Screening of Staphylococcus aureus for Disinfection Evaluation and Transcriptome Analysis of High Tolerance to Chlorine-Containing Disinfectants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening of Standard Strains for Long-Term Bacteriostatic Efficacy Evaluation of Chlorine-Containing Disinfectants
2.1.1. Strain Selection and Bacterial Suspension Preparation
2.1.2. Acid Alkali Resistance Test and Heat Resistance Test
2.1.3. Genome Sequencing, Antimicrobial Resistance Genes Analysis, and Virulence-Related Genes Analysis
2.1.4. Disinfection Test
Neutralizer Identification Test
Minimum Inhibitory Concentration (MIC) Test and Minimal Bactericide Concentration (MBC) Test
Quantitative Germicidal Test
2.2. Differentially Expressed Genes (DEGs) Analysis Intolerance to Chlorine-Containing Disinfectants
3. Results
3.1. Screening of Standard Strains for Long-Term Bacteriostatic Efficacy Evaluation of Chlorine-Containing Disinfectants
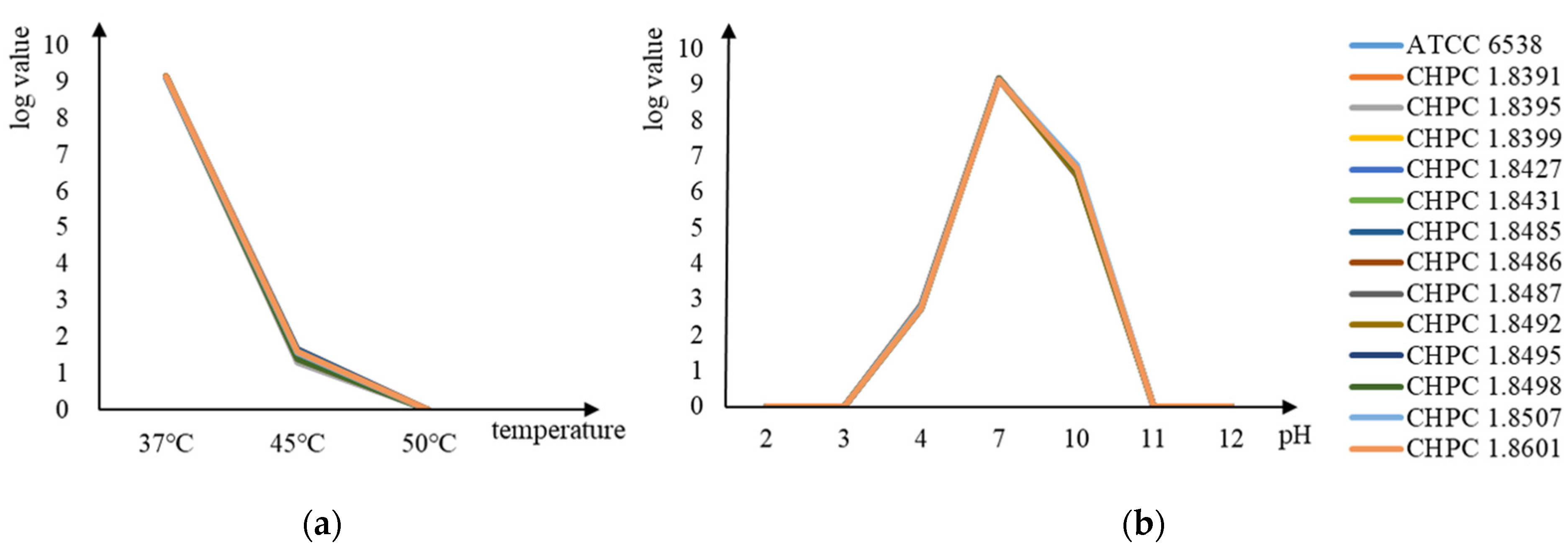
3.1.1. Comparison of Acid–Alkali Resistance and Heat Resistance
3.1.2. Genome Analysis
3.1.3. Disinfection Test Result
Neutralizer Test Result
MIC and MBC Results
Quantitative Germicidal Test Result
3.2. DEGs of Resistance to Chlorine-Containing Disinfectants between S. aureus ATCC6538 and CHPC 1.8487
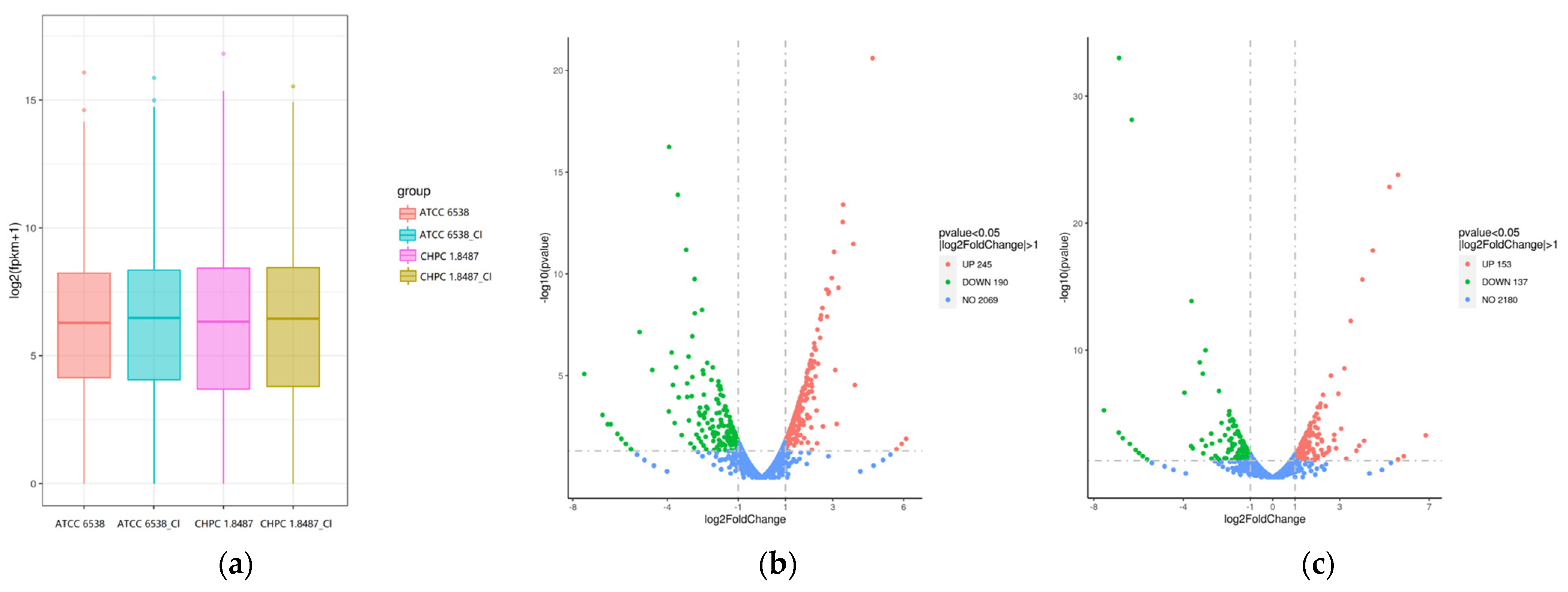
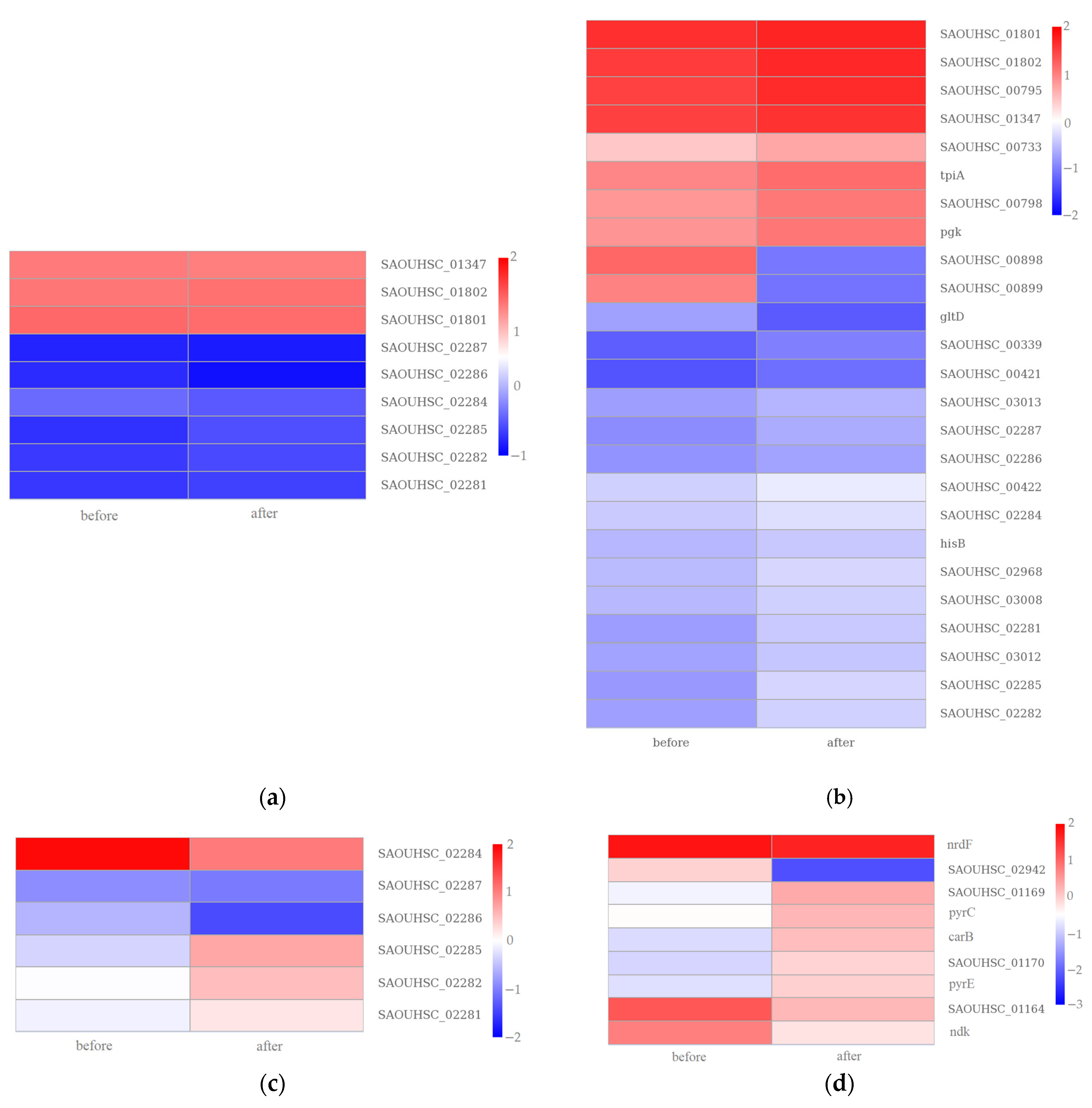
3.2.1. Gene Expression Analysis
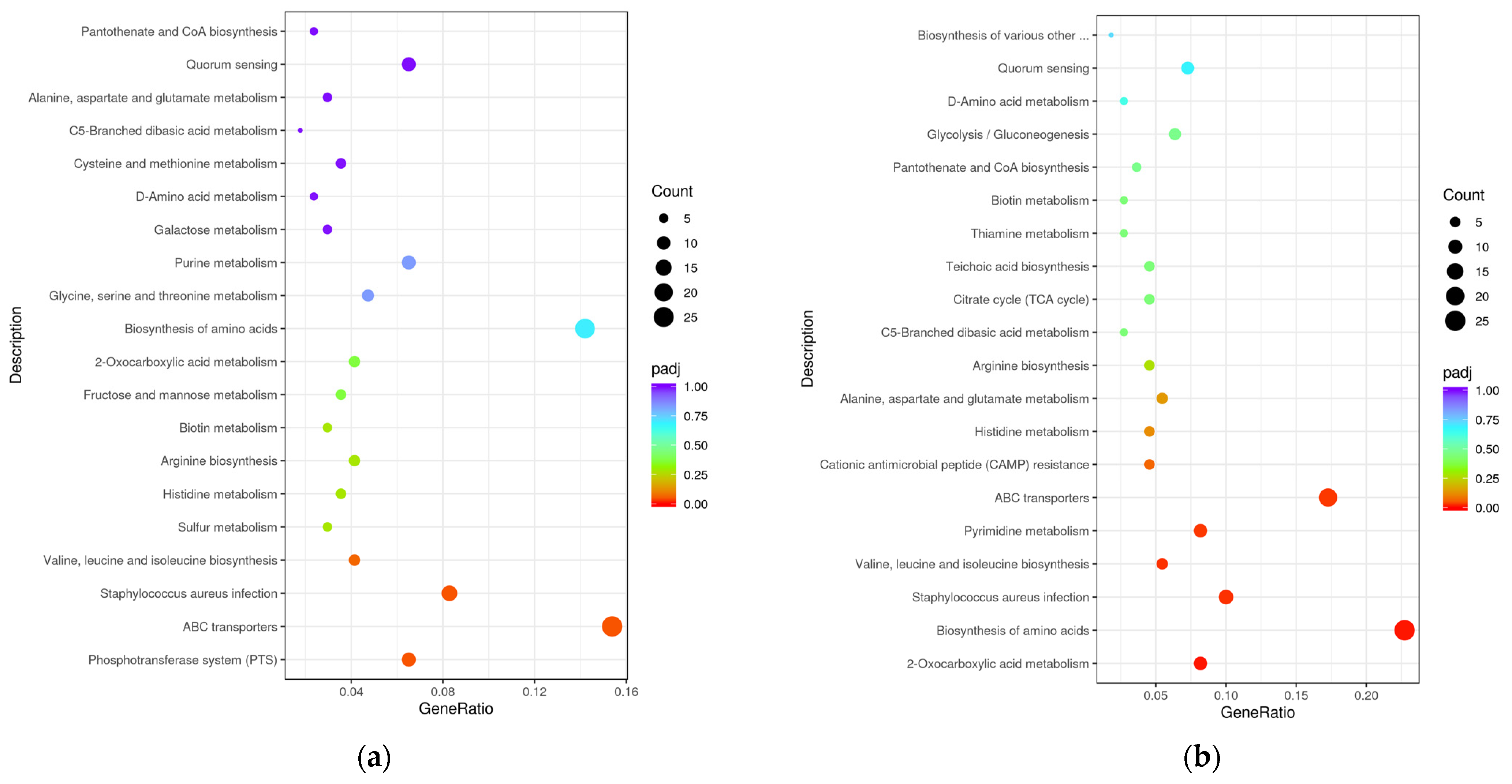
3.2.2. GO Enrichment and KEGG Pathway Analysis
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, G.; Riduan, S.N.; Yuan, Y.; Zhang, Y. Microbicide surface nano-structures. Crit. Rev. Biotechnol. 2019, 39, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.R.; Parikh, R.S. Chemical disinfectants in ophthalmic practice. Indian J. Ophthalmol. 