Screening and Identification of Coastal Chilean Thraustochytrids for Arachidonic Acid Production: Biotechnological Potential of Ulkenia visurgensis Lng2-Strain
Abstract
:1. Introduction
2. Materials and Methods
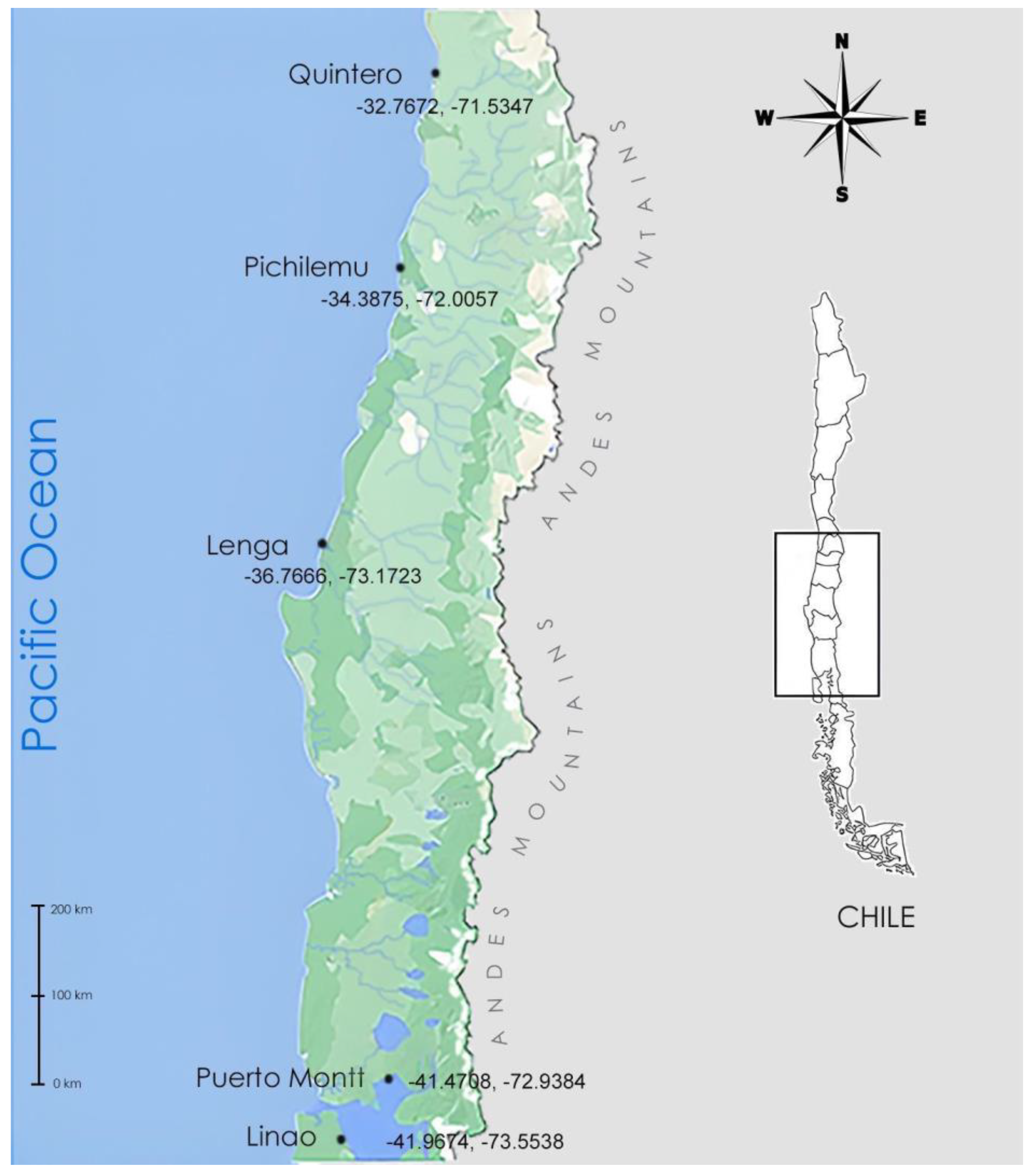
2.1. Isolation and Culture Conditions
2.2. DNA Extraction and PCR Assays
2.3. Screening for High ARA-Producing Strains and Total Biomass Assessment
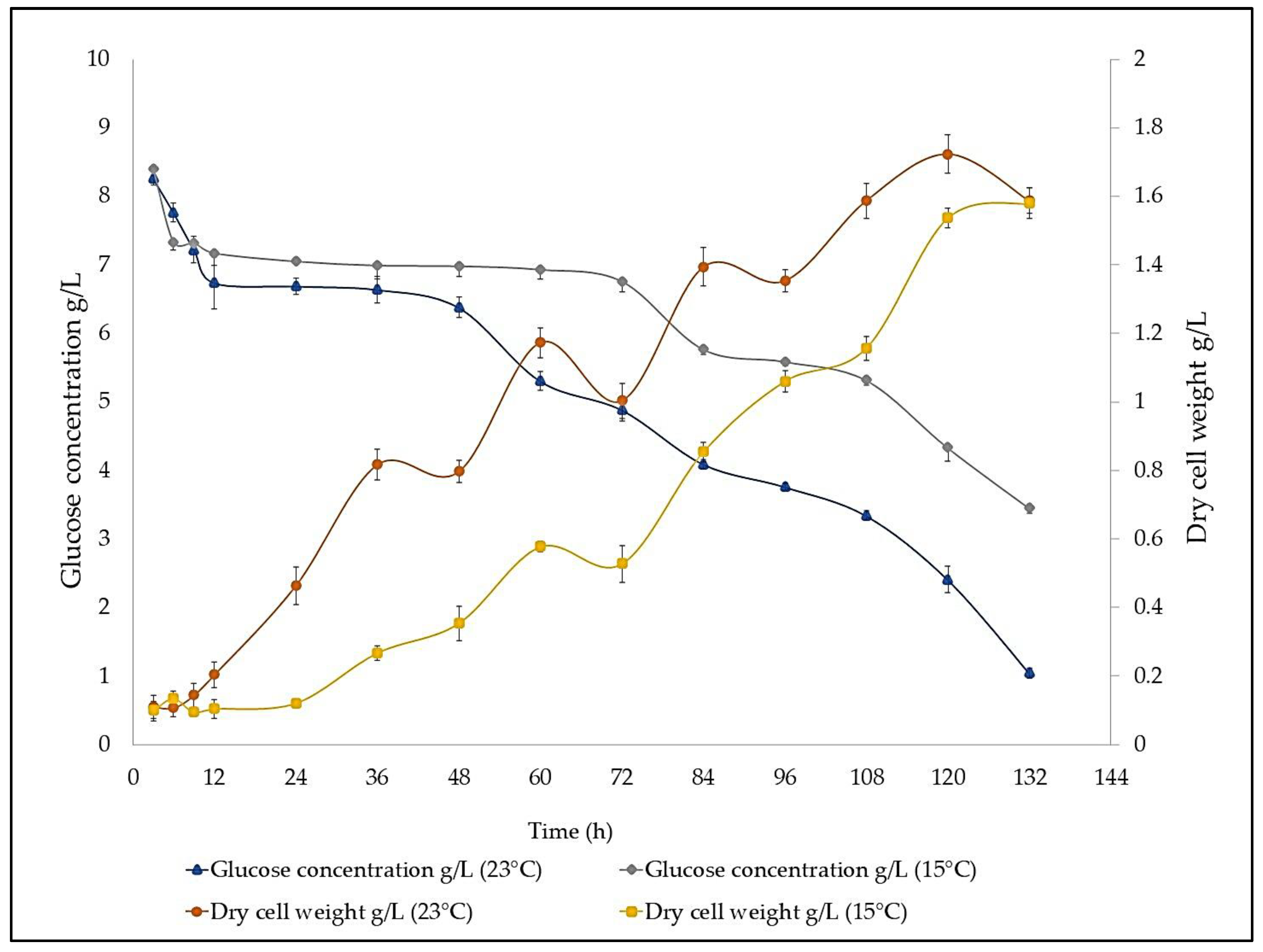
2.4. Temperature Effect on Growth Curve and Glucose Consumption
2.5. C/N Ratio Effect on Productivity de ARA
2.6. Statistical Analysis
3. Results
3.1. Morphological Characteristics and Genetic Identification
3.1.1. Screening for High ARA-Producing Strains
3.1.2. Evaluation of C/N Ratio on the Productivity of ARA
3.1.3. Effect of Culture Conditions on Cell Biomass and ARA Yields
4. Discussion
4.1. Morphological Characteristics and Genetic Identification
4.2. Biomass and Yield in Strain Natives
4.3. Evaluation of the Effect of Temperature on Strain Lng2 (U.visurgensis)
4.4. Evaluation the Reason C/N in the Biomass and Yield of ARA in Strain Lng2 (U. visurgensis)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchan, L.F.; Chang, K.J.L.; Nichols, P.D.; Mitchell, W.J.; Polglase, J.L.; Gutierrez, T. Taxonomy, ecology and biotechnological applications of thraustochytrids: A review. Biotechnol. Adv. 2018, 36, 26–46. [Google Scholar] [CrossRef]
- Leyland, B.; Leu, S.; Boussiba, S. Are Thraustochytrids algae? Fungal Biol. 2017, 121, 835–840. [Google Scholar] [CrossRef]
- Aasen, I.M.; Ertesvåg, H.; Heggeset, T.M.; Liu, B.; Brautaset, T.; Vadstein, O.; Ellingsen, T.E. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids. Appl. Microbiol. Biotechnol. 2016, 100, 4309–4321. [Google Scholar] [CrossRef]
- Qiu, X. Biosynthesis of docosahexaenoic acid (DHA, 22:6-4, 7,10,13,16,19): Two distinct pathways. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 181–186. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Ji, X.J.; Chen, S.L.; Guo, D.S.; Huang, H. Adaptive evolution of Schizochytrium sp. by continuous high oxygen stimulations to enhance docosahexaenoic acid synthesis. Bioresour. Technol. 2016, 211, 374–381. [Google Scholar] [CrossRef]
- Caamaño, E.; Loperena, L.; Hinzpeter, I.; Pradel, P.; Gordillo, F.; Corsini, G.; Tello, M.; Lavín, P.; González, A.R. Isolation and molecular characterization of Thraustochytrium strain isolated from Antarctic Peninsula and its biotechnological potential in the production of fatty acids. Braz. J. Microbiol. 2017, 48, 671–679. [Google Scholar] [CrossRef]
