Antimicrobial Exposure in Critically Ill Patients with Sepsis-Associated Multi-Organ Dysfunction Requiring Extracorporeal Organ Support: A Narrative Review
Abstract
:1. Introduction
2. PK/PD Considerations
- −
- S—susceptible, standard dosing regimen: high likelihood of therapeutic success using a standard dosing regimen of the antimicrobial;
- −
- I—susceptible, increased exposure: high likelihood of therapeutic success if antimicrobial exposure is improved by increasing the dosing regimen in order to reach high drug concentration at the site of infection;
- −
- R—resistant: high likelihood of therapeutic failure even for increased exposure.
2.1. PK Alterations Induced by Sepsis
- ➢
- Solubility is the major determinant of antimicrobial volume of distribution (Vd), which is the theoretical volume necessary to contain the total amount of the drug at the same concentration measured at the steady state in the plasma. The Vd drives the titration of the loading dose of antimicrobials [26].Specifically, hydrophilic antimicrobials (e.g., ß-lactams, aminoglicosides, glycopeptides, daptomycin and polymyxins) are characterized by small Vd (<2 L/kg), being mainly concentrated into the bloodstream. However, sepsis-associated endothelial dysfunction and capillary leak syndrome coupled with fluid overload due to large fluid resuscitation and oliguric acute kidney injury (AKI), may lead to extravascular fluid shift and, consequently, low bloodstream concentration of hydrophilic antimicrobials. This condition implies an increase in the loading dose in order to secure an effective exposure to these drugs. In contrast, lipophilic antimicrobials (e.g., fluoroquinolones, glycocyclines, lincosamides, macrolides) are characterized by large Vd (>2 L/kg) and are not significantly influenced by fluid shift, being mainly concentrated in the adipose tissue.
- ➢
- ➢
- In critically ill patients, hypoalbuminemia has been frequently reported [27], and this condition may alter the PK profile of highly-protein bound antimicrobials (e.g., ß-lactams, glycopeptides, glycylcyclines, lincosamides, daptomycin and macrolides), leading to augmented free fraction, Vd and clearance.
- ➢
- Electrostatic interactions regulate the degree of antimicrobial ionization and free fraction, the amount of which relies on the pKa of the drug. Specifically, the free fraction of weak basis-like antimicrobials is increased by pH of tissues below pKa, while the dissociation of weak acid-like antimicrobials is favored by local pH above the pKa [21].
- ➢
- Molecular size influences drug excretion. Small molecules are preferentially filtered by the kidney into urine and large molecules are secreted by the liver into the bile [21].
- ➢
- Clearance refers to the volume of plasma purified from antimicrobials per unit of time and drives the titration of the maintenance dose [26]. Catabolism and excretion of hydrophilic antimicrobials take place predominantly in the kidney (e.g., ß-lactams, aminoglucosides, glycopeptides, daptomycin, oxazolidones, polymyxins), while the liver plays a role of paramount importance for lipophilic antimicrobials (e.g., glycylcyclines and macrolides). Sepsis-associated MOD may induce alterations of antimicrobial metabolism [21] leading to suboptimal exposure or intoxication. In the early phases of sepsis, hyperdynamic states due to cardiac output increase may enhance glomerular filtration rate, leading to augmented renal clearance [6,14] and suboptimal antimicrobial exposure when prescribed at standard dosing regimens. On the contrary, sepsis is the major cause of AKI [28], and this condition may increase the risk of antimicrobial accumulation and toxicity.
2.2. PK Alterations Induced by ECOS
3. Potential Pitfalls in the Evaluation of ECOS-Related Antimicrobial PK Alterations
4. Renal Replacement Therapy
4.1. Membrane Characteristics
4.2. Setting
4.3. Central Venous Catheters Tip Location
5. Therapeutic Plasma Exchange
6. Coupled Plasma Filtration and Absorption
7. Hemoperfusion
7.1. Polymyxin B-Immoilized Cartridge (Toraymyxin)
7.2. Porous Polystyrene Cartridge (Cytosorb)
7.3. Microbind Affinity Blood Filter (Seraph 100)
8. Extracorporeal CO2 Removal and Extracorporeal Membrane Oxygenation
9. Effective Antimicrobial Dosing Strategies during ECOS
- Unit-level interventions: this strategy includes the administration of antimicrobials driven by the PD characteristics of the drug, leading to the administration of time-dependent antimicrobials (β-lactams [90] and Linezolid [91]) via continuous infusion. However, no evidence exists on the implications of this strategy on the stability of antimicrobials bloodstream concentration during ECOS.
- PK/PD-based antimicrobial dosing program: this strategy includes the use of software that performs a PK assessment based on patient-specific characteristics in conjunction with population pharmacokinetic models, via a Bayesian parametric approach and a Monte Carlo simulation [94]. However, this strategy has never been tested in critically ill patients who receive ECOS.
- Therapeutic Drug Monitoring (TDM): this strategy includes the evaluation of antimicrobial exposure via the assessment of drug concentration at the site of infection (e.g., blood, epithelial lining fluid or cerebrospinal fluid) and implies dose adjustment according to the pathogen susceptibility to the drug (MIC), in order to improve the PK/PD target attainment. In most of the cases, plasma concentration has been used as a surrogate for antimicrobial exposure at the source of infection [17]. TDM may play a role of paramount importance in the dose adjustment of antimicrobials with intra- and/or inter-individual PK variability and narrow therapeutic index [17], especially when PK characteristics are unknown or difficult to predict due to the patient’s clinical severity and instability. Accordingly, recently published guidelines recommended the TDM use for dose titration of ß-lactams, aminoglycosides, linezolid, teicoplanin, vancomycin and voriconazole [17]. In critically ill patients who receive RRT and ECMO [95,96,97], TDM has been largely used for adjusting the dose of ß-lactams, aminoglycosides, linezolid, teicoplanin, glycopeptides and colistin [17]. Furthermore, some evidence has been reported for daptomycin, fluoroquinolones and Tigecycline [17], although it warrants further investigation.
