Identification of Emerging Industrial Biotechnology Chassis Vibrio natriegens as a Novel High Salt-Tolerant and Feedstock Flexibility Electroactive Microorganism for Microbial Fuel Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Media and Reagents
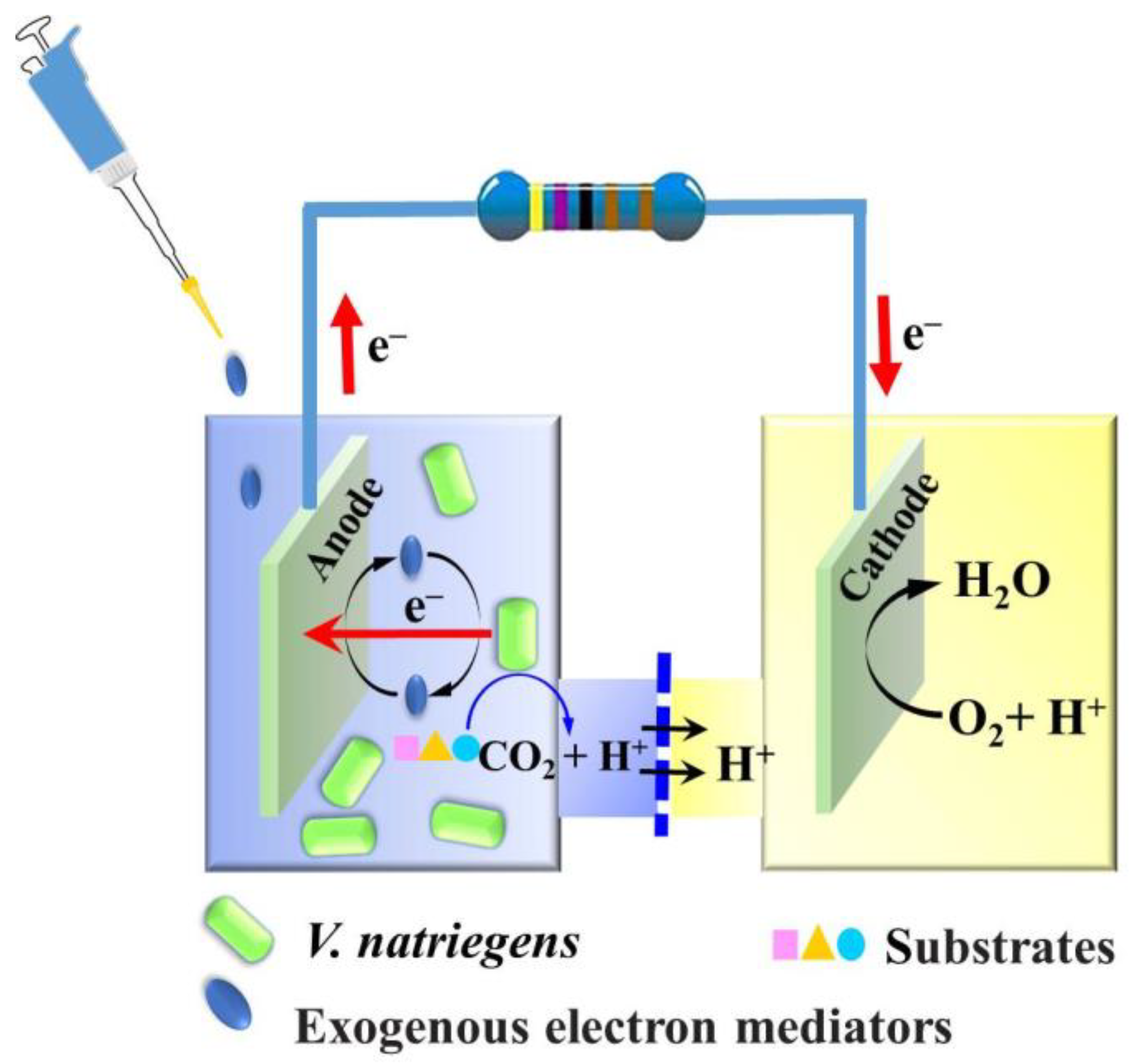
2.2. MFC Setup and Operation
2.3. Culture Condition
2.4. Characterization of Riboflavin, Phenazine-1-Carboxylic Acid, Phenazine-1-Carboxamide, and Hydroxyphenazine by LC-MS/MS
2.5. Data Analysis and Statistics
3. Results
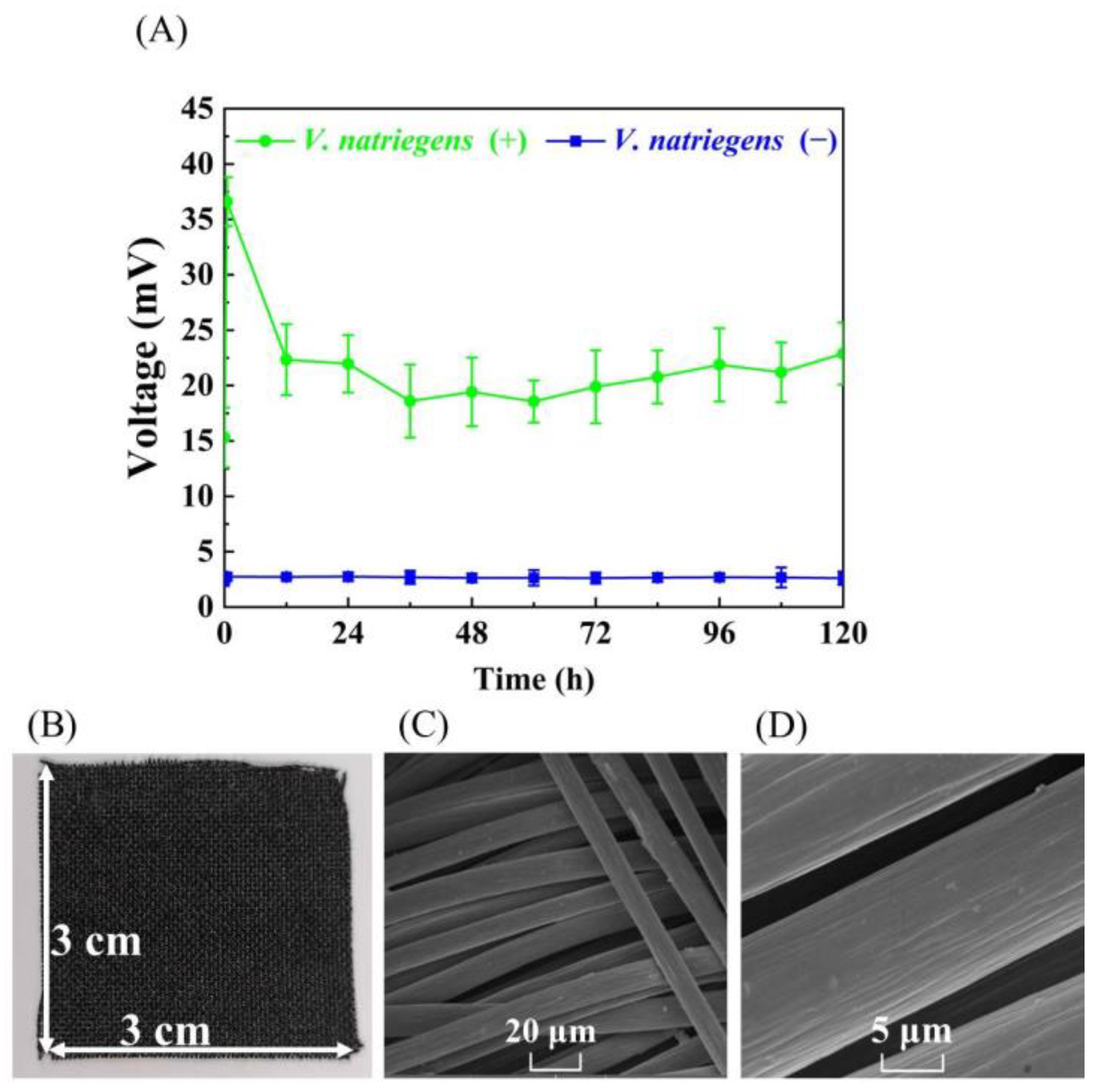
3.1. Evaluating the Bioelectricity Production Capacity of V. natriegens for MFC without Adding Exogenous Electron Mediators
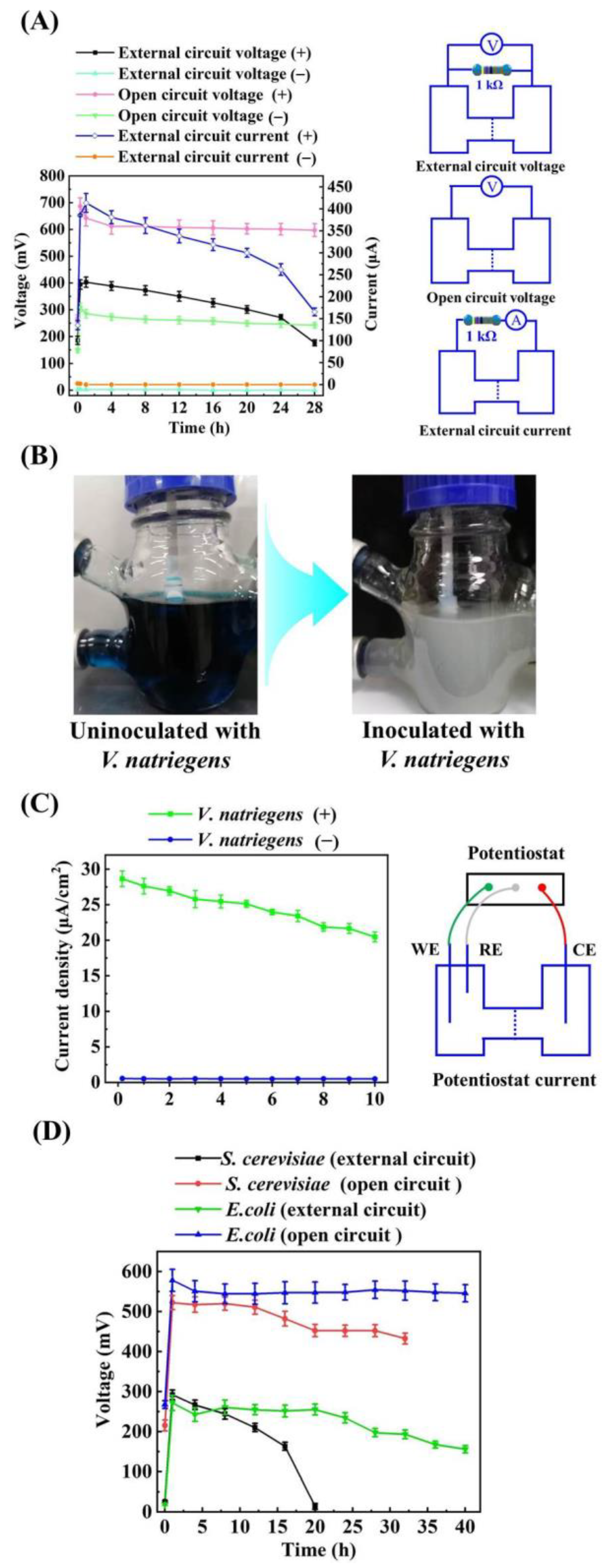
3.2. Effects of Adding Exogenous Electron Mediators on the Bioelectricity Production Capacity of VMFC
3.3. The Performance Evaluation of VMFCs with Media and Carbon Sources Switching
3.4. The Insight of the Endogenous Electronic Mediator Biosynthesis of V. natriegens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Li, B.; Xue, R.; Wang, C.; Cao, W. A systematic bibliometric review of clean energy transition: Implications for low-carbon development. PLoS ONE 2021, 16, e0261091. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Li, Z. Towards world’s low carbon development: The role of clean energy. Appl. Energy 2022, 307, 118160. [Google Scholar] [CrossRef]
- Barua, E.; Hossain, M.S.; Shaha, M.; Islam, E.; Zohora, F.T.; Protity, A.T.; Mukharjee, S.K.; Sarker, P.K.; Salimullah, M.; Hashem, A. Generation of electricity using microbial fuel cell (MFC) from sludge. Bangladesh J. Microbiol. 2018, 35, 23–26. [Google Scholar] [CrossRef]
- Lovley, D.R. The microbe electric: Conversion of organic matter to electricity. Curr. Opin. Biotechnol. 2008, 19, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C.T.; Hassett, D.J.; Gu, T. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol. Adv. 2018, 36, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Z.; Zhao, N.; Zhang, K.; Song, H. Increased power generation from cylindrical microbial fuel cell inoculated with P. aeruginosa. Biosens. Bioelectron. 