2021, 69, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Wales, A.D.; Gosling, R.J.; Bare, H.L.; Davies, R.H. Disinfectant testing for veterinary and agricultural applications: A review. Zoonoses Public Health 2021, 68, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Cabeça, T.K.; Pizzolitto, A.C.; Pizzolitto, E.L. Activity of disinfectants against foodborne pathogens in suspension and adhered to stainless steel surfaces. Braz. J. Microbiol. 2012, 43, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Brühwasser, C.; Heinrich, H.; Lass-Flörl, C.; Mayr, A. Self-disinfecting surfaces and activity against Staphylococcus aureus ATCC 6538 under real-life conditions. J. Hosp. Infect. 2017, 97, 196–199. [Google Scholar] [CrossRef]
- Reybrouck, G. A theoretical approach of disinfectant testing. Zentralbl Bakteriol Orig. B. 1975, 160, 342–367. [Google Scholar] [PubMed]
- Makarova, O.; Johnston, P.; Walther, B.; Rolff, J.; Roesler, U. Complete Genome Sequence of the Disinfectant Susceptibility Testing Reference Strain Staphylococcus aureus subsp. aureus ATCC 6538. Genome Announc. 2017, 5, e00293-17. [Google Scholar] [CrossRef]
- Lu, J.; Guo, J. Disinfection spreads antimicrobial resistance. Science 2021, 371, 474. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Ye, D.; Yan, X.; Zhang, Y.; Yang, W.; Li, X.; Wang, J.; Zhang, L.; Pan, L. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020, 262, 114665. [Google Scholar] [CrossRef]
- Brady, M.J.; Lisay, C.M.; Yurkovetskiy, A.V.; Sawan, S.P. Persistent silver disinfectant for the environmental control of pathogenic bacteria. Am. J. Infect. Control 2003, 31, 208–214. [Google Scholar] [CrossRef]