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.; Chiche, J.; Coopersmith, C.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.; Johnson, S.; Agesa, K.; Shackelford, K.; Tsoi, D.; Kievlan, D.; Colombara, D.; Ikuta, K.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Paul, S.; Akova, M.; Bassetti, M.; Waele, J.D.; Dimopoulos, G.; Kaukonen, K.; Koulenti, D.; Martin, C.; Montravers, P.; et al. Dali: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.; Gesten, F.; Prescott, H.; Friedrich, M.; Iwashyna, T.; Phillips, G.; Lemeshow, S.; Osborn, T.; Terry, K.; Levy, M. Time to treatment and mortality during mandated emergency care for sepsis. N. Engl. J. Med. 2017, 376, 2235–2244. [Google Scholar] [CrossRef]
- Pascale, G.D.; Antonelli, M.; Deschepper, M.; Arvaniti, K.; Blot, K.; Brown, B.; Lange, D.d.; De Waele, J.; Dikmen, Y.; Dimopoulos, G.; et al. Poor timing and failure of source control are risk factors for mortality in critically ill patients with secondary peritonitis. Intensive Care Med. 2022, 48, 1593–1606. [Google Scholar] [CrossRef]
- Udy, A.; Roberts, J.; Boots, R.; Paterson, D.; Lipman, J. Augmented renal clearance: Implications for antibacterial dosing in the critically ill. Clin. Pharmacokinet. 2010, 49, 1–16. [Google Scholar] [CrossRef]
- Jamal, J.; Economou, C.; Lipman, J.; Roberts, J. Improving antibiotic dosing in special situations in the icu: Burns, renal replacement therapy and extracorporeal membrane oxygenation. Curr. Opin. Crit. Care 2012, 18, 460–471. [Google Scholar] [CrossRef]
- Ranieri, V.; Brodie, D.; Vincent, J. Extracorporeal organ support: From technological tool to clinical strategy supporting severe organ failure. JAMA 2017, 318, 1105–1106. [Google Scholar] [CrossRef]
- Husain-Syed, F.; Ricci, Z.; Brodie, D.; Vincent, J.; Ranieri, V.; Slutsky, A.; Taccone, F.; Gattinoni, L.; Ronco, C. Extracorporeal organ support (ecos) in critical illness and acute kidney injury: From native to artificial organ crosstalk. Intensive Care Med. 2018, 44, 1447–1459. [Google Scholar] [CrossRef]
- Villa, G.; Neri, M.; Bellomo, R.; Cerda, J.; De Gaudio, A.; De Rosa, S.; Garzotto, F.; Honore, P.; Kellum, J.; Lorenzin, A.; et al. Nomenclature for renal replacement therapy and blood purification techniques in critically ill patients: Practical applications. Crit. Care 2016, 20, 283. [Google Scholar] [CrossRef]
- Neri, M.; Villa, G.; Garzotto, F.; Bagshaw, S.; Bellomo, R.; Cerda, J.; Ferrari, F.; Guggia, S.; Joannidis, M.; Kellum, J.; et al. Nomenclature for renal replacement therapy in acute kidney injury: Basic principles. Crit. Care 2016, 20, 318. [Google Scholar] [CrossRef]
- Cutuli, S.; Carelli, S.; Grieco, D.; De Pascale, G. Immune modulation in critically ill septic patients. Medicina 2021, 57, 552. [Google Scholar] [CrossRef] [PubMed]
- Tängdén, T.; Martín, V.R.; Felton, T.; Nielsen, E.; Marchand, S.; Brüggemann, R.; Bulitta, J.; Bassetti, M.; Theuretzbacher, U.; Tsuji, B.; et al. The role of infection models and pk/pd modelling for optimising care of critically ill patients with severe infections. Intensive Care Med. 2017, 43, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.; Roberts, J.; Lipman, J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013, 39, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.; French, C.; Machado, F.; Mcintyre, L.; Ostermann, M.; Prescott, H.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Roger, C.; De Waele, J. Personalized antibiotic dosing for the critically ill. Intensive Care Med. 2019, 45, 715–718. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.; Alffenaar, J.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.; Paiva, J.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A position paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Dulhunty, J.; Paterson, D.; Webb, S.; Lipman, J. Antimicrobial utilisation in 37 australian and new zealand intensive care units. Anaesth. Intensive Care 2011, 39, 231–237. [Google Scholar] [CrossRef]
- Vincent, J.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.; Marshall, J.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Cotta, M.; Roberts, J.; Lipman, J. Antibiotic dose optimization in critically ill patients. Med. Intensiva 2015, 39, 563–572. [Google Scholar] [CrossRef]