2019, 141, 111394. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Ethiraj, B.; Cheng, C.K.; Yousuf, A.; Khan, M.M.R. An insight of synergy between Pseudomonas aeruginosa and Klebsiella variicola in a microbial fuel cell. ACS Sustain. Chem. Eng. 2018, 6, 4130–4137. [Google Scholar] [CrossRef]
- Holkar, C.R.; Arora, H.; Halder, D.; Pinjari, D.V. Biodegradation of reactive blue 19 with simultaneous electricity generation by the newly isolated electrogenic Klebsiella sp. C NCIM 5546 bacterium in a microbial fuel cell. Int. Biodeterior. Biodegrad. 2018, 133, 194–201. [Google Scholar] [CrossRef]
- Feng, J.; Qian, Y.; Wang, Z.; Wang, X.; Xu, S.; Chen, K.; Ouyang, P. Enhancing the performance of Escherichia coli-inoculated microbial fuel cells by introduction of the phenazine-1-carboxylic acid pathway. J. Biotechnol. 2018, 275, 1–6. [Google Scholar] [CrossRef]
- Ojima, Y.; Kawaguchi, T.; Fukui, S.; Kikuchi, R.; Terao, K.; Koma, D.; Ohmoto, T.; Azuma, M. Promoted performance of microbial fuel cells using Escherichia coli cells with multiple-knockout of central metabolism genes. Bioprocess Biosyst. Eng. 2020, 43, 323–332. [Google Scholar] [CrossRef]
- Duarte, K.D.; Kwon, Y. In situ carbon felt anode modification via codeveloping Saccharomyces cerevisiae living-template titanium dioxide nanoclusters in a yeast-based microbial fuel cell. J. Power Sources 2020, 474, 228651. [Google Scholar] [CrossRef]
- Christwardana, M.; Kwon, Y. Yeast and carbon nanotube based biocatalyst developed by synergetic effects of covalent bonding and hydrophobic interaction for performance enhancement of membraneless microbial fuel cell. Bioresour. Technol. 2017, 225, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, V.; Knighton, M.C.; Pagaling, E.; Ward, F.B.; Free, A.; Goryanin, I. Novel electrochemically active bacterium phylogenetically related to Arcobacter butzleri, isolated from a microbial fuel cell. Appl. Environ. Microbiol. 