- Shene, C.; Leyton, A.; Esparza, Y.; Flores, L.; Quilodrán, B.; Hinzpeter, I.; Rubilar, M. Microbial oils and fatty acids: Effect of carbon source on docosahexaenoic acid (C22:6 n-3,DHA) production by thraustochytrid strains. J. Soil Sci. Plant Nutr. 2010, 10, 207–216. [Google Scholar] [CrossRef]
- Qiu, X.; Hong, H.; MacKenzie, S.L. Identification of a Δ4 fatty acid desaturase from Thraustochytrium sp. involved in the biosynthesis of docosahexaenoic acid by heterologous expression in Saccharomyces cerevisiae and Brassica juncea. J. Biol. Chem. 2001, 276, 31561–31566. [Google Scholar] [CrossRef]
- Schmidt, E.B.; Christensen, J.H.; Aardestrup, I.; Madsen, T.; Riahi, S.; Hansen, V.E. Marine n-3 fatty acids: Basic features and background. Lipids 2001, 36, S65–S68. [Google Scholar] [CrossRef]
- Ma, W.; Liu, M.; Zhang, Z.; Xu, Y.; Huang, P.; Guo, D.; Sun, X.; Huang, H. Efficient co-production of EPA and DHA by Schizochytrium sp. via regulation of the polyketide synthase pathway. Commun. Biol. 2022, 5, 1356. [Google Scholar] [CrossRef]
- Valenzuela, B.A.; Nieto, K.S. Ácidos grasos omega-6 y omega-3 en la nutrición perinatal: Su importancia en el desarrollo del sistema nervioso y visual. Rev. Chil. Pediatría 2003, 74, 149–157. [Google Scholar] [CrossRef]
- Wendel, K.; Aas, M.F.; Gunnarsdottir, G.; Rossholt, M.E.; Bratlie, M.; Nordvik, T.; Landsend, E.C.S.; Fugelseth, D.; Domellöf, M.; Pripp, A.H.; et al. Effect of arachidonic and docosahexaenoic acid supplementation on respiratory outcomes and neonatal morbidities in preterm infants. Clin. Nutr. 2023, 42, 22–28. [Google Scholar] [CrossRef]
- Hansen, J.; Schade, D.; Harris, C.; Merkel, K.; Adamkin, D. Docosahexaenoic acid plus arachidonic acid enhance preterm infant growth. Prostaglandins Leukot. Essent. Fat. Acids 1997, 57, 196. [Google Scholar]
- Clandinin, M.T.; van Aerde, J.E.; Merkel, K.L.; Harris, C.L.; Springer, M.A.; Hansen, J.W.; Diersen-Schade, D.A. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J. Pediatr. 2005, 146, 461–468. [Google Scholar] [CrossRef]
- Brenna, J.T.; Diau, G.Y. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 247–250. [Google Scholar] [CrossRef]
- Orellana, P.; Valenzuela, R.; Valenzuela, A.; Morales, G.I. Efectos neuroprotectores del ácido araquidónico y del ácido docosahexaenoico en las etapas extremas de la vida: Una visión integradora. Rev. Chil. Nutr. 2018, 45, 80–88. [Google Scholar] [CrossRef]