- Charlton, M.; Thompson, J. Pharmacokinetics in sepsis. BJA Educ. 2019, 19, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Taccone, F.; Lipman, J. Understanding pk/pd. Intensive Care Med. 2016, 42, 1797–1800. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.; Muller, A.; Canton, R.; Giske, C.; Kahlmeter, G.; Turnidge, J. Mic-based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobrial Susceptibility Testing (EUCAST). Clinical Breakpoints and Dosing of Antibiotics 2023. Available online: https://www.eucast.org/ (accessed on 4 January 2023).
- Mouton, J.; Muller, A.; Canton, R.; Giske, C.; Kahlmeter, G.; Turnidge, J. Mic-based dose adjustment: Facts and fables-authors’ response. J. Antimicrob. Chemother. 2018, 73, 2585–2586. [Google Scholar] [CrossRef]
- Pistolesi, V.; Morabito, S.; Di Mario, F.; Regolisti, G.; Cantarelli, C.; Fiaccadori, E. A guide to understanding antimicrobial drug dosing in critically ill patients on renal replacement therapy. Antimicrob. Agents Chemother. 2019, 63, e00583-19. [Google Scholar] [CrossRef]
- Vincent, J.; Russell, J.; Jacob, M.; Martin, G.; Guidet, B.; Wernerman, J.; Ferrer, R.; McCluskey, S.; Gattinoni, L. Albumin administration in the acutely ill: What is new and where next? Crit. Care 2014, 18, 231. [Google Scholar] [CrossRef]
- Uchino, S.; Kellum, J.; Bellomo, R.; Doig, G.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Hoff, B.; Maker, J.; Dager, W.; Heintz, B. Antibiotic dosing for critically ill adult patients receiving intermittent hemodialysis, prolonged intermittent renal replacement therapy, and continuous renal replacement therapy: An update. Ann. Pharmacother. 2020, 54, 43–55. [Google Scholar] [CrossRef]
- Thompson, A.; Li, F.; Gross, A. Considerations for medication management and anticoagulation during continuous renal replacement therapy. AACN Adv. Crit. Care 2017, 28, 51–63. [Google Scholar] [CrossRef]
- Bauer, P.; Ostermann, M.; Russell, L.; Robba, C.; David, S.; Ferreyro, B.; Castro, J.; Juffermans, N.; Montini, L.; Pirani, T.; et al. Plasma exchange in the intensive care unit: A narrative review. Intensive Care Med. 2022, 48, 1382–1396. [Google Scholar] [CrossRef]
- Mariano, F.; Leporati, M.; Carignano, P.; Stella, M.; Vincenti, M.; Biancone, L. Efficient removal of colistin a and b in critically ill patients undergoing cvvhdf and sorbent technologies. J. Nephrol. 2015, 28, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Scharf, C.; Weinelt, F.; Schroeder, I.; Paal, M.; Weigand, M.; Zoller, M.; Irlbeck, M.; Kloft, C.; Briegel, J.; Liebchen, U. Does the cytokine adsorber cytosorb® reduce vancomycin exposure in critically ill patients with sepsis or septic shock? A prospective observational study. Ann. Intensive Care 2022, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Fraser, J.; Smith, M.; Roberts, J. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J. Crit. Care 2012, 27, 741. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.; Veita, J.; Laudanski, K. Antibiotics and ecmo in the adult population-persistent challenges and practical guides. Antibiotics 2022, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Gomersall, C.; Tian, Q.; Joynt, G.; Freebairn, R.; Lipman, J. Principles of antibacterial dosing in continuous renal replacement therapy. Crit. Care Med. 2009, 37, 2268–2282. [Google Scholar] [CrossRef]
- Wilson, R.; Soullier, G. The validity of two-hour creatinine clearance studies in critically ill patients. Crit. Care Med. 1980, 8, 281–284. [Google Scholar] [CrossRef]
- Hoste, E.J.; Kellum, J.; Selby, N.; Zarbock, A.; Palevsky, P.; Bagshaw, S.; Goldstein, S.; Cerdá, J.; Chawla, L. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- Bagshaw, S.; Uchino, S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin. J. Am. Soc. Nephrol. 2007, 2, 431–439. [Google Scholar] [CrossRef]
- Roberts, J.; Joynt, G.; Lee, A.; Choi, G.; Bellomo, R.; Kanji, S.; Mudaliar, M.Y.; Peake, S.; Stephens, D.; Taccone, F.; et al. The effect of renal replacement therapy and antibiotic dose on antibiotic concentrations in critically ill patients: Data from the multinational sampling antibiotics in renal replacement therapy study. Clin. Infect. Dis. 2021, 72, 1369–1378. [Google Scholar] [CrossRef]
- Legrand, M.; Darmon, M.; Joannidis, M.; Payen, D. Management of renal replacement therapy in icu patients: An international survey. Intensive Care Med. 2013, 39, 101–108. [Google Scholar] [CrossRef]