2009, 75, 7326–7334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Mayagoitia, S.; Fernández-Luqueño, F.; Morales-Acosta, D.; Carrillo-Rodríguez, J.; García-Lobato, M.; De La Torre-Saenz, L.; Alonso-Lemus, I.; Rodrıíguez-Varela, F. Energy generation from pharmaceutical residual water in microbial fuel cells using ordered mesoporous carbon and bacillus subtilis as bioanode. ACS Sustain. Chem. Eng. 2019, 7, 12179–12187. [Google Scholar] [CrossRef]
- Oh, S.; Logan, B.E. Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res. 2005, 39, 4673–4682. [Google Scholar] [CrossRef]
- Yong, Y.-C.; Yu, Y.-Y.; Yang, Y.; Li, C.M.; Jiang, R.; Wang, X.; Wang, J.-Y.; Song, H. Increasing intracellular releasable electrons dramatically enhances bioelectricity output in microbial fuel cells. Electrochem. Commun. 2012, 19, 13–16. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, S.; Jia, L.; Ji, A.; Chatterjee, S.G. Co-generation system of bioethanol and electricity with microbial fuel cell technology. Energy Fuels 2020, 34, 6414–6422. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kumar, A.N.; Kumar, G.; Kim, D.-H.; Song, Y.-C.; Kim, S.-H. Electro-fermentation for biofuels and biochemicals production: Current status and future directions. Bioresour. Technol. 2021, 323, 124598. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, R.J. Bidirectional extracellular electron transfers of electrode-biofilm: Mechanism and application. Bioresour. Technol. 2019, 271, 439–448. [Google Scholar] [CrossRef]
- Grattieri, M.; Minteer, S.D. Microbial fuel cells in saline and hypersaline environments: Advancements, challenges and future perspectives. Bioelectrochemistry 2018, 120, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Berkessa, Y.W.; Lang, Q.; Yan, B.; Kuang, S.; Mao, D.; Shu, L.; Zhang, Y. Anion exchange membrane organic fouling and mitigation in salt valorization process from high salinity textile wastewater by bipolar membrane electrodialysis. Desalination 2019, 465, 94–103. [Google Scholar] [CrossRef]