- Li, D.; Zeng, S.; He, M.; Gu, A.Z. Water Disinfection Byproducts Induce Antibiotic Resistance-Role of Environmental Pollutants in Resistance Phenomena. Environ. Sci. Technol. 2016, 50, 3193–3201. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely Used Benzalkonium Chloride Disinfectants Can Promote Antibiotic Resistance. Appl. Environ. Microbiol. 2018, 84, e01201-18. [Google Scholar] [CrossRef] [PubMed]
- Furi, L.; Ciusa, M.L.; Knight, D.; Lorenzo, D.V.; Tocci, N.; Cirasola, D.; Aragones, L.; Coelho, J.R.; Freitas, A.T.; Marchi, E.; et al. Evaluation of reduced susceptibility to quaternary ammonium compounds and bisbiguanides in clinical isolates and laboratory-generated mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Winward, G.P.; Avery, L.M.; Stephenson, T.; Jefferson, B. Chlorine disinfection of grey water for reuse: Effect of organics and particles. Water Res. 2008, 42, 483–491. [Google Scholar] [CrossRef]
- Carlie, M.S.; Boucher, C.E.; Bragg, R.R. Molecular basis of bacterial disinfectant resistance. Drug Resist. Updat. 2020, 48, 100672. [Google Scholar] [CrossRef]
- Ofori, I.; Maddila, S.; Lin, J.; Jonnalagadda, S.B. Chlorine dioxide oxidation of Escherichia coli in water—A study of the disinfection kinetics and mechanism. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2017, 52, 598–606. [Google Scholar] [CrossRef]
- Daer, S.; Goodwill, J.E.; Ikuma, K. Effect of ferrate and monochloramine disinfection on the physiological and transcriptomic response of Escherichia coli at late stationary phase. Water Res. 2021, 189, 116580. [Google Scholar] [CrossRef]
- Dukan, S.; Dadon, S.; Smulski, D.R.; Belkin, S. Hypochlorous acid activates the heat shock and soxRS systems of Escherichia coli. Appl. Environ. Microbiol. 1996, 62, 4003–4008. [Google Scholar] [CrossRef]
- Vaezi, S.S.; Poorazizi, E.; Tahmourespour, A.; Aminsharei, F. Application of artificial neural networks to describe the combined effect of pH, time, NaCl and ethanol concentrations on the biofilm formation of Staphylococcus aureus. Microb. Pathog. 2020, 141, 103986. [Google Scholar] [CrossRef]