- Kinsella, J.E.; Lokesh, B.; Broughton, S.; Whelan, J. Dietary polyunsaturated fatty acids and eicosanoids: Potential effects on the modulation of inflammatory and immune cells: An overview. Nutrition 1990, 6, 2415. [Google Scholar]
- Hallahan, B.; Garland, M.R. Essential fatty acids and mental health. Br. J. Psychiatry 2005, 186, 275–277. [Google Scholar] [CrossRef]
- Valenzuela, R.; Sanhueza, J.; Valenzuela, A. Docosahexaenoic Acid (DHA), an Important Fatty Acid in Aging and the Protection of Neurodegenerative Diseases. J. Nutr. Ther. 2012, 1, 63–72. [Google Scholar] [CrossRef]
- Xu, H.; Meng, X.; Wei, Y.; Ma, Q.; Liang, M.; Turchini, G.M. Arachidonic acid matters. Rev. Aquac. 2022, 14, 1912–1944. [Google Scholar] [CrossRef]
- Araújo, B.C.; Skrzynska, A.K.; Marques, V.H.; Tinajero, A.; del Rio-Zaragoza, O.B.; Viana, M.T.; Mata-Sotres, J.A. Dietary Arachidonic Acid (20:4n-6) Levels and Its Effect on Growth Performance, Fatty Acid Profile, Gene Expression for Lipid Metabolism, and Health Status of Juvenile California Yellowtail (Seriola dorsalis). Fishes 2022, 7, 185. [Google Scholar] [CrossRef]
- Li, C.; Xing, X.; Qi, H.; Liu, Y.; Jian, F.; Wang, J. The arachidonic acid and its metabolism pathway play important roles for Apostichopus japonicus infected by Vibrio splendens. Fish Shellfish. Immunol. 2022, 125, 152–160. [Google Scholar] [CrossRef]
- Dantagnan, P.; Gonzalez, K.; Hevia, M.; Betancor, M.B.; Hernández, A.J.; Borquez, A.; Montero, D. Effect of the arachidonic acid/vitamin E interaction on the immune response of juvenile Atlantic salmon (Salmo salar) challenged against Piscirickettsia salmonis. Aquac. Nutr. 2017, 23, 710–720. [Google Scholar] [CrossRef]
- Alonso, D.L.; Maroto, F.G. Plants as ‘chemical factories’ for the production of polyunsaturated fatty acids. Biotechnol. Adv. 2000, 18, 481–497. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Abdo, S.M.; Ali, G. Potential production of omega fatty acids from microalgae. Int. J. Pharm. Sci. Rev. Res. 2018, 34, 210–215. [Google Scholar]
- Gupta, A.; Barrow, C.J.; Puri, M. Omega-3 biotechnology: Thraustochytrids as a novel source of omega-3 oils. Biotechnol. Adv. 2012, 30, 1733–1745. [Google Scholar] [CrossRef]
- Shanab, S.M.M.; Hafez, R.M.; Fouad, A.S. A Review on algae and plants as potential source of arachidonic acid. J. Adv. Res. 2018, 11, 3–13. [Google Scholar] [CrossRef]