- Lefebvre, O.; Habouzit, F.; Bru, V.; Delgenès, J.-P.; Godon, J.-J.; Moletta, R. Treatment of hypersaline industrial wastewater by a microbial consortium in a sequencing batch reactor. Environ. Technol. 2004, 25, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Schaetzle, O.; Barrière, F.; Baronian, K. Bacteria and yeasts as catalysts in microbial fuel cells: Electron transfer from micro-organisms to electrodes for green electricity. Energy Environ. Sci. 2008, 1, 607–620. [Google Scholar] [CrossRef]
- Weinstock, M.T.; Hesek, E.D.; Wilson, C.M.; Gibson, D.G. Vibrio natriegens as a fast-growing host for molecular biology. Nat. Methods 2016, 13, 849–851. [Google Scholar] [CrossRef]
- Lee, H.H.; Ostrov, N.; Wong, B.G.; Gold, M.A.; Khalil, A.S.; Church, G.M. Functional genomics of the rapidly replicating bacterium Vibrio natriegens by CRISPRi. Nat. Microbiol. 2019, 4, 1105–1113. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Chen, W.; Peng, Y.; Tu, R.; Lin, Y.; Xing, J.; Wang, Q. Design and Reconstruction of Regulatory Parts for Fast-Growing Vibrio natriegens Synthetic Biology. ACS Synth. Biol. 2020, 9, 2399–2409. [Google Scholar] [CrossRef]
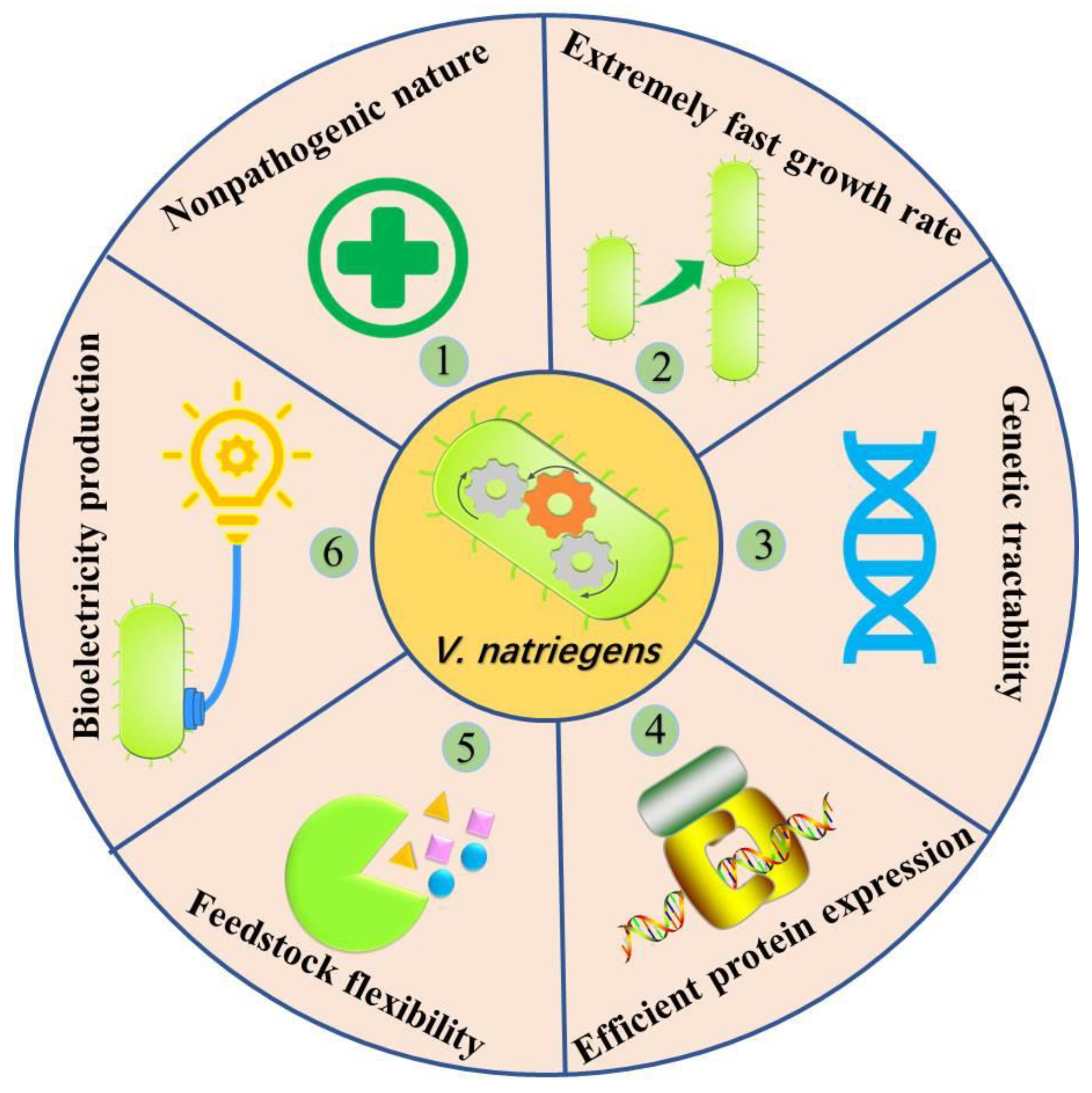
- Ellis, G.A.; Tschirhart, T.; Spangler, J.; Walper, S.A.; Medintz, I.L.; Vora, G.J. Exploiting the feedstock flexibility of the emergent synthetic biology chassis Vibrio natriegens for engineered natural product production. Mar. Drugs 2019, 17, 679. [Google Scholar] [CrossRef] [Green Version]
- Hoffart, E.; Grenz, S.; Lange, J.; Nitschel, R.; Müller, F.; Schwentner, A.; Feith, A.; Lenfers-Lücker, M.; Takors, R.; Blombach, B. High substrate uptake rates empower Vibrio natriegens as production host for industrial biotechnology. Appl. Environ. Microbiol. 2017, 83, e01614–e01617. [Google Scholar] [CrossRef] [Green Version]
- Becker, W.; Wimberger, F.; Zangger, K. Vibrio natriegens: An alternative expression system for the high-yield production of isotopically labeled proteins. Biochemistry 2019, 58, 2799–2803. [Google Scholar] [CrossRef]