- Tango, C.N.; Akkermans, S.; Hussain, M.S.; Khan, I.; Impe, V.J.; Jin, Y.G.; Oh, D.H. Modeling the effect of pH, water activity, and ethanol concentration on biofilm formation of Staphylococcus aureus. Food Microbiol. 2018, 76, 287–295. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Lai, Q.; Zeng, R.; Ye, D.; Xu, J.; Shao, Z. Proposal of nine novel species of the Bacillus cereus group. Int. J. Syst. Evol. Microbiol. 2017, 8, 2499–2508. [Google Scholar] [CrossRef]
- Song, Y.; Xu, X.; Huang, Z.; Xiao, Y.; Yu, K.; Jiang, M.; Yin, S.; Zheng, M.; Meng, H.; Han, Y.; et al. Genomic Characteristics Revealed Plasmid-Mediated Pathogenicity and Ubiquitous Rifamycin Resistance of Rhodococcus equi. Front. Cell Infect. Microbiol. 2022, 12, 807610. [Google Scholar] [CrossRef] [PubMed]
- Honaas, L.A.; Wafula, E.K.; Wickett, N.J.; Der, J.P.; Zhang, Y.; Edger, P.P.; Altman, N.S.; Pires, J.C.; Leebens-Mack, J.H.; de Pamphilis, C.W. Selecting Superior De Novo Transcriptome Assemblies: Lessons Learned by Leveraging the Best Plant Genome. PLoS ONE 2016, 1, e0146062. [Google Scholar] [CrossRef] [PubMed]
- EN 1276:2009/AC; Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Bactericidal Activity of Chemical Disinfectants and Antiseptics Used in Food, Industrial, Domestic and Institutional Areas—Test Method and Requirements (Phase 2, Step 1). European Committee for Standardisation: Brussels, Belgium, 2010.
- Fernández-Crehuet, M.; Espigares, M.; Moreno, E.; Espigares, E. Specificity of the neutralizers as the cause of errors in evaluating disinfectant efficacy: An assessment of triclosan. Lett. Appl. Microbiol. 2013, 6, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health P.R. China. Technical Standard for Disinfection; Ministry of Health P.R. China: Beijing, China, 2002.
