What Is the Best Vancomycin Therapeutic Drug Monitoring Parameter to Assess Efficacy? A Critical Review of Experimental Data and Assessment of the Need for Individual Patient Minimum Inhibitory Concentration Value
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Paucity of Peer Reviewed Published Studies from In Vivo PKPD Studies
3.2. Is Evidence from In Vitro Models Consistent with In Vivo Studies?
3.3. Is the Use of MIC Required in PKPD Parameter Based TDM?
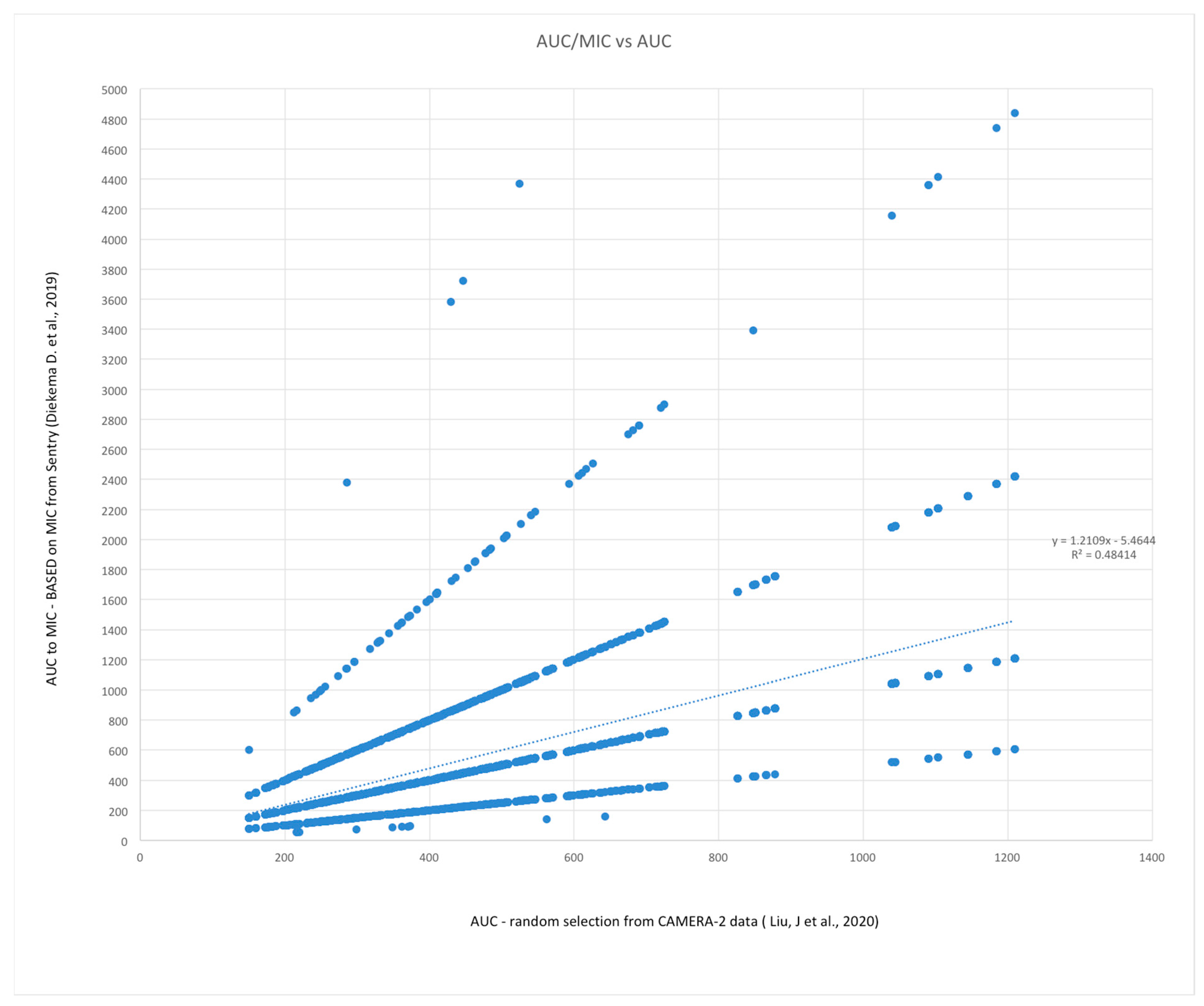
3.4. Monte Carlo Simulations of AUC24 and AUC24:MIC (Methods Described above)
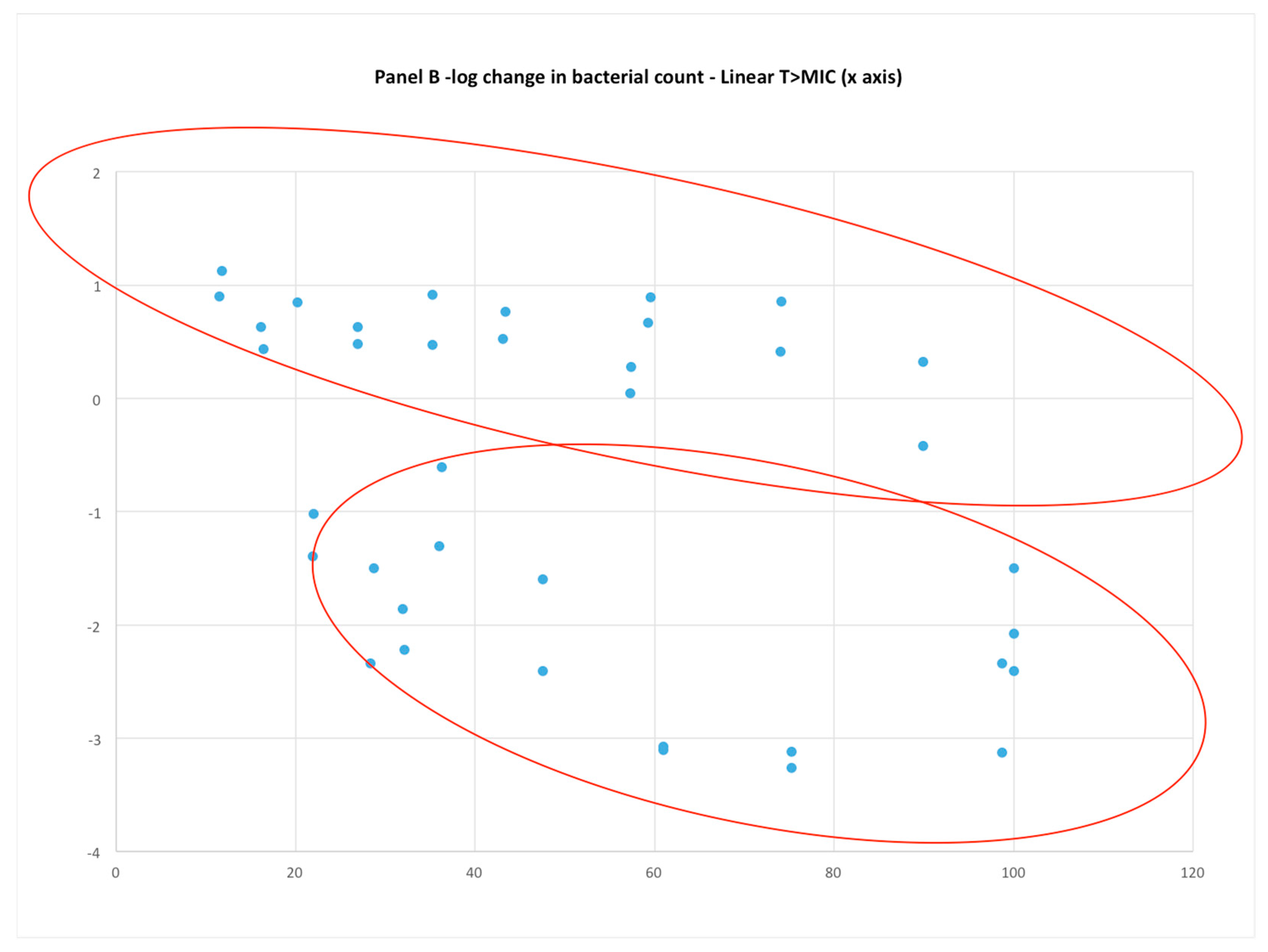
3.5. Does %T > MIC Represent the Activity of troughs of Vancomycin in the Clinical Setting?
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Rybak, M.J.; Lomaestro, B.M.; Rotscahfer, J.C.; Moellering, J.R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin Therapeutic Guidelines: A Summary of Consensus Recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef]
- Rocha, J.L.L.; Kondo, W.; Baptista, M.I.D.K.; Da Cunha, C.A.; Martins, L.T.F. Uncommon vancomycin: Induced side effects. Braz. J. Infect. Dis. 2002, 6, 196–200. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.; Levine, D.; Bradley, J.; Liu, C.; Mueller, B.; Pai, M.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Executive Summary: Therapeutic Monitoring of Vancomycin for Serious Methicillin-Resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. J. Pediatr. Infect. Dis. Soc. 2020, 9, 281–284. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Executive Summary: Therapeutic Monitoring of Vancomycin for Serious Methicillin-Resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 363–367. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic Monitoring of Vancomycin for Serious Methicillin-resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2020, 71, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Oda, K.; Shoji, K.; Hanai, Y.; Takahashi, Y.; Fujii, S.; Hamada, Y.; Kimura, T.; Mayumi, T.; Ueda, T.; et al. Clinical Practice Guidelines for Therapeutic Drug Monitoring of Vancomycin in the Framework of Model-Informed Precision Dosing: A Consensus Review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Pharmaceutics 2022, 14, 489. [Google Scholar] [CrossRef]