- Lim, H.G.; Kwak, D.H.; Park, S.; Woo, S.; Yang, J.-S.; Kang, C.W.; Kim, B.; Noh, M.H.; Seo, S.W.; Jung, G.Y. Vibrio sp. dhg as a platform for the biorefinery of brown macroalgae. Nat. Commun. 2019, 10, 2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Yang, S.; Yang, L. Vibrio natriegens as a host for rapid biotechnology. Trends Biotechnol. 2021, 40, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Liu, Y.; Cen, X.; Liu, D.; Chen, Z. Systems metabolic engineering of Vibrio natriegens for the production of 1, 3-propanediol. Metab. Eng. 2021, 65, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Erian, A.M.; Freitag, P.; Gibisch, M.; Pflügl, S. High rate 2, 3-butanediol production with Vibrio natriegens. Bioresour. Technol. Rep. 2020, 10, 100408. [Google Scholar] [CrossRef]
- Dalia, T.N.; Hayes, C.A.; Stolyar, S.; Marx, C.J.; McKinlay, J.B.; Dalia, A.B. Multiplex genome editing by natural transformation (MuGENT) for synthetic biology in Vibrio natriegens. ACS Synth. Biol. 2017, 6, 1650–1655. [Google Scholar] [CrossRef] [Green Version]
- Thoma, F.; Schulze, C.; Gutierrez-Coto, C.; Hädrich, M.; Huber, J.; Gunkel, C.; Thoma, R.; Blombach, B. Metabolic engineering of Vibrio natriegens for anaerobic succinate production. Microb. Biotechnol. 2021, 15, 1671–1684. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Najafpour, G.; Ghoreyshi, A.; Shakeri, M.; Zare, H. Methylene blue as electron promoters in microbial fuel cell. Int. J. Hydrogen Energy 2011, 36, 13335–13341. [Google Scholar] [CrossRef]
- Yong, Y.-C.; Cai, Z.; Yu, Y.-Y.; Chen, P.; Jiang, R.; Cao, B.; Sun, J.-Z.; Wang, J.-Y.; Song, H. Increase of riboflavin biosynthesis underlies enhancement of extracellular electron transfer of Shewanella in alkaline microbial fuel cells. Bioresour. Technol. 2013, 130, 763–768. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Head, I.M.; Curtis, T.P.; Scott, K.; Lloyd, J.R.; Von Canstein, H. The effect of flavin electron shuttles in microbial fuel cells current production. Appl. Microbiol. Biotechnol. 