- Kanamori, H.; Rutala, W.A.; Gergen, M.F.; Sickbert-Bennett, E.E.; Weber, D.J. Germicidal Activity against Carbapenem/Colistin-Resistant Enterobacteriaceae Using a Quantitative Carrier Test Method. Antimicrob. Agents Chemother. 2018, 7, e00318-18. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, K.; Fang, Y.; Dai, H.; Cai, H.; Li, Z.; Kan, B.; Wei, Q.; Wang, D. Comparative Genomics and Transcriptomics Analyses Reveal a Unique Environmental Adaptability of Vibrio fujianensis. Microorganisms 2020, 8, 555. [Google Scholar] [CrossRef]
- Goodwin, K.D.; McNay, M.; Cao, Y.; Ebentier, D.; Madison, M.; Griffith, J.F. A multi-beach study of Staphylococcus aureus, MRSA, and enterococci in seawater and beach sand. Water Res. 2012, 13, 4195–4207. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 4, e00020-18. [Google Scholar] [CrossRef]
- Budzyńska, A.; Skowron, K.; Kaczmarek, A.; Wietlicka-Piszcz, M.; Gospodarek-Komkowska, E. Virulence Factor Genes and Antimicrobial Susceptibility of Staphylococcus aureus Strains Isolated from Blood and Chronic Wounds. Toxins 2021, 13, 491. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, B.; Wei, Q. Pathogenic microorganism biobanking in China. J. Biosaf. Biosecur. 2019, 1, 31–33. [Google Scholar] [CrossRef]
- Brian, G.; Zhiquan, S.; Shen, R.; Dayong, G. Biobanking: A foundation of life-science research and advancement. Biosaf. Health 2022, 4, 285–289. [Google Scholar] [CrossRef]
- Kozlakidis, Z.; Cheong, H.I.; Wei, Q. Supporting the scientific advancement from pathogenic microorganisms biobank. Biosaf. Health 2022, 5, 283–284. [Google Scholar] [CrossRef]
- Briaud, P.; Frey, A.; Marino, E.C.; Bastock, R.A.; Zielinski, R.E.; Wiemels, R.E.; Keogh, R.A.; Murphy, E.R.; Shaw, L.N.; Carroll, R.K. Temperature Influences the Composition and Cytotoxicity of Extracellular Vesicles in Staphylococcus aureus. mSphere 2021, 5, e0067621. [Google Scholar] [CrossRef] [PubMed]