- Onichimowski, D.; Ziółkowski, H.; Nosek, K.; Jaroszewski, J.; Rypulak, E.; Czuczwar, M. Comparison of adsorption of selected antibiotics on the filters in continuous renal replacement therapy circuits: In vitro studies. J. Artif. Organs 2020, 23, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Ulldemolins, M.; Martín-Loeches, I.; Llauradó-Serra, M.; Fernández, J.; Vaquer, S.; Rodríguez, A.; Pontes, C.; Calvo, G.; Torres, A.; Soy, D. Piperacillin population pharmacokinetics in critically ill patients with multiple organ dysfunction syndrome receiving continuous venovenous haemodiafiltration: Effect of type of dialysis membrane on dosing requirements. J. Antimicrob. Chemother. 2016, 71, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, S.; Grieco, D.; De Pascale, G.; Antonelli, M. Hemadsorption. Curr. Opin. Anaesthesiol. 2021, 34, 113–118. [Google Scholar] [CrossRef]
- Wong, W.; Choi, G.; Gomersall, C.; Lipman, J. To increase or decrease dosage of antimicrobials in septic patients during continuous renal replacement therapy: The eternal doubt. Curr. Opin. Pharmacol. 2015, 24, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Pinder, M.; Bellomo, R.; Lipman, J. Pharmacological principles of antibiotic prescription in the critically ill. Anaesth. Intensive Care 2002, 30, 134–144. [Google Scholar] [CrossRef]
- Roberts, D.; Roberts, J.; Roberts, M.; Liu, X.; Nair, P.; Cole, L.; Lipman, J.; Bellomo, R.; Investigators, R.R.T.S. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: A multicentre pharmacokinetic study. Crit. Care Med. 2012, 40, 1523–1528. [Google Scholar] [CrossRef]
- Jamal, J.; Udy, A.; Lipman, J.; Roberts, J. The impact of variation in renal replacement therapy settings on piperacillin, meropenem, and vancomycin drug clearance in the critically ill: An analysis of published literature and dosing regimens. Crit. Care Med. 2014, 42, 1640–1650. [Google Scholar] [CrossRef]
- Roberts, D.; Liu, X.; Roberts, J.; Nair, P.; Cole, L.; Roberts, M.; Lipman, J.; Bellomo, R.; Investigators, R.R.T.S. A multicenter study on the effect of continuous hemodiafiltration intensity on antibiotic pharmacokinetics. Crit. Care 2015, 19, 84. [Google Scholar] [CrossRef]
- Joannes-Boyau, O.; Honoré, P.; Perez, P.; Bagshaw, S.; Grand, H.; Canivet, J.; Dewitte, A.; Flamens, C.; Pujol, W.; Grandoulier, A.; et al. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (ivoire study): A multicentre randomized controlled trial. Intensive Care Med. 2013, 39, 1535–1546. [Google Scholar] [CrossRef]
- Bakdach, D.; Elajez, R.; Bakdach, A.; Awaisu, A.; De Pascale, G.; Hssain, A. Pharmacokinetics, pharmacodynamics, and dosing considerations of novel β-lactams and β-lactam/β-lactamase inhibitors in critically ill adult patients: Focus on obesity, augmented renal clearance, renal replacement therapies, and extracorporeal membrane oxygenation. J. Clin. Med. 2022, 11, 6898. [Google Scholar]
- KDIGO AKI Work Group. Kdigo clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 17, 1–138. [Google Scholar]
- Vicka, V.; Vickiene, A.; Tutkus, J.; Stanaitis, J.; Bandzeviciute, R.; Ringaitiene, D.; Vosylius, S.; Sipylaite, J. Immediate aspiration of the drug infused via central venous catheter through the distally positioned central venous dialysis catheter: An experimental study. J. Vasc. Access 2021, 22, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kam, K.; Mari, J.; Wigmore, T. Adjacent central venous catheters can result in immediate aspiration of infused drugs during renal replacement therapy. Anaesthesia 2012, 67, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Frithiof, R.; Bandert, A.; Larsson, A.; Lipcsey, M.; Smekal, D. Central venous line and dialysis catheter position affects drug clearance during continuous renal replacement therapy in an animal model. ASAIO J. 2019, 65, 408–413. [Google Scholar] [CrossRef]
- Knaup, H.; Stahl, K.; Schmidt, B.; Idowu, T.; Busch, M.; Wiesner, O.; Welte, T.; Haller, H.; Kielstein, J.; Hoeper, M.; et al. Early therapeutic plasma exchange in septic shock: A prospective open-label nonrandomized pilot study focusing on safety, hemodynamics, vascular barrier function, and biologic markers. Crit. Care 2018, 22, 285. [Google Scholar] [CrossRef]
- Stahl, K.; Schmidt, J.; Seeliger, B.; Schmidt, B.; Welte, T.; Haller, H.; Hoeper, M.; Budde, U.; Bode, C.; David, S. Effect of therapeutic plasma exchange on endothelial activation and coagulation-related parameters in septic shock. Crit. Care 2020, 24, 71. [Google Scholar] [CrossRef]
- Krzych, Ł.; Czok, M.; Putowski, Z. Is antimicrobial treatment effective during therapeutic plasma exchange? Investigating the role of possible interactions. Pharmaceutics 2020, 12, 395. [Google Scholar] [CrossRef]