2010, 85, 1373–1381. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, L.; Su, W.; Gao, P.; Li, D.; He, X.; Zhang, Y. The direct electrocatalysis of phenazine-1-carboxylic acid excreted by Pseudomonas alcaliphila under alkaline condition in microbial fuel cells. Bioresour. Technol. 2011, 102, 7099–7102. [Google Scholar] [CrossRef]
- Boon, N.; De Maeyer, K.; Höfte, M.; Rabaey, K.; Verstraete, W. Use of Pseudomonas species producing phenazine-based metabolites in the anodes of microbial fuel cells to improve electricity generation. Appl. Microbiol. Biotechnol. 2008, 80, 985–993. [Google Scholar]
- Yong, X.-Y.; Shi, D.-Y.; Chen, Y.-L.; Jiao, F.; Lin, X.; Zhou, J.; Wang, S.-Y.; Yong, Y.-C.; Sun, Y.-M.; OuYang, P.-K. Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells. Bioresour. Technol. 2014, 152, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mittal, Y.; Tamta, P.; Srivastava, P.; Yadav, A.K. Textile Wastewater Treatment Using Microbial Fuel Cell and Coupled Technology: A Green Approach for Detoxification and Bioelectricity Generation. In Integrated Microbial Fuel Cells for Wastewater Treatmen; Elsevier: Amsterdam, The Netherlands, 2020; pp. 73–92. [Google Scholar]
- Sun, J.; Li, W.; Li, Y.; Hu, Y.; Zhang, Y. Redox mediator enhanced simultaneous decolorization of azo dye and bioelectricity generation in air-cathode microbial fuel cell. Bioresour. Technol. 2013, 142, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Kadivarian, M.; Karamzadeh, M. Electrochemical modeling of microbial fuel cells performance at different operating and structural conditions. Bioprocess Biosyst. Eng. 2020, 43, 393–401. [Google Scholar] [CrossRef]
- Laursen, J.B.; Nielsen, J. Phenazine natural products: Biosynthesis, synthetic analogues, and biological activity. Chem. Rev. 2004, 104, 1663–1686. [Google Scholar] [CrossRef]