- Ganchev, I. Biofilm Formation Between Bacillus Subtilis and Escherichia Coli K-12 Strains at Acidic and Oxidative Stress. SJC 2019, 7, 15–18. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Truong, V.K.; Wang, J.Y.; Berndt, C.C.; Jones, R.T.; Yusuf, I.I.; Peake, I.; Schmidt, H.W.; Fluke, C.; Barnes, D.; et al. Impact of nanoscale roughness of titanium thin film surfaces on bacterial retention. Langmuir 2010, 3, 1973–1982. [Google Scholar] [CrossRef]
- Nostro, A.; Cellini, L.; Giulio, M.D.; D’Arrigo, M.; Marino, A.; Blanco, A.R.; Favaloro, A.; Cutroneo, G.; Bisignano, G. Effect of alkaline pH on staphylococcal biofilm formation. APMIS 2012, 9, 733–742. [Google Scholar] [CrossRef]
- Li, Y.X.; Song, Y.; Mei, L.; Jiang, M.N.; Wang, D.C.; Wei, Q. Screening Pathogenic Microorganism Standard Strains for Disinfection Efficacy Evaluation. Biomed. Environ. Sci. 2022, 11, 1070–1073. [Google Scholar] [CrossRef]
- Pereira, B.M.P.; Wang, X.; Tagkopoulos, I. Short- and Long-Term Transcriptomic Responses of Escherichia coli to Biocides: A Systems Analysis. Appl. Environ. Microbiol. 2020, 14, e00708-20. [Google Scholar] [CrossRef]
- Ye, C.; Lin, H.; Zhang, M.; Chen, S.; Yu, X. Characterization and potential mechanisms of highly antibiotic tolerant VBNC Escherichia coli induced by low level chlorination. Sci. Rep. 2020, 1, 1957. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, D.; Pan, X.; Xia, B.; Weng, Y.; Long, Y.; Ren, H.; Zhou, J.; Jin, Y.; Bai, F.; et al. TpiA is a Key Metabolic Enzyme That Affects Virulence and Resistance to Aminoglycoside Antibiotics through CrcZ in Pseudomonas aeruginosa. mBio 2020, 1, e02079-19. [Google Scholar] [CrossRef] [Green Version]