- Kikukawa, H.; Sakuradani, E.; Ando, A.; Shimizu, S.; Ogawa, J. Arachidonic acid production by the oleaginous fungus Mortierella alpina 1S-4: A review. J. Adv. Res. 2018, 11, 15–22. [Google Scholar] [CrossRef]
- Ghobadi, Z.; Hamidi-Esfahani, Z.; Azizi, M.H. Statistical optimization of arachidonic acid synthesis by Mortierella alpina CBS 754.68 in a solid-state fermenter. Food Sci. Nutr. 2022, 10, 436–444. [Google Scholar] [CrossRef]
- Li, Y.-W.; Guo, Q.; Peng, Q.-Q.; Shen, Q.; Nie, Z.-K.; Ye, C.; Shi, T.-Q. Recent Development of Advanced Biotechnology in the Oleaginous Fungi for Arachidonic Acid Production. ACS Synth. Biol. 2022, 11, 3163–3173. [Google Scholar] [CrossRef]
- Nichols, D.S.; McMeekin, T.A. Biomarker techniques to screen for bacteria that produce polyunsaturated fatty acids. J. Microbiol. Methods 2002, 48, 161–170. [Google Scholar] [CrossRef]
- Bigogno, C.; Khozin-goldberg, I.; Cohen, Z. accumulation of arachidonic acid-rich triacylglycerols in the microalga Parietochloris Incisa (Trebuxiophyceae, Chlorophyta). Phytochemistry 2002, 60, 135–143. [Google Scholar] [CrossRef]
- Jiao, K.; Xiao, W.; Xu, Y.; Zeng, X.; Ho, S.H.; Laws, E.A.; Lu, Y.; Ling, X.; Shi, T.; Sun, Y.; et al. Using a trait-based approach to optimize mixotrophic growth of the red microalga Porphyridium Purpureum towards fatty acid production. Biotechnol. Biofuels 2018, 11, 273. [Google Scholar] [CrossRef]
- Lee, Y.-K. Microalgal mass culture systems and methods: Their limitations and potential. J. Appl. Phycol. 2001, 13, 307–315. [Google Scholar] [CrossRef]
- Pérez, A.; Labbé, J. Microalgas, Cultivo y Beneficios. Rev. Biol. Mar. Oceanogr. 2014, 49, 157–173. [Google Scholar] [CrossRef]
- Ganuza, E.; Benítez-Santana, T.; Atalah, E.; Vega-Orellana, O.; Ganga, R.; Izquierdo, M.S. Crypthecodinium cohnii and Schizochytrium sp. as potential substitutes to fisheries-derived oils from seabream (Sparus aurata) microdiets. Aquaculture 2008, 277, 109–116. [Google Scholar] [CrossRef]
- Fan, K.W.; Chen, F. Production of high-value products by marine microalgae Thraustochytrids. In Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2007; pp. 293–323. [Google Scholar]
- Quilodrán, B.; Hinzpeter, I.; Hormazabal, E.; Quiroz, A.; Shene, C. Docosahexaenoic acid (C22:6n−3, DHA) and astaxanthin production by Thraustochytriidae sp. AS4-A1 a native strain with high similitude to Ulkenia sp.: Evaluation of liquid residues from food industry as nutrient sources. Enzym. Microb. Technol. 2010, 47, 24–30. [Google Scholar] [CrossRef]