- Reuter, S.E.; Stocker, S.L.; Alffenaar, J.-W.C.P.; Baldelli, S.C.; Cattaneo, D.P.; Jones, G.M.; Koch, B.C.P.; Kocic, D.; Mathew, S.K.; Molinaro, M.M.; et al. Optimal Practice for Vancomycin Therapeutic Drug Monitoring: Position Statement From the Anti-infectives Committee of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther. Drug Monit. 2021, 44, 121–132. [Google Scholar] [CrossRef]
- Moise-Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of Vancomycin and Other Antimicrobials in Patients with Staphylococcus aureus Lower Respiratory Tract Infections. Clin. Pharmacokinet. 2004, 43, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Davis, S.L.; Levine, D.P.; Rybak, M.J. Impact of Vancomycin Exposure on Outcomes in Patients With Methicillin-Resistant Staphylococcus aureus Bacteremia: Support for Consensus Guidelines Suggested Targets. Clin. Infect. Dis. 2011, 52, 975–981. [Google Scholar] [CrossRef]
- Brown, J.; Brown, K.; Forrest, A. Vancomycin AUC 24 /MIC Ratio in Patients with Complicated Bacteremia and Infective Endocarditis Due to Methicillin-Resistant Staphylococcus aureus and Its Association with Attributable Mortality during Hospitalization. Antimicrob. Agents Chemother. 2012, 56, 634–638. [Google Scholar] [CrossRef]
- Holmes, N.E.; Turnidge, J.D.; Munckhof, W.J.; Robinson, J.O.; Korman, T.M.; O’Sullivan, M.V.N.; Anderson, T.L.; Roberts, S.A.; Warren, S.J.C.; Gao, W.; et al. Vancomycin AUC/MIC Ratio and 30-Day Mortality in Patients with Staphylococcus aureus Bacteremia. Antimicrob. Agents Chemother. 2013, 57, 1654–1663. [Google Scholar] [CrossRef]
- Gawronski, K.; Goff, D.A.; Brown, J.; Khadem, T.M.; Bauer, K.A. A Stewardship Program’s Retrospective Evaluation of Vancomycin AUC24/MIC and Time to Microbiological Clearance in Patients with Methicillin-Resistant Staphylococcus aureus Bacteremia and Osteomyelitis. Clin. Ther. 2013, 35, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Song, K.-H.; Cho, J.E.; Kim, H.-S.; Kim, N.-H.; Kim, T.S.; Choe, P.G.; Chung, J.-Y.; Park, W.B.; Bang, J.H.; et al. Area under the concentration–time curve to minimum inhibitory concentration ratio as a predictor of vancomycin treatment outcome in methicillin-resistant Staphylococcus aureus bacteraemia. Int. J. Antimicrob. Agents 2014, 43, 179–183. [Google Scholar] [CrossRef]