- Guttenberger, N.; Blankenfeldt, W.; Breinbauer, R. Recent developments in the isolation, biological function, biosynthesis, and synthesis of phenazine natural products. Bioorg. Med. Chem. 2017, 25, 6149–6166. [Google Scholar] [CrossRef] [PubMed]
- Mavrodi, D.V.; Blankenfeldt, W.; Thomashow, L.S. Phenazine compounds in fluorescent Pseudomonas spp. biosynthesis and regulation. Annu. Rev. Phytopathol. 2006, 44, 417–445. [Google Scholar] [CrossRef]
- Mavrodi, D.V.; Ksenzenko, V.N.; Bonsall, R.F.; Cook, R.J.; Boronin, A.M.; Thomashow, L.S. A seven-gene locus for synthesis of phenazine-1-carboxylic acid by Pseudomonas fluorescens 2-79. J. Bacteriol. 1998, 180, 2541–2548. [Google Scholar] [CrossRef] [Green Version]
- Chin-A-Woeng, T.F.; Thomas-Oates, J.E.; Lugtenberg, B.J.; Bloemberg, G.V. Introduction of the phzH gene of Pseudomonas chlororaphis PCL1391 extends the range of biocontrol ability of phenazine-1-carboxylic acid-producing Pseudomonas spp. strains. Mol. Plant-Microbe Interact. 2001, 14, 1006–1015. [Google Scholar] [CrossRef] [Green Version]
- Park, D.H.; Zeikus, J.G. Electricity generation in microbial fuel cells using neutral red as an electronophore. Appl. Environ. Microbiol. 2000, 66, 1292–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, M.; Cai, B.; He, K.; Wang, M.; Chen, B.; Tan, T. De novo biosynthesis of α-aminoadipate via multi-strategy metabolic engineering in Escherichia coli. MicrobiologyOpen 2022, 11, e1301. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, N.; Du, M.; Zhang, Y.; Hao, C.; Hu, R. Study on the effect of synergy effect between the mixed cultures on the power generation of microbial fuel cells. Bioengineered 2021, 12, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Nandy, A.; Jana, S.; Khamrai, M.; Kumar, V.; Mukherjee, S.; Bhattacharyya, A.; Kundu, P.P. Cloning and expression of α-amylase in E. coli: Genesis of a superior biocatalyst for substrate-specific MFC. Int. J. Green Energy 2019, 16, 309–316. [Google Scholar] [CrossRef]
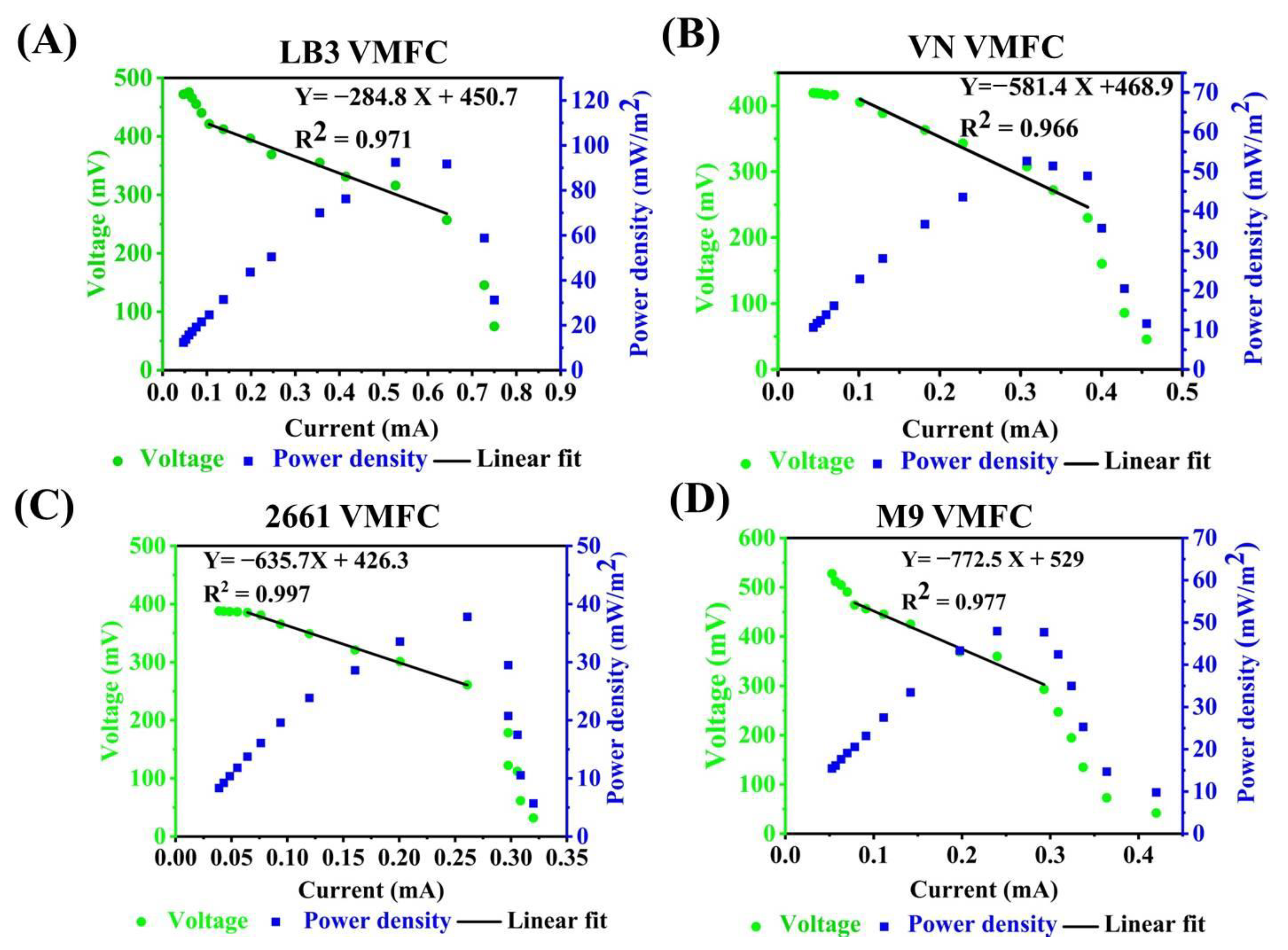
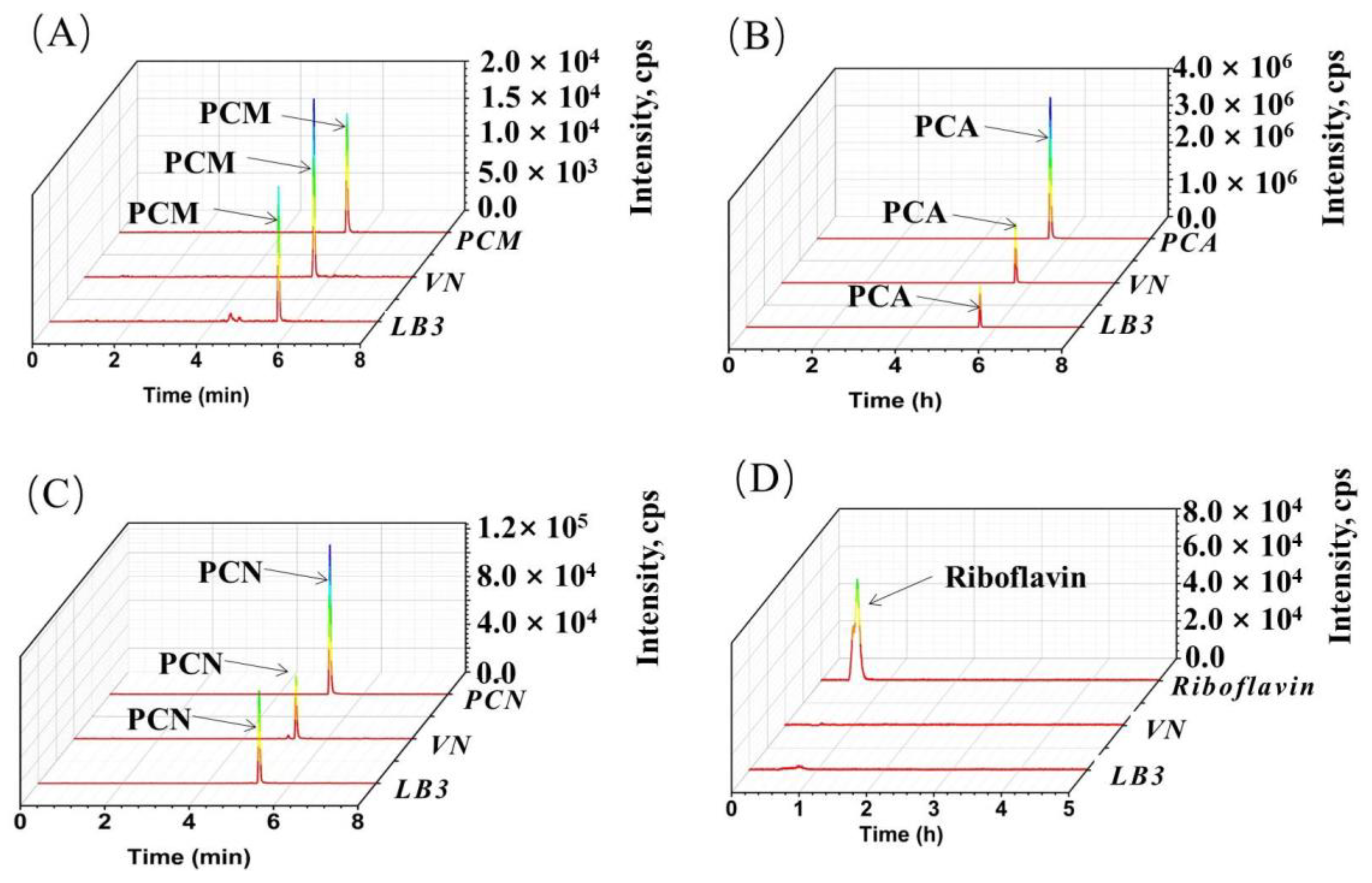
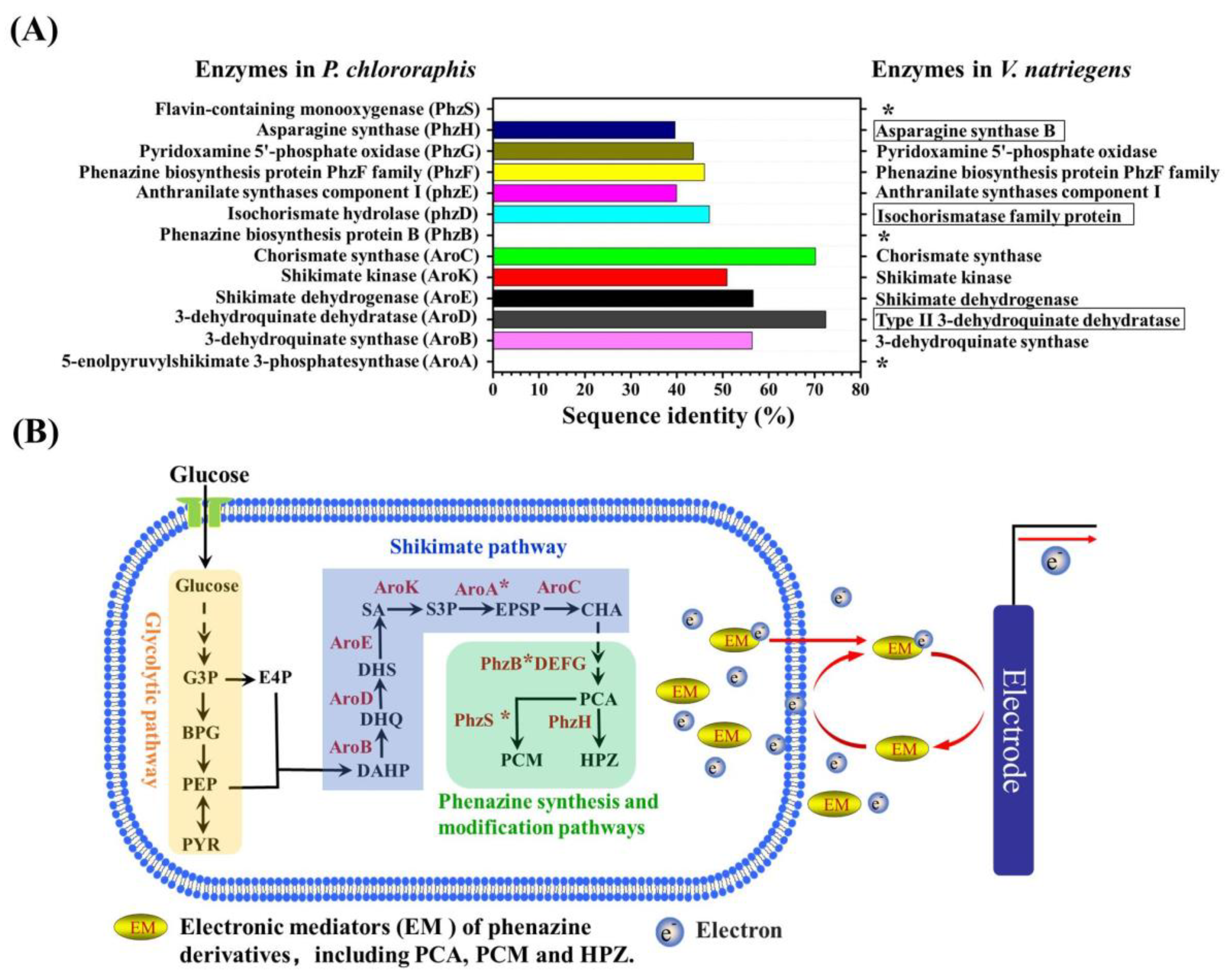