- Colonia, B.S.O.; de Melo Pereira, G.V.; de Carvalho, J.C.; dos Santos Sousa, P.H.; Fanka, L.S.; Rodrigues, C.; Medeiros, A.B.P.; Soccol, C.R. Lipids produced by microalgae and thraustochytrids. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 191–217. [Google Scholar]
- Bai, M.; Sen, B.; Wen, S.; Ye, H.; He, Y.; Zhang, X.; Wang, G. Culturable Diversity of Thraustochytrids from Coastal Waters of Qingdao and Their Fatty Acids. Mar. Drugs 2022, 20, 229. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Patel, A.K.; Chen, C.-W.; Chang, J.-S.; Michaud, P.; Dong, C.-D.; Singhania, R.R. Enhanced production of high-value polyunsaturated fatty acids (PUFAs) from potential thraustochytrid Aurantiochytrium sp. Bioresour. Technol. 2023, 370, 128536. [Google Scholar] [CrossRef]
- Morabito, C.; Bournaud, C.; Maës, C.; Schuler, M.; Aiese Cigliano, R.; Dellero, Y.; Maréchal, E.; Amato, A.; Rébeillé, F. The lipid metabolism in thraustochytrids. Prog. Lipid Res. 2019, 76, 101007. [Google Scholar] [CrossRef]
- Orozco Colonia, B.S.; Vinícius de Melo Pereira, G.; Soccol, C.R. Omega-3 microbial oils from marine thraustochytrids as a sustainable and technological solution: A review and patent landscape. Trends Food Sci. Technol. 2020, 99, 244–256. [Google Scholar] [CrossRef]
- Zeng, Y.; Ji, X.J.; Lian, M.; Ren, L.J.; Jin, L.J.; Ouyang, P.K.; Huang, H. Development of a temperature shift strategy for efficient docosahexaenoic acid production by a marine fungoid protist, Schizochytrium sp. HX-308. Appl. Biochem. Biotechnol. 2011, 164, 249–255. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Ji, X.J.; Huang, H. Enhancing biomass and lipid accumulation in the microalgae Schizochytrium sp. by addition of fulvic acid and EDTA. AMB Express 2018, 8, 150. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Katapodis, P.; Sharma, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Bioprospecting of thraustochytrids for omega-3 fatty acids: A sustainable approach to reduce dependency on animal sources. Trends Food Sci. Technol. 2021, 115, 433–444. [Google Scholar] [CrossRef]
- Hinzpeter, I.; Shene, C.; Masson Salaué, L. Alternativas Biotecnológicas Para la Producción de Ácidos Grasos Poliinsaturados Omega-3. September 2006. Available online: https://repositorio.uchile.cl/handle/2250/120596 (accessed on 1 November 2021).