- Lodise, T.P.; Drusano, G.L.; Zasowski, E.; Dihmess, A.; Lazariu, V.; Cosler, L.; McNutt, L.-A. Vancomycin Exposure in Patients With Methicillin-Resistant Staphylococcus aureus Bloodstream Infections: How Much Is Enough? Clin. Infect. Dis. 2014, 59, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Rosenkranz, S.L.; Finnemeyer, M.; Evans, S.; Sims, M.; Zervos, M.J.; Creech, C.B.; Patel, P.C.; Keefer, M.; Riska, P.; et al. The Emperor’s New Clothes: PRospective Observational Evaluation of the Association Between Initial VancomycIn Exposure and Failure Rates Among ADult HospitalizEd Patients With Methicillin-resistant Staphylococcus aureus Bloodstream Infections (PROVIDE). Clin. Infect. Dis. 2019, 70, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Casapao, A.M.; Lodise, T.P.; Davis, S.L.; Claeys, K.C.; Kullar, R.; Levine, D.P.; Rybak, M.J. Association between Vancomycin Day 1 Exposure Profile and Outcomes among Patients with Methicillin-Resistant Staphylococcus aureus Infective Endocarditis. Antimicrob. Agents Chemother. 2015, 59, 2978–2985. [Google Scholar] [CrossRef]
- Dalton, B.R.; Rajakumar, I.; Langevin, A.; Ondro, C.; Sabuda, D.; Griener, T.P.; Dersch-Mills, D.; Rennert-May, E. Vancomycin area under the curve to minimum inhibitory concentration ratio predicting clinical outcome: A systematic review and meta-analysis with pooled sensitivity and specificity. Clin. Microbiol. Infect. 2019, 26, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.; Jorgensen, S.C.; Dresser, L.; Lau, T.T.; Gin, A.; Thirion, D.J.; Nishi, C.; Dalton, B. A Canadian perspective on the revised 2020 ASHP–IDSA–PIDS–SIDP guidelines for vancomycin AUC-based therapeutic drug monitoring for serious MRSA infections. Off. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2021, 6, 3–9. [Google Scholar] [CrossRef]