| Electron Mediators | Media | Maximum Power Density (mW/cm2) | Carbon Sources | Mediators | Maximum Power Density (mW/cm2) |
|---|---|---|---|---|---|
| MB (50 mg/L) | LB3 | 91.1 ± 4.5 | Glucose (10 g/L) | MB | 71.9 ± 6.3 |
| Riboflavin (40 mg/L) | LB3 | 0.9 ± 0.3 | Glycerol (10 g/L) | MB | 111.9 ± 6.7 |
| PCA (20 mg/L) | LB3 | 8.0 ± 0.2 | SA (2.5 g/L) | MB | 81.6 ± 3.3 |
| PCM (20 mg/L) | LB3 | 3.2 ± 0.2 | PG (2.5 g/L) | MB | 46.4 ± 2.1 |
| HPZ (60 mg/L) | LB3 | 2.4 ± 0.1 | Sucrose (10 g/L) | MB | 99.1 ± 4.8 |
| DAD (41 mg/L) | LB3 | 1.8 ± 0.1 | FA (2.5 g/L) | MB | 22.4 ± 1.2 |
| HA (1.3 g/L) | LB3 | 2.3 ± 0.1 | Fructose (10 g/L) | MB | 85.5 ± 3.9 |
| Media | Mediator | Maximum Power Density (mW/cm2) | ME (10 g/L) | MB | 75.1 ± 2.8 |
| Arabinose (10 g/L) | MB | 31.0 ± 2.0 | |||
| LB3 | MB | 91.1 ± 8.5 | Media | Mediator | Maximum Power Density (mW/cm2) |
| M9 | MB | 56.4 ± 3.3 | |||
| VN | MB | 71.9 ± 3.8 | LB | MB | 41.1 ± 3.5 |
| 2661 | MB | 40.4 ± 2.5 | YPD | MB | 47.4 ± 3.3 |
| LB6 | MB | 108.6 ± 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Xie, R.; Zhang, Y.; Wang, M.; Tan, T. Identification of Emerging Industrial Biotechnology Chassis Vibrio natriegens as a Novel High Salt-Tolerant and Feedstock Flexibility Electroactive Microorganism for Microbial Fuel Cell. Microorganisms 2023, 11, 490. https://doi.org/10.3390/microorganisms11020490
Gong Z, Xie R, Zhang Y, Wang M, Tan T. Identification of Emerging Industrial Biotechnology Chassis Vibrio natriegens as a Novel High Salt-Tolerant and Feedstock Flexibility Electroactive Microorganism for Microbial Fuel Cell. Microorganisms. 2023; 11(2):490. https://doi.org/10.3390/microorganisms11020490
Chicago/Turabian StyleGong, Zhijin, Rong Xie, Yang Zhang, Meng Wang, and Tianwei Tan. 2023. "Identification of Emerging Industrial Biotechnology Chassis Vibrio natriegens as a Novel High Salt-Tolerant and Feedstock Flexibility Electroactive Microorganism for Microbial Fuel Cell" Microorganisms 11, no. 2: 490. https://doi.org/10.3390/microorganisms11020490