- Honda, D.; Yokochi, T.; Nakahara, T.; Raghukumar, S.; Nakagiri, A.; Schaumann, K.; Higashihara, T. Molecular phylogeny of labyrinthulids and thraustochytrids based on the sequencing of 18S ribosomal RNA gene. J. Eukaryot. Microbiol. 1999, 46, 637–647. [Google Scholar] [CrossRef]
- Mo, C.; Douek, J.; Rinkevich, B. Development of a PCR strategy for thraustochytrid identification based on 18S rDNA sequence. Mar. Biol. 2002, 140, 883–889. [Google Scholar] [CrossRef]
- Yokoyama, R.; Honda, D. Taxonomic rearrangement of the genus Schizochytrium sensu lato based on morphology, chemotaxonomic characteristics, and 18S RRNA Gene Phylogeny (Thraustochytriaceae, Labyrinthulomycetes): Emendation for Schizochytrium and erection of Aurantiochytrium and Oblongichytrium Gen. Nov. Mycoscience 2007, 48, 199–211. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Posada, D. Selection of Models of DNA Evolution with jModelTest. Methods Mol. Biol. 2009, 537, 93–112. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride-Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, H.; Xie, Y.; He, Y.; Sen, B.; Wang, G. Culturable Diversity and Lipid Production Profile of Labyrinthulomycete Protists Isolated from Coastal Mangrove Habitats of China. Mar. Drugs 2019, 17, 268. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.A.; El-Samawaty, A.E.-R.M.A.; Elgorban, A.M.; Bahkali, A.H. Fatty acid production of thraustochytrids from Saudi Arabian mangroves. Saudi J. Biol. Sci. 2021, 28, 855–864. [Google Scholar] [CrossRef]
- Pino, N.L.; Socias, C.; González, R.R. Marine fungoid producers of DHA, EPA and carotenoids from central and southern Chilean marine ecosystems. Rev. Biol. Mar. Oceanogr. 2015, 50, 507–520. [Google Scholar] [CrossRef]
- Leano, E.; Gapasin, R.; Polohan, B.; Vrijmoed, L.; Vrijmoed, B. Growth and fatty acid production of thraustochytrids from Panay mangroves, Philippines. Fungal Divers 2003, 12, 111–122. [Google Scholar]
- Taoka, Y.; Nagano, N.; Okita, Y.; Izumida, H.; Sugimoto, S.; Hayashi, M. Influences of culture temperature on the growth, lipid content and fatty acid composition of Aurantiochytrium sp. Strain mh0186. Mar. Biotechnol. 2009, 11, 368–374. [Google Scholar] [CrossRef]
- Ma, Z.; Tan, Y.; Cui, G.; Feng, Y.; Cui, Q.; Song, X. Transcriptome and gene expression analysis of DHA producer Aurantiochytrium under low temperature conditions. Sci. Rep. 2015, 5, 14446. [Google Scholar] [CrossRef]
- Sarathy, P.P.; Bharathidasan, V.; Murugesan, P.; Selvaraj, P.; Punniyamoorthy, R. Seasonal carbonate system vis-à-vis pH and Salinity in selected tropical estuaries: Implications on polychaete diversity and composition towards predicting ecological health. Oceanologia 2022, 64, 346–362. [Google Scholar] [CrossRef]
- Amon, J.P. Thraustochytrids and Labyrinthulids of Terrestrial, Aquatic and Hypersaline Environments of the Great Salt Lake, USA. Mycologia 1978, 70, 1299. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Bi, Z.Q.; Ji, X.J.; Zhao, Q.Y.; Huang, H. Adaptive evolution of microalgae Schizochytrium sp. under high salinity stress to alleviate oxidative damage and improve lipid biosynthesis. Bioresour. Technol. 2018, 267, 438–444. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, X.; Ji, L.; Song, X.; Kuang, C. Changes of lipid content and fatty acid composition of Schizochytrium limacinum in response to different temperatures and salinities. Process Biochem. 2007, 42, 210–214. [Google Scholar] [CrossRef]