- Wright, W.F.; Jorgensen, S.C.J.; Spellberg, B. Heaping the Pelion of Vancomycin on the Ossa of Methicillin-resistant Staphylococcus aureus: Back to Basics in Clinical Care and Guidelines. Clin. Infect. Dis. 2020, 72, e682–e684. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, T.J.; Schulz, L.T.; Rose, W.E. Vancomycin Advanced Therapeutic Drug Monitoring: Exercise in Futility or Virtuous Endeavor to Improve Drug Efficacy and Safety? Clin. Infect. Dis. 2020, 72, e675–e681. [Google Scholar] [CrossRef]
- Dalton, B.R.; Krishnan, A.; Stewart, J.J.; Jorgensen, S.C.J. Limitations of classification and regression tree analysis in vancomycin exposure-response relationship studies: Insights from data simulation (Letter). Clin. Microbiol. Infect. 2021, 27, 1701–1703. [Google Scholar] [CrossRef]
- Rybak, M.J. The Pharmacokinetic and Pharmacodynamic Properties of Vancomycin. Clin. Infect. Dis. 2006, 42, S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. State-of-the-Art Clinical Article: Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef]
- Craig, W.A. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 2003, 17, 479–501. [Google Scholar] [CrossRef]
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat. Rev. Genet. 2004, 2, 289–300. [Google Scholar] [CrossRef]
- Gunderson, B.W.; Ross, G.H.; Ibrahim, K.H.; Rotschafer, J.C. What Do We Really Know About Antibiotic Pharmacodynamics? Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001, 21, 302S–318S. [Google Scholar] [CrossRef]
- Avedissian, S.N.; Pais, G.; Liu, J.; O’Donnell, J.N.; Lodise, T.P.; Neely, M.; Prozialeck, W.C.; Lamar, P.C.; Becher, L.; Scheetz, M.H. The Pharmacodynamic-Toxicodynamic Relationship of AUC and Cmax in Vancomycin-Induced Kidney Injury in an Animal Model. Antimicrob. Agents Chemother. 2021, 65, e01945-20. [Google Scholar] [CrossRef]
- Ebert, S.; Leggett, J.; Vogelman, B. In Vivo Cidal Activity and Pharmacokinetic Parameters (PKPs) for Vancomycin (VAN) against Methicillin-Susceptible and Methicillin-Resistant S. aureus. In Proceedings of the Program and Abstracts of the Twenty-Seventh Interscience Conference of Antimicrobial Agents and Chemotherapy, New York, NY, USA, 4–7 October 1987; p. 173. [Google Scholar]