- Page, M.; Cohen, S.; Ber, C.; Allaouchiche, B.; Kellum, J.; Rimmelé, T. In vivo antibiotic removal during coupled plasma filtration adsorption: A retrospective study. ASAIO J. 2014, 60, 70–75. [Google Scholar] [CrossRef]
- Ricci, Z.; Romagnoli, S.; Reis, T.; Bellomo, R.; Ronco, C. Hemoperfusion in the intensive care unit. Intensive Care Med. 2022, 48, 1397–1408. [Google Scholar] [CrossRef]
- Fujii, T.; Ganeko, R.; Kataoka, Y.; Furukawa, T.; Featherstone, R.; Doi, K.; Vincent, J.; Pasero, D.; Robert, R.; Ronco, C.; et al. Polymyxin b-immobilized hemoperfusion and mortality in critically ill adult patients with sepsis/septic shock: A systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2018, 44, 167–178. [Google Scholar] [CrossRef]
- Early Use of Polymyxin B Hemoperfusion in the Abdominal Sepsis 2 Collaborative Group. Polymyxin b hemoperfusion in clinical practice: The picture from an unbound collaborative registry. Blood Purif. 2014, 37, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, S.; Artigas, A.; Fumagalli, R.; Monti, G.; Ranieri, V.; Ronco, C.; Antonelli, M.; EUPHAS 2 Collaborative Group. Polymyxin-B hemoperfusion in septic patients: Analysis of a multicenter registry. Ann. Intensive Care 2016, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, K.; Takakuwa, R.; Taya, K.; Wada, Y.; Yamazaki, N.; Murata, M.; Hirata, K.; Masuno, T.; Yokota, H.; Ishii, F. Adsorption of various antimicrobial agents to endotoxin removal polymyxin-b immobilized fiber (toraymyxin®). Colloids Surf. B Biointerfaces 2012, 90, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, K.; Takakuwa, R.; Wada, Y.; Yamazaki, N.; Ishii, F. Adsorption of various antimicrobial agents to endotoxin removal polymyxin-b immobilized fiber (toraymyxin®). Part 2: Adsorption of two drugs to toraymyxin pmx-20r cartridges. Colloids Surf. B Biointerfaces 2013, 101, 350–352. [Google Scholar] [CrossRef]
- Singhan, W.; Vadcharavivad, S.; Areepium, N.; Wittayalertpanya, S.; Chaijamorn, W.; Srisawat, N. The effect of direct hemoperfusion with polymyxin b immobilized cartridge on meropenem in critically ill patients requiring renal support. J. Crit. Care 2019, 51, 71–76. [Google Scholar] [CrossRef]
- Schneider, A.; André, P.; Scheier, J.; Schmidt, M.; Ziervogel, H.; Buclin, T.; Kindgen-Milles, D. Pharmacokinetics of anti-infective agents during cytosorb hemoadsorption. Sci. Rep. 2021, 11, 10493. [Google Scholar] [CrossRef] [PubMed]
- Schädler, D.; Pausch, C.; Heise, D.; Meier-Hellmann, A.; Brederlau, J.; Weiler, N.; Marx, G.; Putensen, C.; Spies, C.; Jörres, A.; et al. The effect of a novel extracorporeal cytokine hemoadsorption device on il-6 elimination in septic patients: A randomized controlled trial. PLoS ONE 2017, 12, e0187015. [Google Scholar] [CrossRef]
- Paul, R.; Sathe, P.; Kumar, S.; Prasad, S.; Aleem, M.; Sakhalvalkar, P. Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (cytosorb®) in patients with sepsis and septic shock. World J. Crit. Care Med. 2021, 10, 22–34. [Google Scholar] [CrossRef]
- Berlot, G.; Bella, S.D.; Tomasini, A.; Roman-Pognuz, E. The effects of hemoadsorption on the kinetics of antibacterial and antifungal agents. Antibiotics 2022, 11, 180. [Google Scholar] [CrossRef]
- Seffer, M.; Cottam, D.; Forni, L.; Kielstein, J. Heparin 2.0: A new approach to the infection crisis. Blood Purif. 2021, 50, 28–34. [Google Scholar] [CrossRef]
- Schmidt, J.; Eden, G.; Seffer, M.; Winkler, M.; Kielstein, J. In vitro elimination of anti-infective drugs by the seraph® 100 microbind® affinity blood filter. Clin. Kidney J. 2020, 13, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Auzinger, G.; Capellier, G.; Du Cheyron, D.; Clement, I.; Consales, G.; Dabrowski, W.; De Bels, D.; Ortiz, F.d.M.; Gottschalk, A.; et al. ECCO2R therapy in the icu: Consensus of a european round table meeting. Crit. Care 2020, 24, 490. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Coppola, S.; Camporota, L. Physiology of extracorporeal CO2 removal. Intensive Care Med. 2022, 48, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Mosier, J.; Kelsey, M.; Raz, Y.; Gunnerson, K.; Meyer, R.; Hypes, C.; Malo, J.; Whitmore, S.; Spaite, D. Extracorporeal membrane oxygenation (ecmo) for critically ill adults in the emergency department: History, current applications, and future directions. Crit. Care 2015, 19, 431. [Google Scholar] [CrossRef]
