ± 12.02 | 45.09 ± 18.27 | 58.43 ± 13.55 |
| Source of Variance | Sum of Squares | Freedom | Variance | F Value | p | Significance |
|---|---|---|---|---|---|---|
| Regression model | 16,817.49 | 14 | 1201.25 | 25.42 | <0.0001 | significant |
| A | 1663.1 | 1 | 1663.1 | 35.19 | <0.0001 | ** |
| B | 2353.12 | 1 | 2353.12 | 49.79 | <0.0001 | ** |
| C | 3072.96 | 1 | 3072.96 | 65.02 | <0.0001 | ** |
| D | 796.42 | 1 | 796.42 | 16.85 | 0.0011 | ** |
| AB | 1.69 | 1 | 1.69 | 0.036 | 0.8527 | |
| AC | 34.11 | 1 | 34.11 | 0.72 | 0.4099 | |
| AD | 427.04 | 1 | 427.04 | 9.04 | 0.0094 | ** |
| BC | 105.37 | 1 | 105.37 | 2.23 | 0.1576 | |
| BD | 263.58 | 1 | 263.58 | 5.58 | 0.0332 | * |
| CD | 9.12 | 1 | 9.12 | 0.19 | 0.6672 | |
| A2 | 3182.61 | 1 | 3182.61 | 67.34 | <0.0001 | ** |
| B2 | 3451.35 | 1 | 3451.35 | 73.02 | <0.0001 | ** |
| C2 | 4437.53 | 1 | 4437.53 | 93.89 | <0.0001 | ** |
| D2 | 911.14 | 1 | 911.14 | 19.28 | 0.0006 | ** |
| Residual | 661.68 | 14 | 47.26 | |||
| Misfit term | 523.36 | 10 | 52.34 | 1.51 | 0.3668 | |
| Pure error | 138.32 | 4 | 34.58 | |||
| Total | 17,479.17 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-L.; Qiu, S.-Y.; Zhou, S.-Q.; Xu, Z.-H.; Liu, X.-T. Phosphate-Solubilizing Capacity of Paecilomyces lilacinus PSF7 and Optimization Using Response Surface Methodology. Microorganisms 2023, 11, 454. https://doi.org/10.3390/microorganisms11020454
Wang X-L, Qiu S-Y, Zhou S-Q, Xu Z-H, Liu X-T. Phosphate-Solubilizing Capacity of Paecilomyces lilacinus PSF7 and Optimization Using Response Surface Methodology. Microorganisms. 2023; 11(2):454. https://doi.org/10.3390/microorganisms11020454
Chicago/Turabian StyleWang, Xue-Li, Shu-Yi Qiu, Shao-Qi Zhou, Zhi-Hu Xu, and Xue-Ting Liu. 2023. "Phosphate-Solubilizing Capacity of Paecilomyces lilacinus PSF7 and Optimization Using Response Surface Methodology" Microorganisms 11, no. 2: 454. https://doi.org/10.3390/microorganisms11020454