- Busch, S.; Hoffmann, B.; Valerius, O.; Starke, K.; Düvel, K.; Braus, G.H. Regulation of the Aspergillus nidulans hisB gene by histidine starvation. Curr. Genet. 2001, 6, 314–322. [Google Scholar] [CrossRef]
- Xia, Y.; Li, S.; Xu, G.; Xie, S.; Liu, X.; Lin, X.; Wu, H.; Gao, X. The carB Gene of Escherichia coli BL21(DE3) is Associated with Nematicidal Activity against the Root-Knot Nematode Meloidogyne javanica. Pathogens 2021, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 1, 34–44. [Google Scholar] [CrossRef]



| VF Class | Virulence Factors | Related Genes |
|---|---|---|
| Adherence | Cell wall-associated fibronectin-binding protein | ebh |
| Clumping factor A | clfA | |
| Clumping factor B | clfB | |
| Collagen adhesion | cna | |
| Extracellular adherence protein/MHC analogous protein | eap/map | |
| Intercellular adhesin | icaD | |
| Ser-Asp rich fibrinogen-binding proteins | sdrC | |
| sdrD | ||
| Enzyme | Serine protease | splA |
| splB | ||
| splC | ||
| splD | ||
| splE | ||
| splF | ||
| Immune evasion | AdsA | adsA |
| CHIPS | chp | |
| SCIN | scn | |
| Secretion system | Type VII secretion system | esaB |
| esaD | ||
| esaE | ||
| esxB | ||
| esxC | ||
| esxD | ||
| Toxin | Enterotoxin A | sea |
| Enterotoxin B | seb | |
| Enterotoxin0like K | selk | |
| Enterotoxin0like Q | selq | |
| Exotoxin | set | |
| Leukotoxin D | lukD |
| No. | Average Colony Count of Each Group (cfu/mL) | Error Rate among Groups 3, 4, and 5 (%) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 1 | 0 | 18 | 22,300,000 | 24,700,000 | 25,300,000 | 0 | 4.98 |
| 2 | 0 | 9 | 16,800,000 | 17,000,000 | 18,700,000 | 0 | 4.57 |
| 3 | 12 | 180 | 25,800,000 | 27,500,000 | 29,100,000 | 0 | 2.19 |
| Strain | Control Group | Experimental Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Average Bacteria Concentration (cfu/mL) | N0 | 10 min | 30 min | |||||