- Wildschut, E.; Ahsman, M.; Allegaert, K.; Mathot, R.; Tibboel, D. Determinants of drug absorption in different ecmo circuits. Intensive Care Med. 2010, 36, 2109–2116. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, H.; Zhang, Q.; Ou, Q.; Zhou, H.; Sha, T.; Zeng, Z.; Wu, J.; Lu, J.; Chen, Z. Effects of ex vivo extracorporeal membrane oxygenation circuits on sequestration of antimicrobial agents. Front. Med. 2021, 8, 748769. [Google Scholar] [CrossRef]
- Raffaeli, G.; Cavallaro, G.; Allegaert, K.; Koch, B.; Mosca, F.; Tibboel, D.; Wildschut, E. Sequestration of voriconazole and vancomycin into contemporary extracorporeal membrane oxygenation circuits: An in vitro study. Front. Pediatr. 2020, 8, 468. [Google Scholar] [CrossRef]
- Cheng, V.; Abdul-Aziz, M.; Roberts, J.; Shekar, K. Optimising drug dosing in patients receiving extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10, S629–S641. [Google Scholar] [CrossRef]
- Wi, J.; Noh, H.; Min, K.; Yang, S.; Jin, B.; Hahn, J.; Bae, S.; Kim, J.; Park, M.; Choi, D.; et al. Population pharmacokinetics and dose optimization of teicoplanin during venoarterial extracorporeal membrane oxygenation. Antimicrob. Agents Chemother. 2017, 61, e01015–e01017. [Google Scholar] [CrossRef]
- Daele, R.V.; Bekkers, B.; Lindfors, M.; Broman, L.; Schauwvlieghe, A.; Rijnders, B.; Hunfeld, N.; Juffermans, N.; Taccone, F.; Sousa, C.; et al. A large retrospective assessment of voriconazole exposure in patients treated with extracorporeal membrane oxygenation. Microorganisms 2021, 9, 1543. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.; Kim, J.; Jung, W.; Heo, W.; Kim, Y.; Kim, S.; No, T.; Jo, K.; Ko, J.; et al. Pharmacokinetics and monte carlo simulation of meropenem in critically ill adult patients receiving extracorporeal membrane oxygenation. Front. Pharmacol. 2021, 12, 2984. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, W.; Wang, Q.; Li, M.; Zhang, Z.; Cui, G.; Li, P.; Zhang, X.; Ma, Y.; Zhan, Q.; et al. Influence of venovenous extracorporeal membrane oxygenation on pharmacokinetics of vancomycin in lung transplant recipients. J. Clin. Pharm. Ther. 2020, 45, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Donadello, K.; Roberts, J.; Cristallini, S.; Beumier, M.; Shekar, K.; Jacobs, F.; Belhaj, A.; Vincent, J.; de Backer, D.; Taccone, F. Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: A matched cohort study. Crit. Care 2014, 18, 632. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Halwick, D.; Dodson, B.; Thompson, J.; Arnold, J. Potential drug sequestration during extracorporeal membrane oxygenation: Results from an ex vivo experiment. Intensive Care Med. 2007, 33, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Roberts, J.; Mcdonald, C.; Fisquet, S.; Barnett, A.; Mullany, D.; Ghassabian, S.; Wallis, S.; Fung, Y.; Smith, M.; et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit. Care 2012, 16, R194. [Google Scholar] [CrossRef] [PubMed]
- Spriet, I.; Annaert, P.; Meersseman, P.; Hermans, G.; Meersseman, W.; Verbesselt, R.; Willems, L. Pharmacokinetics of caspofungin and voriconazole in critically ill patients during extracorporeal membrane oxygenation. J. Antimicrob. Chemother. 2009, 63, 767–770. [Google Scholar] [CrossRef]
- Shekar, K.; Abdul-Aziz, M.; Cheng, V.; Burrows, F.; Buscher, H.; Cho, Y.; Corley, A.; Diehl, A.; Gilder, E.; Jakob, S.; et al. Antimicrobial exposures in critically ill patients receiving extracorporeal membrane oxygenation. Am. J. Respir. Crit. Care Med. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Roberts, J.; Kruger, P.; Paterson, D.; Lipman, J. Antibiotic resistance—What’s dosing got to do with it? Crit. Care Med. 2008, 36, 2433–2440. [Google Scholar] [CrossRef]
- Dulhunty, J.; Roberts, J.; Davis, J.; Webb, S.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.; Myburgh, J.; Paterson, D.; et al. A multicenter randomized trial of continuous versus intermittent β-lactam infusion in severe sepsis. Am. J. Respir. Crit. Care Med. 2015, 192, 1298–1305. [Google Scholar] [CrossRef]
- De Pascale, G.; Fortuna, S.; Tumbarello, M.; Cutuli, S.; Vallecoccia, M.; Spanu, T.; Bello, G.; Montini, L.; Pennisi, M.; Navarra, P.; et al. Linezolid plasma and intrapulmonary concentrations in critically ill obese patients with ventilator-associated pneumonia: Intermittent vs continuous administration. Intensive Care Med. 2015, 41, 103–110. [Google Scholar] [CrossRef]