- Hu, F.; Clevenger, A.L.; Zheng, P.; Huang, Q.; Wang, Z. Low-temperature effects on docosahexaenoic acid biosynthesis in Schizochytrium sp. TIO01 and its proposed underlying mechanism. Biotechnol. Biofuels 2020. 13, 172. [CrossRef]
- Jiao, K.; Chang, J.; Zeng, X.; Ng, I.S.; Xiao, Z.; Sun, Y.; Tang, X.; Lin, L. 5-Aminolevulinic acid promotes arachidonic acid biosynthesis in the red microalga Porphyridium purpureum. Biotechnol. Biofuels 2017, 10, 168. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, G. Mechanisms of fatty acid synthesis in marine fungus-like protists. Appl. Microbiol. Biotechnol. 2015, 99, 8363–8375. [Google Scholar] [CrossRef]
- Villarroel Hipp, M.P.; Silva Rodríguez, D. Bioremediation of piggery slaughterhouse wastewater using the marine protist, Thraustochytrium kinney VAL-B1. J. Adv. Res. 2018, 12, 21–26. [Google Scholar] [CrossRef]
- Shamzi Mohamed, M.; Zee Wei, L.; Ariff, A. Heterotrophic Cultivation of Microalgae for Production of Biodiesel. Recent Pat. Biotechnol. 2011, 5, 95–107. [Google Scholar] [CrossRef]
- Contreras, P.; Oviedo, C. Potential and Future Perspectives of Thraustochytrids in Bioremediation. Int. J. Environ. Sci. Technol. 2023; in press. [Google Scholar] [CrossRef]
- Kikukawa, H.; Watanabe, K.; Kishino, S.; Takeuchi, M.; Ando, A.; Izumi, Y.; Sakuradani, E. Recent trends in the field of lipid engineering. J. Biosci. Bioeng. 2022, 133, 405–413. [Google Scholar] [CrossRef]
- Soccol, C.R.; Colonia, B.S.O.; de Melo Pereira, G.V.; Mamani, L.D.; Karp, S.G.; Thomaz Soccol, V.; de Oliveira Penha, R.; Dalmas Neto, C.J.; César de Carvalho, J. Bioprospecting lipid-producing microorganisms: From metagenomic-assisted isolation techniques to industrial application and innovations. Bioresour. Technol. 2022, 346, 126455. [Google Scholar] [CrossRef]




| Reagents | Total Organic Carbon TOC (%) | Total N (%) |
|---|---|---|
| Glucose (Gibco) | 40 | - |
| Peptone BactoTM (Gibco) | 41 | 10 |
| Yeast extract (Gibco) | 25.35 | 7.22 |
| Sampling Site | Sampling Date | Isolation | Number of Strains | Genus Level Classification |
|---|---|---|---|---|
| Quintero (Quint1) | January 2020 | Water column | 1 | Ulkenia sp. |
| Pichilemu (PiCh3-PiCh4) | September 2019 | Water column | 2 | Thraustochytrium sp. |
| Lenga Estuario (Lng 1-Lng2-Lng3-Lng6) | April 2019 | Water column | 4 | Thraustochytrium sp. Ulkenia visurgensis Botryochytrium sp. |
| Puerto Montt (PtoM) | April 2021 | Water column | 1 | Ulkenia sp. |
| Linao (LNO) | April 2021 | Marine sediment | 1 | Ulkenia sp. |
| Strain | Pich3 | Pich4 | Quint1 | Lng1 | Lng2 | Lng3 | Lng6 | LNO | PtoM |
|---|---|---|---|---|---|---|---|---|---|
| Biomass (g-DW/L) | 0.35 ± 0.25 | 0.54 + 0.03 | 0.55 + 0.80 | 0.55 ± 0.67 | 0.45 ± 0.13 | 1.12 ± 0.05 | 0.72 ± 0.34 | 0.11 ± 0.01 | 0.5 ± 0.17 |
| * Total Lipids (%) | 8.35 ± 1.62 | 9.14 ± 0.99 | 7.16 ± 2.54 | 10.66 ± 0.75 | 10.68 ± 0.98 | 16.35 ± 0.89 | 4.88 ± 2.96 | 3.89 ± 3.63 | 6.29 ± 0.93 |
| ARA 1(%) | 2.59 ± 0.77 | 3.11 ± 0.97 | 5.13 ± 0.17 | 4.25 ± 1.42 | 5.44 ± 0.75 | 2.97 ± 1.89 | 8.24 ± 2.35 | 9.31 ± 1.28 | 4.46 ± 0.65 |
| EPA 1 (%) | 11.66 ± 1.20 | 12.1 ± 0.87 | 4.46 ± 0.32 | 8.66 ± 0.86 | 16.26 ± 1.17 | 6.03 ± 0.49 | 15.17 ± 0.57 | 11.76 ± 2.18 | 3.63 ± 0.19 |
| DHA 1(%) | 57.01 ± 1.18 | 58.23 ± 1.15 | 17.8 + 0.96 | 31.46 ± 0.73 | 44.78 ± 0.84 | 22.16 ± 0.91 | 28.42 ± 2.99 | 32.58 ± 2.01 | 14.28 ± 0.55 |