- Lepak, A.J.; Zhao, M.; Andes, D.R. Comparative Pharmacodynamics of Telavancin and Vancomycin in the Neutropenic Murine Thigh and Lung Infection Models against Staphylococcus aureus. Antimicrob. Agents Chemother. 2017, 61, e00281-17. [Google Scholar] [CrossRef]
- Liu, J.; Tong, S.Y.C.; Davis, J.S.; Rhodes, N.J.; Scheetz, M.H.; Anagostou, N.; Andresen, D.; Archuleta, S.; Bak, N.; Cass, A.; et al. Vancomycin Exposure and Acute Kidney Injury Outcome: A Snapshot From the CAMERA2 Study. Open Forum Infect. Dis. 2020, 7, ofaa538. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Pfaller, M.; Shortridge, D.; Zervos, M.; Jones, R.N. Twenty-Year Trends in Antimicrobial Susceptibilities Among Staphylococcus aureus From the SENTRY Antimicrobial Surveillance Program. Open Forum Infect. Dis. 2019, 6, S47–S53. [Google Scholar] [CrossRef]
- Dudley, M.; Griffith, D.; Corcoran, E.; Liu, C.; Sorensen, K.; Tembe, V.; Cotter, D.; Chamberland, S.; Chen, S. 2031 Pharmacokinetic-Pharmacodynamic (PK-PD) Indices for Vancomycin treatment of Susceptible and Intermediate S. aureus in the Neutropenic Mouse Thich Model. In Proceedings of the Program and Abstracts of the Thirty-Nineth Interscience Conference of Antimicrobial Agents and Chemotherapy, San Francisco, CA, USA, 26–29 September 1999; p. 49. [Google Scholar]
- Lee, D.-G.; Murakami, Y.; Andes, D.R.; Craig, W.A. Inoculum Effects of Ceftobiprole, Daptomycin, Linezolid, and Vancomycin with Staphylococcus aureus and Streptococcus pneumoniae at Inocula of 10 5 and 10 7 CFU Injected into Opposite Thighs of Neutropenic Mice. Antimicrob. Agents Chemother. 2013, 57, 1434–1441. [Google Scholar] [CrossRef]
- Ackerman, B.H.; Vannier, A.M.; Eudy, E.B. Analysis of vancomycin time-kill studies with Staphylococcus species by using a curve stripping program to describe the relationship between concentration and pharmacodynamic response. Antimicrob. Agents Chemother. 1992, 36, 1766–1769. [Google Scholar] [CrossRef]
- Cantoni, L.; Glauser, M.P.; Bille, J. Comparative efficacy of daptomycin, vancomycin, and cloxacillin for the treatment of Staphylococcus aureus endocarditis in rats and role of test conditions in this determination. Antimicrob. Agents Chemother. 1990, 34, 2348–2353. [Google Scholar] [CrossRef]