- Pea, F.; Viale, P.; Cojutti, P.; Furlanut, M. Dosing nomograms for attaining optimum concentrations of meropenem by continuous infusion in critically ill patients with severe gram-negative infections: A pharmacokinetics/pharmacodynamics-based approach. Antimicrob. Agents Chemother. 2012, 56, 6343–6348. [Google Scholar] [CrossRef] [PubMed]
- Cristallini, S.; Hites, M.; Kabtouri, H.; Roberts, J.; Beumier, M.; Cotton, F.; Lipman, J.; Jacobs, F.; Vincent, J.; Creteur, J.; et al. New regimen for continuous infusion of vancomycin in critically ill patients. Antimicrob. Agents Chemother. 2016, 60, 4750–4756. [Google Scholar] [CrossRef] [PubMed]
- Heil, E.; Nicolau, D.; Farkas, A.; Roberts, J.; Thom, K. Pharmacodynamic target attainment for cefepime, meropenem, and piperacillin-tazobactam using a pharmacokinetic/pharmacodynamic-based dosing calculator in critically ill patients. Antimicrob. Agents Chemother. 2018, 62, e01008–e01018. [Google Scholar] [CrossRef] [PubMed]
- Bouglé, A.; Dujardin, O.; Lepère, V.; Hamou, N.; Vidal, C.; Lebreton, G.; Salem, J.; El-Helali, N.; Petijean, G.; Amour, J. Pharmecmo: Therapeutic drug monitoring and adequacy of current dosing regimens of antibiotics in patients on extracorporeal life support. Anaesth. Crit. Care Pain Med. 2019, 38, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Kühn, D.; Metz, C.; Seiler, F.; Wehrfritz, H.; Roth, S.; Alqudrah, M.; Becker, A.; Bracht, H.; Wagenpfeil, S.; Hoffmann, M.; et al. Antibiotic therapeutic drug monitoring in intensive care patients treated with different modalities of extracorporeal membrane oxygenation (ecmo) and renal replacement therapy: A prospective, observational single-center study. Crit. Care 2020, 24, 664. [Google Scholar] [CrossRef]
- Matusik, E.; Boidin, C.; Friggeri, A.; Richard, J.; Bitker, L.; Roberts, J.; Goutelle, S. Therapeutic drug monitoring of antibiotic drugs in patients receiving continuous renal replacement therapy or intermittent hemodialysis: A critical review. Ther. Drug Monit. 2022, 44, 86–102. [Google Scholar] [CrossRef]
- Tabah, A.; Waele, J.D.; Lipman, J.; Zahar, J.; Cotta, M.; Barton, G.; Timsit, J.; Roberts, J.; Working Group for Antimicrobial Use in the ICU within the Infection Section of the European Society of Intensive Care Medicine (ESICM). The ADMIN-ICU survey: A survey on antimicrobial dosing and monitoring in icus. J. Antimicrob. Chemother. 2015, 70, 2671–2677. [Google Scholar] [CrossRef]
- Wong, G.; Brinkman, A.; Benefield, R.; Carlier, M.; De Waele, J.; Helali, N.E.; Frey, O.; Harbarth, S.; Huttner, A.; McWhinney, B.; et al. An international, multicentre survey of β-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J. Antimicrob. Chemother. 2014, 69, 1416–1423. [Google Scholar] [CrossRef]

| Antimicrobials | PK/PD Index | Free Fraction% | Volume of Distribution (L kg−1) | Route of Elimination |
|---|---|---|---|---|
| ANTIBIOTICS | ||||
| β-lactams | ||||
| Amoxicillin/clavulanate | fT>MIC | 82/75 | 0.36/0.21 | R/L |
| Piperacillin/Tazobactam | fT>MIC | 70/78 | 0.24/0.40 | R |
| Oxacillin | fT>MIC | 6–10 | 0.4 | R |
| Ceftriaxone | fT>MIC | 10 | 0.1–0.2 | R/L |
| Cefepime | fT>MIC | 84 | 0.3 | R |
| Ceftaroline | fT>MIC | 80 | 0.29 | R |
| Ceftazidime | fT>MIC | 90 | 0.28–0.40 | R |
| Ceftazidime/Avibactam | fT>MIC | 90/92 | 0.28/0.31 | R |
| Ceftolozane/Tazobactam | fT>MIC | 80/78 | 0.19/0.40 | R |
| Cefiderocol | fT>MIC | 60 | 0.26 | R |
| Meropenem | fT>MIC | 98 | 0.35 | R |
| Imipenem/Cilastatin | fT>MIC | 80/56 | 0.22–0.24 | R |
| Meropenem/Vaborbactam | fT>MIC | 98/77 | 0.28/0.25 | R |
| Aminoglycosides | ||||
| Amikacin | Cmax/MIC | >95 | 0.22–0.5 | R |
| Gentamicin | Cmax/MIC | >95 | 0.36 | R |
| Glyco-, glycolipo- and Lipopeptides | ||||
| Daptomycin | AUC24/MIC | 20 | 0.1–0.13 | R |
| Teicoplanin | AUC24/MIC | 10–40 | 0.5–1.2 | R |
| Vancomycin | AUC24/MIC | 50–90 | 0.47–1.1 | R |
| Glycylcycline | ||||
| Tigecycline | AUC24/MIC | 11–29 | 0.12 | L |
| Lincosamides | ||||
| Clindamycin | AUC24/MIC | 5–15 | 1.1 | L |
| Macrolides | ||||
| Azithromycin | AUC24/MIC | 50–93 | 0.47 | L |
| Monobactam | ||||
| Aztreonam | fT>MIC | 44 | 0.18 | R |
| Nitroimidazoles | ||||
| Metronidazole | AUC24/MIC | 80 | 0.6–0.85 | R |
| Oxazolidinones | ||||
| Linezolid | AUC24/MIC | 70 | 0.5–0.8 | L |
| Tedizolid | AUC24/MIC | 50–90 | 0.95–1.14 | L |
| Polymyxins | ||||
| Colistin | AUC24/MIC | 59–74 | 0.3–0.4 | R |
| Quinolones | ||||
| Ciprofloxacin | AUC24/MIC | 60–80 | 2.5 | R/L |
| Levofloxacin | AUC24/MIC | 60–75 | 1.1–1.5 | R |