| Average Bacteria Concentration (cfu/mL) | NX | KL | Average Bacteria Concentration (cfu/mL) | NX | KL | |||
| ATCC 6538 | 13,900,000 | 7.143 | 590 | 2.771 | 4.372 | 0 | / | >7 |
| CHPC 1.8391 | 15,000,000 | 7.176 | 1420 | 3.152 | 4.024 | 0 | / | >7 |
| CHPC 1.8395 | 11,900,000 | 7.076 | 410 | 2.613 | 4.463 | 0 | / | >7 |
| CHPC 1.8399 | 11,700,000 | 7.068 | 40 | 1.602 | 5.466 | 0 | / | >7 |
| CHPC 1.8427 | 16,300,000 | 7.212 | 110 | 2.041 | 5.171 | 0 | / | >7 |
| CHPC 1.8431 | 13,900,000 | 7.143 | 140 | 2.146 | 4.997 | 0 | / | >7 |
| CHPC 1.8485 | 14,000,000 | 7.146 | 0 | / | >7 | 0 | / | >7 |
| CHPC 1.8486 | 13,800,000 | 7.140 | 30 | 1.477 | 5.663 | 0 | / | >7 |
| CHPC 1.8487 | 16,900,000 | 7.228 | 10 | 1.000 | 6.228 | 0 | / | >7 |
| CHPC 1.8492 | 12,400,000 | 7.094 | 30 | 1.477 | 5.617 | 0 | / | >7 |
| CHPC 1.8495 | 19,000,000 | 7.279 | 0 | / | >7 | 0 | / | >7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Song, Y.; Huang, Z.; Mei, L.; Jiang, M.; Wang, D.; Wei, Q. Screening of Staphylococcus aureus for Disinfection Evaluation and Transcriptome Analysis of High Tolerance to Chlorine-Containing Disinfectants. Microorganisms 2023, 11, 475. https://doi.org/10.3390/microorganisms11020475
Li Y, Song Y, Huang Z, Mei L, Jiang M, Wang D, Wei Q. Screening of Staphylococcus aureus for Disinfection Evaluation and Transcriptome Analysis of High Tolerance to Chlorine-Containing Disinfectants. Microorganisms. 2023; 11(2):475. https://doi.org/10.3390/microorganisms11020475
Chicago/Turabian StyleLi, Yixiao, Yang Song, Zhenzhou Huang, Li Mei, Mengnan Jiang, Duochun Wang, and Qiang Wei. 2023. "Screening of Staphylococcus aureus for Disinfection Evaluation and Transcriptome Analysis of High Tolerance to Chlorine-Containing Disinfectants" Microorganisms 11, no. 2: 475. https://doi.org/10.3390/microorganisms11020475