- Duffull, S.B.; Begg, E.J.; Chambers, S.T.; Barclay, M.L. Efficacies of different vancomycin dosing regimens against Staphylococcus aureus determined with a dynamic in vitro model. Antimicrob. Agents Chemother. 1994, 38, 2480–2482. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.J.; Walker, K.J.; Raddatz, J.K.; Rotschafer, J.C. The concentration-independent effect of monoexponential and biexponential decay in vancomycin concentrations on the killing of Staphylococcus aureus under aerobic and anaerobic conditions. J. Antimicrob. Chemother. 1996, 38, 589–597. [Google Scholar] [CrossRef]
- Löwdin, E.; Odenholt, I.; Cars, O. In Vitro Studies of Pharmacodynamic Properties of Vancomycin against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 1998, 42, 2739–2744. [Google Scholar] [CrossRef]
- Peetermans, W.E.; Hoogeterp, J.J.; Dokkum, A.M.H.-V.; Broek, P.V.D.; Mattie, H. Antistaphylococcal activities of teicoplanin and vancomycin in vitro and in an experimental infection. Antimicrob. Agents Chemother. 1990, 34, 1869–1874. [Google Scholar] [CrossRef]
- Khatib, R.; Riederer, K.; Shemes, S.; Musta, A.C.; Szpunar, S. Correlation of methicillin-resistant Staphylococcus aureus vancomycin minimal inhibitory concentration results by Etest and broth microdilution methods with population analysis profile: Lack of Etest overestimation of the MIC. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 803–806. [Google Scholar] [CrossRef]
- Kruzel, M.C.; Lewis, C.T.; Welsh, K.J.; Lewis, E.M.; Dundas, N.E.; Mohr, J.F.; Armitige, L.Y.; Wanger, A. Determination of Vancomycin and Daptomycin MICs by Different Testing Methods for Methicillin-Resistant Staphylococcus aureus. J. Clin. Microbiol. 2011, 49, 2272–2273. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Nation, R.L. Limitations of Antibiotic MIC-Based PK-PD Metrics: Looking Back to Move Forward. Front. Pharmacol. 2021, 12, 770518. [Google Scholar] [CrossRef]
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 2017, 73, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, J.M.; McKinnon, P.S.; Zimmer, G.S.; Schentag, J.J. The Importance of Pharmacokinetic/Pharmacodynamic Surrogate Markers to Outcome. Clin. Pharmacokinet. 1995, 28, 143–160. [Google Scholar] [CrossRef] [PubMed]