| Rifamycins | ||||
| Rifampin | AUC24/MIC | 20 | 0.65 | R/L |
| Tetracyclines | ||||
| Doxycycline | AUC24/MIC | 7 | 0.75–1.91 | R/L |
| ANTIMYCOTICS | ||||
| Liposomal Amphotericin B | Cmax/MIC | 10 | 4 | R/L |
| Fluconazole | AUC24/MIC | 88 | 0.7 | R |
| Isavuconazole | AUC24/MIC | <1 | 6.42 | R/L |
| Itraconazole | AUC24/MIC | <1 | 0.14 | L |
| Posaconazole | AUC24/MIC | <1 | 3.22–4.21 | L |
| Voriconazole | AUC24/MIC | 40 | 4.6 | L |
| Anidulafungin | AUC24/MIC | <1 | 0.4–0.7 | L |
| Caspofungin | AUC24/MIC | 3 | 0.11 | L |
| ANTIVIRALS | ||||
| Acyclovir | - | 91–67 | 0.7 | R |
| Ganciclovir | - | 99–98 | 0.7 | R |
| Oseltamivir | - | 97 | 23–26 | R |
| Remdesivir | - | <20 | 2.05 | R |
| Extracorporeal Organ Support Therapy | References | |||
|---|---|---|---|---|
| Extracorporeal Blood Purification Therapies |  | CRRT | Continuous Renal Replacement Therapy (CRRT) allows blood purification from the life-threatening waste product overload occurring during acute kidney injury. The three main mechanisms of solute and fluid removal during CRRT are:
| [29,30] |
 | TPE | Therapeutic plasma exchange (TPE) allows plasma filtration via high cut-off membranes and subsequent replacement with solutions of donor plasma, colloids, crystalloids or a mixture thereof. Main indications for this therapy are represented by immunopathological conditions such as myasthenia gravis, Guillain–Barré syndrome and Waldenström macroglobulinemia. However, TPE has been used in patients with sepsis, although no definitive evidence exists in this field.Antimicrobial PK characteristics that may favour drug removal during TPE are low Vd and high protein binding (>80%). | [31] | |
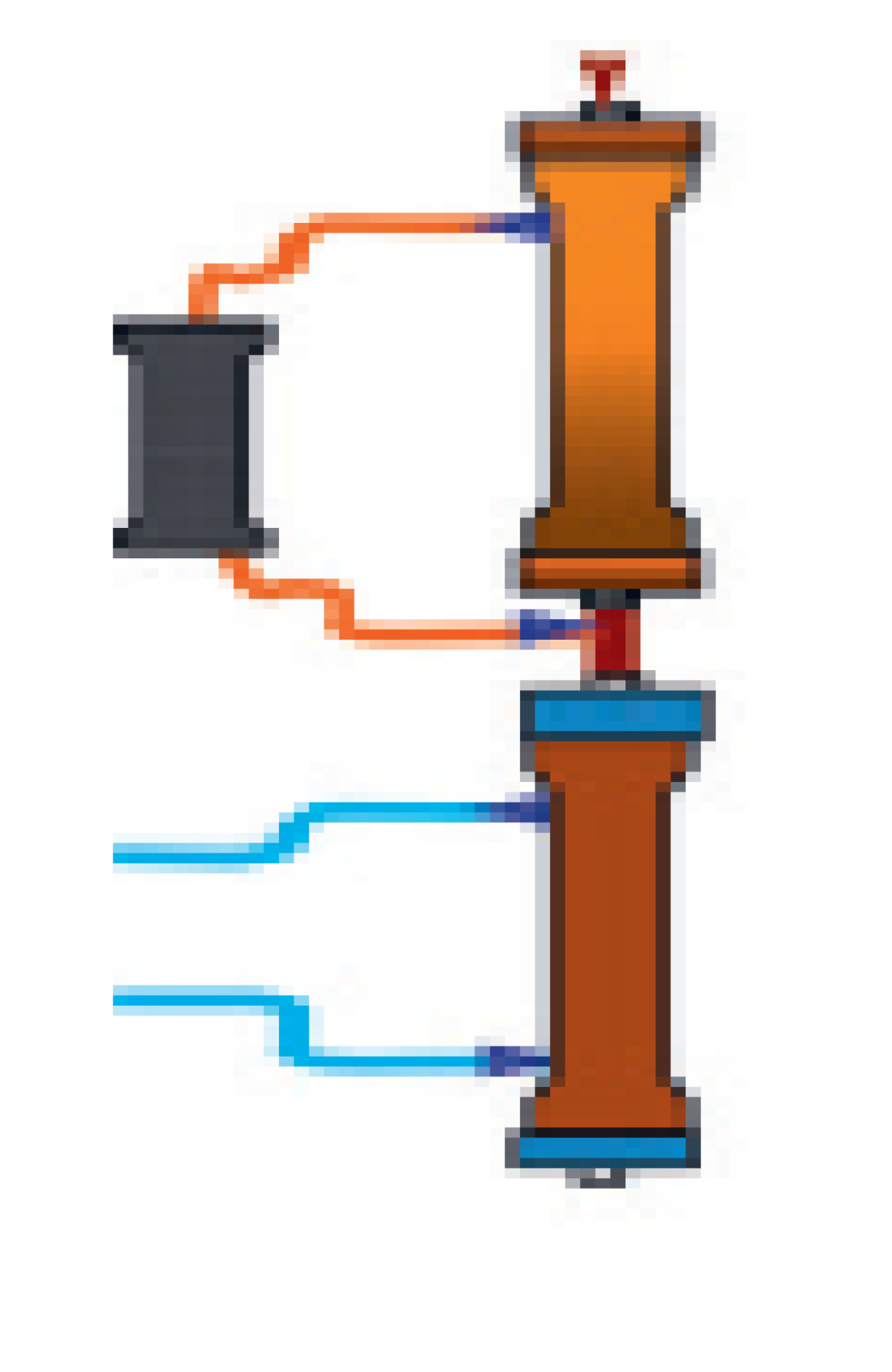 | CPFA | Coupled plasma filtration and adsorption (CPFA) combines plasma filtration via high cut-off membranes with subsequent adsorption via styrene resin. The volume plasma purified by waste products is then reinfused into the blood line and the whole blood passes across an hemofilter for further solute removal. This therapy has been used in septic patents, although clear benefit has never been demonstrated.CPFA was demonstrated to significantly lower the bloodstream concentration of colistin, whose amount was directly proportional to the volume of plasma filtered over time. | [32] | |
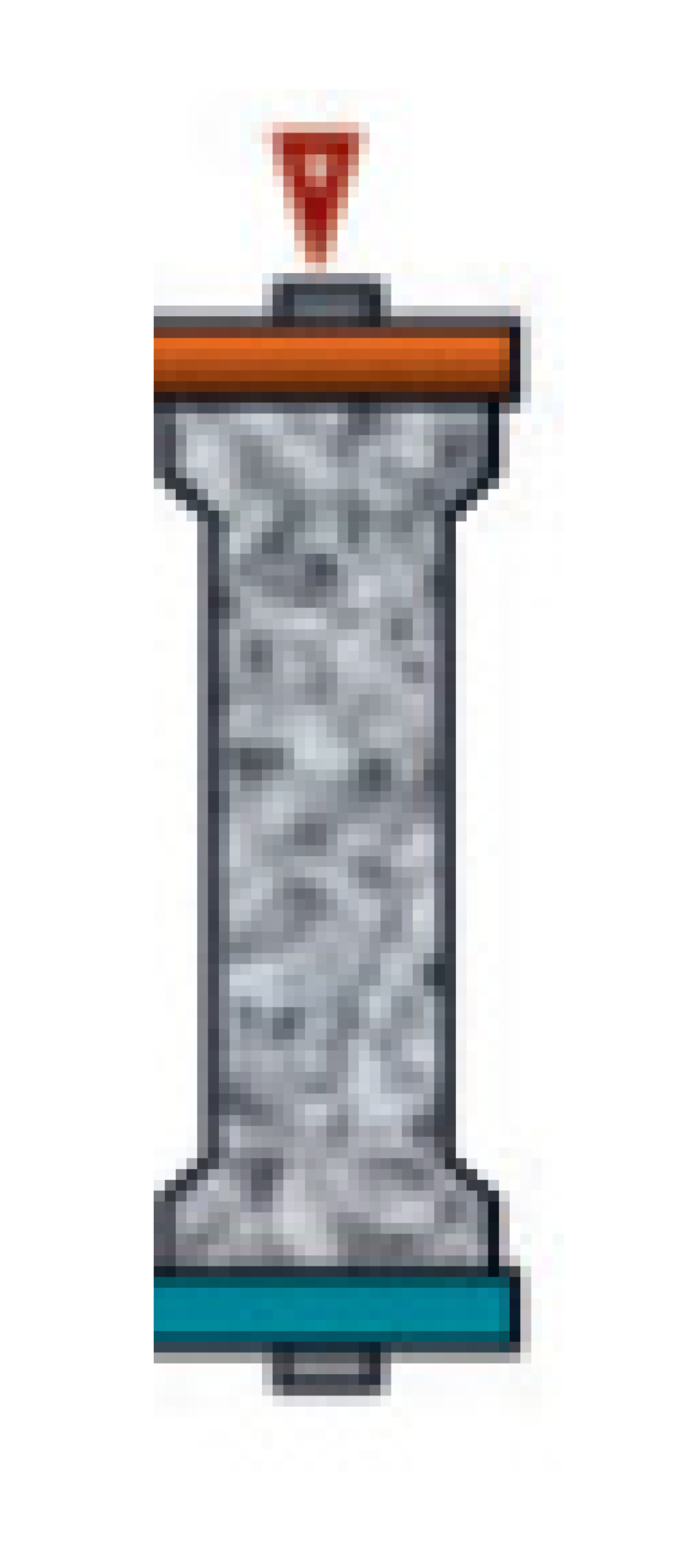 | HP | Hemoperfusion allows extracorporeal removal of mediators via absorption and, according to cartridge characteristics, may be classified as: Selective
| [33] | |
| Cardio-pulmonary support | 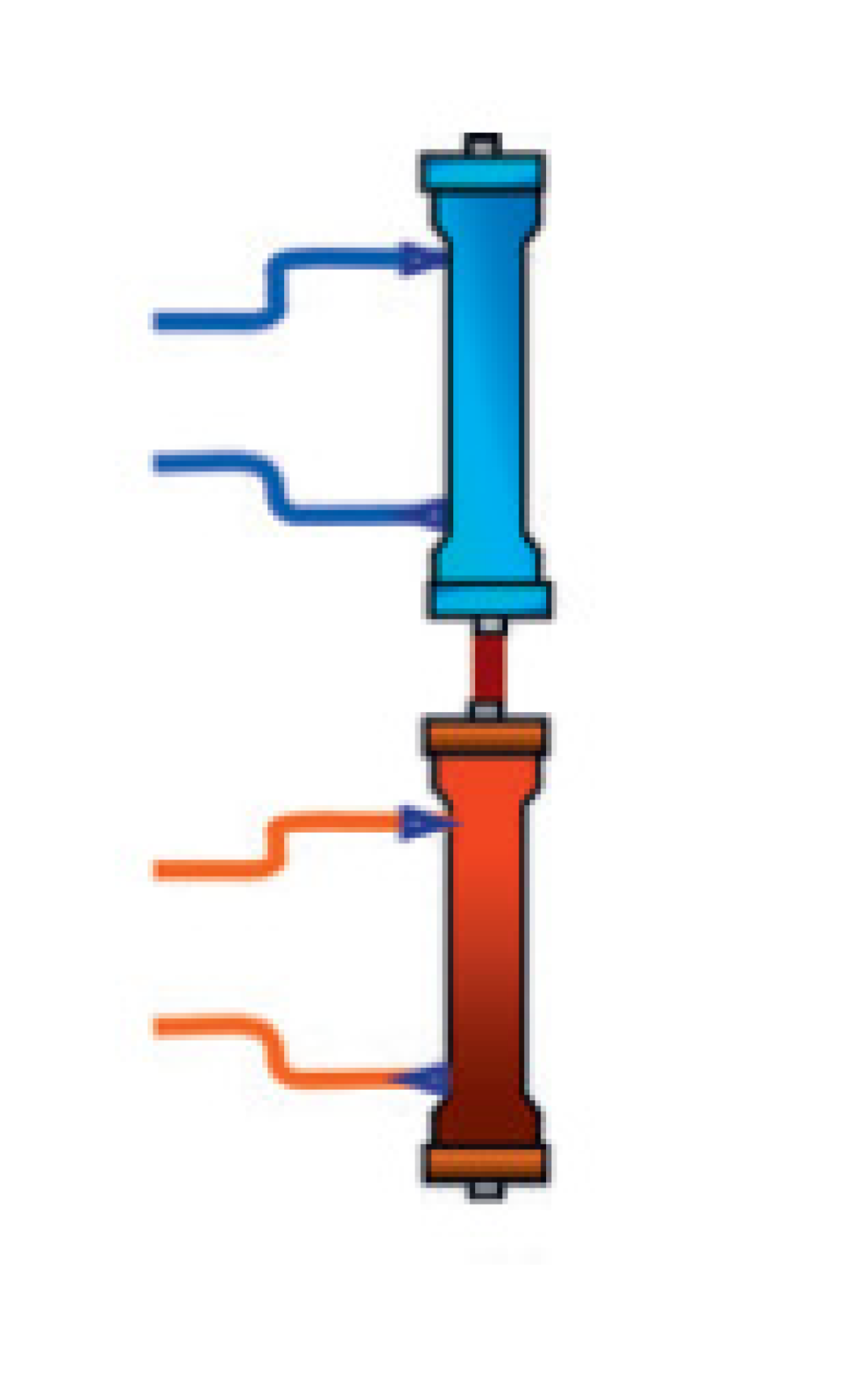 | ECCO2R | Extracorporeal CO2 removal (ECCO2R) allows CO2 removal in hypercapnic respiratory diseases (e.g., COPD and ARDS); no evidence of significant antimicrobial removal in vivo. | |
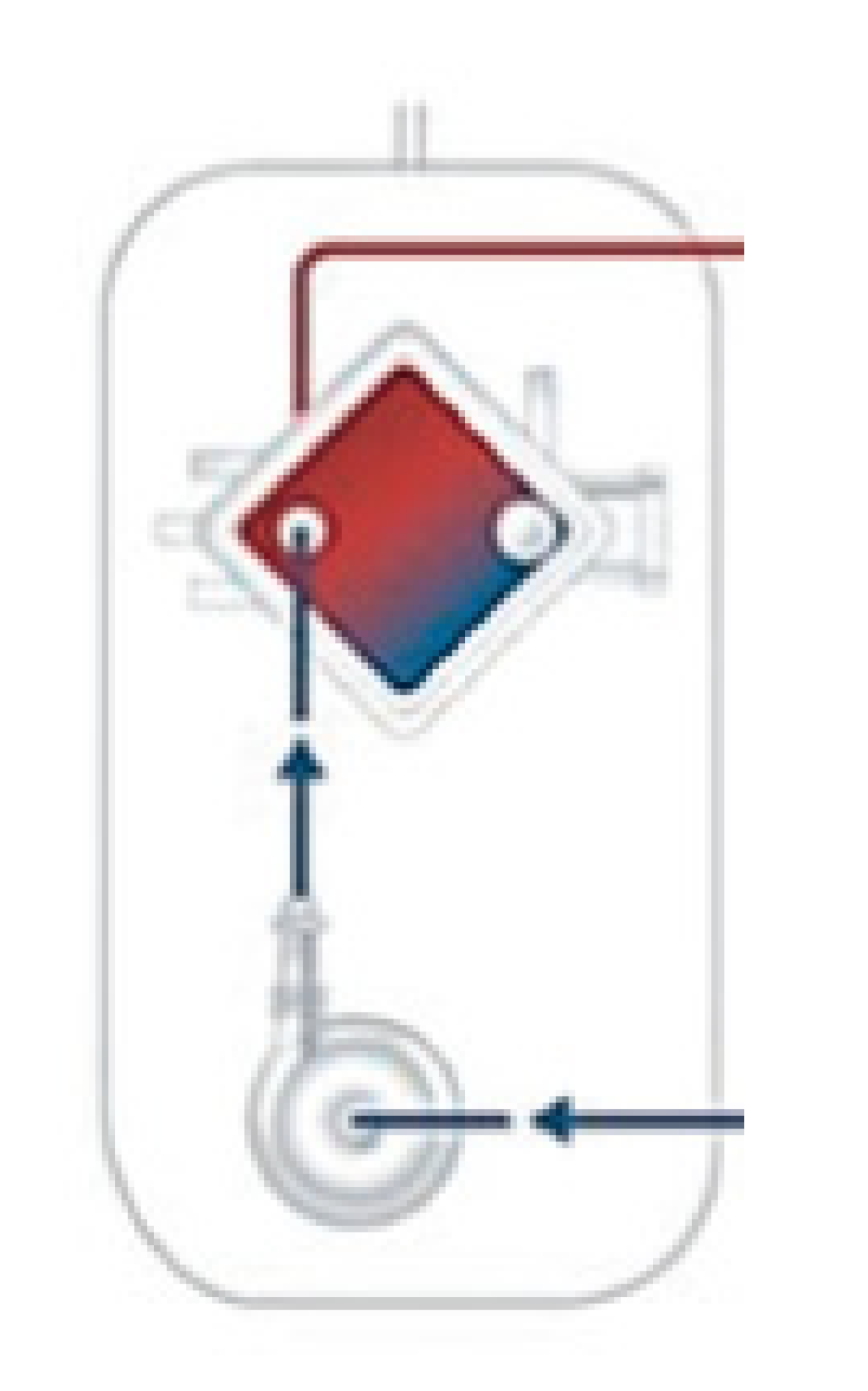 | ECMO | Extracorporeal membrane oxygenation (ECMO) may be set as:
| [34,35] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutuli, S.L.; Cascarano, L.; Lazzaro, P.; Tanzarella, E.S.; Pintaudi, G.; Grieco, D.L.; De Pascale, G.; Antonelli, M. Antimicrobial Exposure in Critically Ill Patients with Sepsis-Associated Multi-Organ Dysfunction Requiring Extracorporeal Organ Support: A Narrative Review. Microorganisms 2023, 11, 473. https://doi.org/10.3390/microorganisms11020473
Cutuli SL, Cascarano L, Lazzaro P, Tanzarella ES, Pintaudi G, Grieco DL, De Pascale G, Antonelli M. Antimicrobial Exposure in Critically Ill Patients with Sepsis-Associated Multi-Organ Dysfunction Requiring Extracorporeal Organ Support: A Narrative Review. Microorganisms. 2023; 11(2):473. https://doi.org/10.3390/microorganisms11020473
Chicago/Turabian StyleCutuli, Salvatore Lucio, Laura Cascarano, Paolo Lazzaro, Eloisa Sofia Tanzarella, Gabriele Pintaudi, Domenico Luca Grieco, Gennaro De Pascale, and Massimo Antonelli. 2023. "Antimicrobial Exposure in Critically Ill Patients with Sepsis-Associated Multi-Organ Dysfunction Requiring Extracorporeal Organ Support: A Narrative Review" Microorganisms 11, no. 2: 473. https://doi.org/10.3390/microorganisms11020473
APA StyleCutuli, S. L., Cascarano, L., Lazzaro, P., Tanzarella, E. S., Pintaudi, G., Grieco, D. L., De Pascale, G., & Antonelli, M. (2023). Antimicrobial Exposure in Critically Ill Patients with Sepsis-Associated Multi-Organ Dysfunction Requiring Extracorporeal Organ Support: A Narrative Review. Microorganisms, 11(2), 473. https://doi.org/10.3390/microorganisms11020473