| ARA mg/biomass g-DW | 2.16 ± 0.89 | 2.84 ± 1.02 | 3.67 ± 0.62 | 4.53 ± 0.90 | 5.81 ± 1.17 | 4.86 ± 0.95 | 4.02 ± 0.76 | 3.62 ± 0.91 | 2.8 ± 2.01 |
| EPA mg/biomass g DW | 9.74 ± 1.07 | 11.06 ± 0.75 | 3.19 ± 0.87 | 9.23 ± 0.56 | 17.37 ± 0.95 | 9.86 ± 1.02 | 7.4 ± 0.89 | 4.57 ± 0.57 | 2.28 ± 0.83 |
| DHA mg/biomass g DW | 47.61 ± 1.08 | 53.22 ± 2.91 | 12.74 ± 1.19 | 33.54 ± 0.03 | 47.83 ± 0.05 | 36.23 ± 0.82 | 13.87 ± 0.23 | 12.66 ± 0.76 | 8.98 ± 0.79 |
| Strain LNG2 | 15 °C | 23 °C | ||
|---|---|---|---|---|
| 72 h | 120 h | 72 h | 120 h | |
| Biomass g-DW/L | 1.14 ± 0.23 | 1.24 ± 0.11 | 1.46 ± 0.45 | 1.09 ± 0.25 |
| 1 Total Lipids (%) | 8.52 ± 1.05 | 9.65 ± 1.79 | 7.79 ± 0.98 | 8.99 ± 1.45 |
| 1 C12:0 | 0.16 ± 0.04 | 0.21 ± 0.05 | 0.3 ± 0.17 | 0.28 ± 0.13 |
| 1 C14:0 | 1.58 ± 0.86 | 1.92 ± 1.26 | 2.29 ± 0.60 | 1.98 ± 0.09 |
| 1 C15:0 | 1.10 ± 0.19 | 1.57 ± 0.36 | 1.45 ± 0.42 | 1.37 ± 0.37 |
| 1 C16:0 | 33.79 ± 1.09 | 39.98 ± 4.28 | 43.36 ± 3.79 | 38.78 ± 4.62 |
| 1 C17:0 | 0.61 ± 0.23 | 1.04 ± 0.44 | 0.76 ± 0.27 | 0.77 ± 0.34 |
| 1 C18:0 | 6.23 ± 1.09 | 8.40 ± 1.46 | 7.76 ± 3.15 | 7.86 ± 3.65 |
| 1 C18:3n3 | 0.03 ± 0.03 | 0.03 ± 0.02 | 0 ± 0.00 | 0.03 ± 0.02 |
| 1 C20:2 | 0.09 ± 0.04 | 0.11 ± 0.01 | 0.07 ± 0.06 | 0.1 ± 0.02 |
| 1 C20:4n6 ARA | 3.99 ± 1.49 | 3.10 ± 1.59 | 1.88 ± 1.12 | 3.07 ± 1.07 |
| 1 C20:5n3 | 4.67 ± 2.82 | 3.34 ± 1.76 | 4.44 ± 2.86 | 4.81 ± 3.17 |
| 1 C22:6n3 | 33.65 ± 0.89 | 26.97 ± 1.10 | 21.81 ± 0.10 | 25.09 ± 2.14 |
| 2 ARA mg/biomass g-DW | 3.40 ± 0.89 | 2.99 ± 1.00 | 1.46 ± 0.98 | 2.79 ± 0.40 |
| 1 Total PUFAs | 44.00 ± 3.53 | 29.48 ± 4.21 | 34.87 ± 0.30 | 34.45 ± 3.86 |
| 1 Total SFAs | 45.38 ± 2.29 | 56.87 ± 1.83 | 54.56 ± 7.48 | 52.27 ± 4.30 |
| 1 Total MFAs | 10.63 ± 1.80 | 13.65 ± 1.97 | 10.56 ± 2.01 | 13.28 ± 3.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasquez-Sandoval, C.; Navarrete, J.; Herrera-Herrera, P.; Dantagnan, P.; Diaz-Navarrete, P.; Arancibia-Avila, P.; Oviedo, C. Screening and Identification of Coastal Chilean Thraustochytrids for Arachidonic Acid Production: Biotechnological Potential of Ulkenia visurgensis Lng2-Strain. Microorganisms 2023, 11, 559. https://doi.org/10.3390/microorganisms11030559
Vasquez-Sandoval C, Navarrete J, Herrera-Herrera P, Dantagnan P, Diaz-Navarrete P, Arancibia-Avila P, Oviedo C. Screening and Identification of Coastal Chilean Thraustochytrids for Arachidonic Acid Production: Biotechnological Potential of Ulkenia visurgensis Lng2-Strain. Microorganisms. 2023; 11(3):559. https://doi.org/10.3390/microorganisms11030559
Chicago/Turabian StyleVasquez-Sandoval, Cinthia, José Navarrete, Paula Herrera-Herrera, Patricio Dantagnan, Paola Diaz-Navarrete, Patricia Arancibia-Avila, and Claudia Oviedo. 2023. "Screening and Identification of Coastal Chilean Thraustochytrids for Arachidonic Acid Production: Biotechnological Potential of Ulkenia visurgensis Lng2-Strain" Microorganisms 11, no. 3: 559. https://doi.org/10.3390/microorganisms11030559
APA StyleVasquez-Sandoval, C., Navarrete, J., Herrera-Herrera, P., Dantagnan, P., Diaz-Navarrete, P., Arancibia-Avila, P., & Oviedo, C. (2023). Screening and Identification of Coastal Chilean Thraustochytrids for Arachidonic Acid Production: Biotechnological Potential of Ulkenia visurgensis Lng2-Strain. Microorganisms, 11(3), 559. https://doi.org/10.3390/microorganisms11030559