| Pharmacokinetic/Pharmacodynamic (PKPD) Parameter | Therapeutic Drug Monitoring (TDM) Parameter | |
|---|---|---|
| Purpose | Understanding of drug mechanism, empiric dosing for general therapeutic target | Adjusting dose to individualized target |
| Setting | Experimental model | Clinical |
| Use of MIC | Use of MIC–reference method(s) | Delay to obtain MIC, usually from automated susceptibility method(s). In the case of vancomycin; assumed to equal 1 mg/L |
| Range of dosing | Very wide (orders of magnitude) | Human therapeutic range |
| Relevant outcomes | Bacterial kill Animal survival Organ damage/toxicity markers | Clinical cure Patient survival Toxicity |
| Specific examples | Time above the minimum inhibitory concentration (%T > MIC) Maximum or peak concentration (Cmax) to MIC ratio Area under concentration time curve (AUC, various time intervals) to MIC ratio | Trough or minimum concentration (Cmin) Cmax Twenty-four hour AUC (AUC24) |
| Citation/Ref Number | Model | Vancomycin Concentrations (mg/L) | Results |
|---|---|---|---|
| Ackerman B et al., 1992 [35] | Static concentration— 24 h observation. Staphylococcal spp. | 10, 20, 30 and 50 | Statistically and visually no difference in bacterial kill in Staphylococcus aureus. |
| Cantoni L et al., 1990 [36] | Static concentration— 48 h observation Staphylococcus aureus | 2, 10, 40 | Visually slightly less activity of 2 mg/L vs. 10 and 40. No statistical test result reported. |
| Duffull S et al., 1994 [37] | Dynamic in vitro model—24 h observation Staphylococcus aureus | 48→3 (one dose) 30→7.5 (two doses) 16.2 mg/L constant | No difference in rate or extent of bacterial killing. |
| Larsson A et al., 1996 [38] | Dynamic in vitro—12 h observation Staphylococcus aureus T1/2 = 6 h | 5, 10, 20 or 40 mg/L peak concentration | 5 and 20 mg/L slightly lower extent of killing than 10 and 40 mg/L by visual inspection. Statistically NS. |
| Lowdin E et al., 1998 [39] | Dynamic in vitro—24 h observation Staphylococcus aureus and Staphylococcus epidermidis. T1/2 = 5 h | 2, 4, 8, 16 and 64× MIC | No concentration-dependent killing observed by visual inspection. |
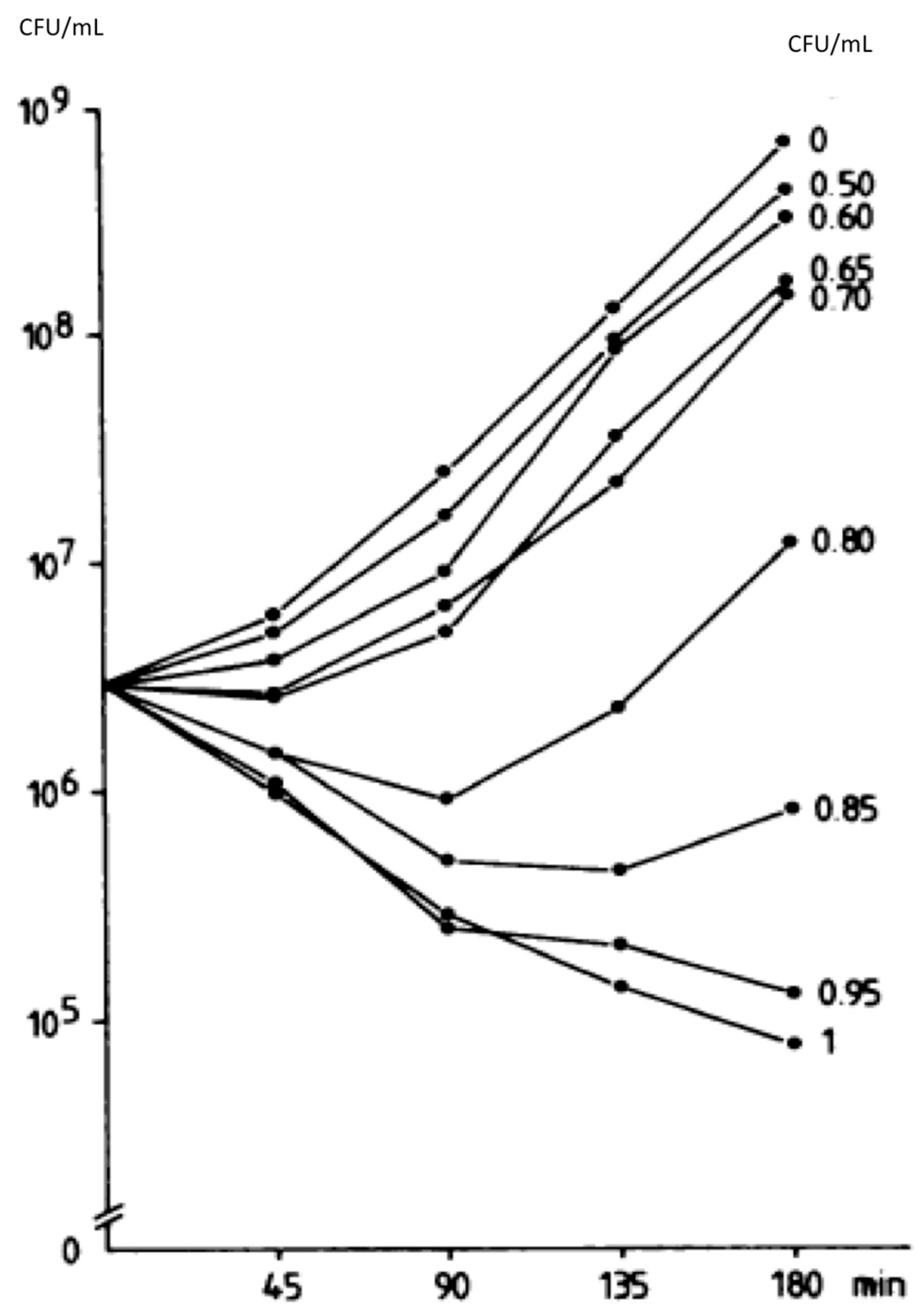
| Peetermans W et al., 1990 [40] | Static concentrations—3 h observation, Staphylococcus aureus | 0.5, 0.6, 0.65, 0.7, 0.8, 0.85, 0.95 and 1 | Progressive effect on bacterial killing by increasing concentrations. Higher than 1 mg/L did not lead to more killing. |
| Simulation Number | R-Square (Pearson, Correlation) | Bias (Linear Regression Coefficient) |
|---|---|---|
| 1 | 0.498 | 1.198 |
| 2 | 0.508 | 1.210 |
| 3 | 0.500 | 1.202 |
| 4 | 0.492 | 1.170 |
| 5 | 0.484 | 1.211 |
| AUC:MIC High | AUC:MIC Therapeutic | AUC:MIC Low | |
|---|---|---|---|
| AUC High | 3171 (15.9) | 44 (0.2) | 120 (0.6) |
| AUC Therapeutic | 1430 (7.2) | 5312 (26.6) | 359 (1.8) |
| AUC Low | 994 (5.0) | 825 (4.1) | 7745 (38.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalton, B.R. What Is the Best Vancomycin Therapeutic Drug Monitoring Parameter to Assess Efficacy? A Critical Review of Experimental Data and Assessment of the Need for Individual Patient Minimum Inhibitory Concentration Value. Microorganisms 2023, 11, 567. https://doi.org/10.3390/microorganisms11030567
Dalton BR. What Is the Best Vancomycin Therapeutic Drug Monitoring Parameter to Assess Efficacy? A Critical Review of Experimental Data and Assessment of the Need for Individual Patient Minimum Inhibitory Concentration Value. Microorganisms. 2023; 11(3):567. https://doi.org/10.3390/microorganisms11030567
Chicago/Turabian StyleDalton, Bruce R. 2023. "What Is the Best Vancomycin Therapeutic Drug Monitoring Parameter to Assess Efficacy? A Critical Review of Experimental Data and Assessment of the Need for Individual Patient Minimum Inhibitory Concentration Value" Microorganisms 11, no. 3: 567. https://doi.org/10.3390/microorganisms11030567
APA StyleDalton, B. R. (2023). What Is the Best Vancomycin Therapeutic Drug Monitoring Parameter to Assess Efficacy? A Critical Review of Experimental Data and Assessment of the Need for Individual Patient Minimum Inhibitory Concentration Value. Microorganisms, 11(3), 567. https://doi.org/10.3390/microorganisms11030567





