Association between Gut Dysbiosis and the Occurrence of SIBO, LIBO, SIFO and IMO
Abstract
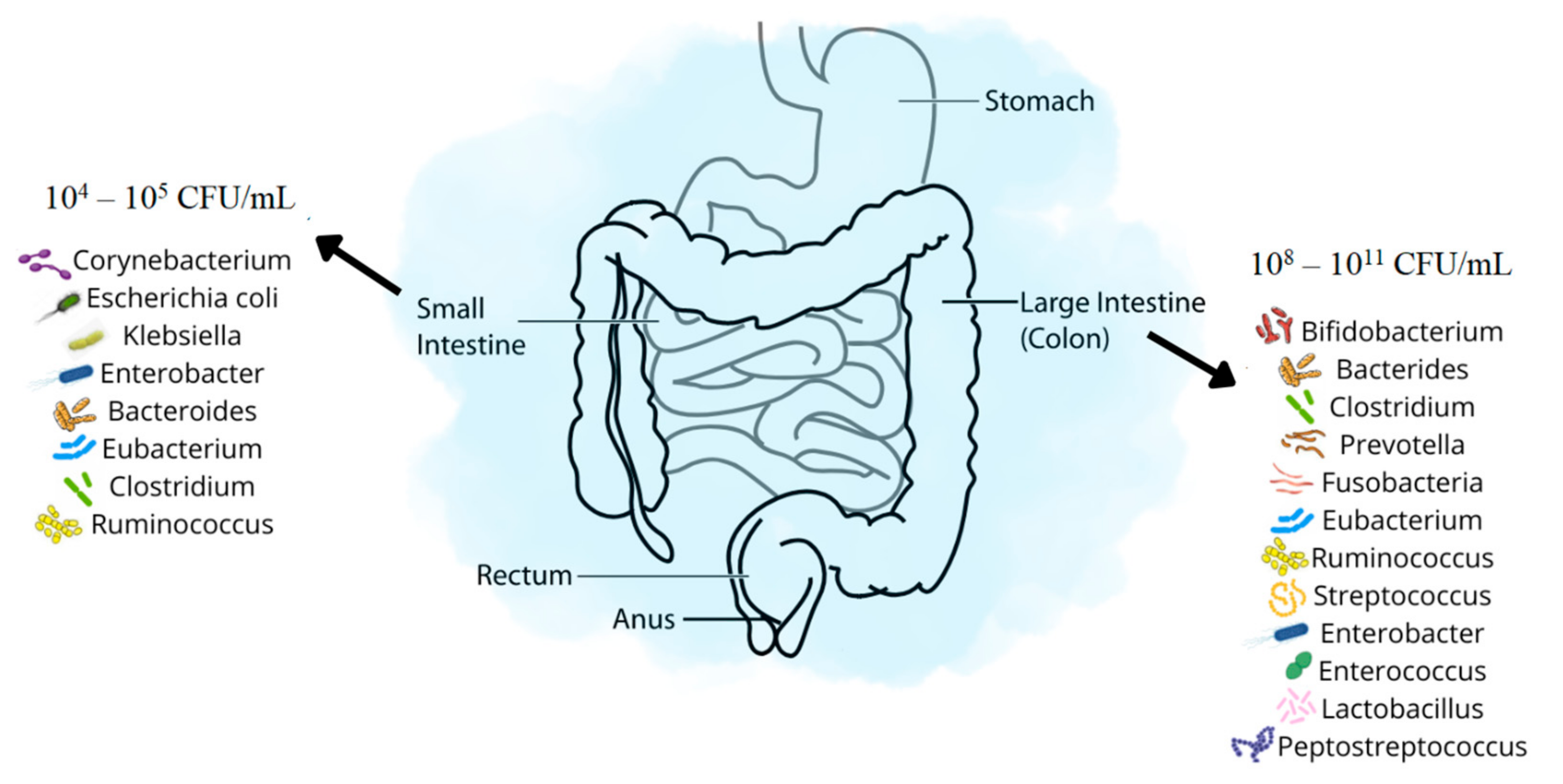
1. Introduction
2. Small Intestinal Bacterial Overgrowth—SIBO
3. Large Intestinal Bacterial Overgrowth—LIBO
4. Small Intestinal Fungal Overgrowth—SIFO
5. Intestinal Methanogen Overgrowth—IMO
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sheh, A.; Fox, J.G. The Role of the Gastrointestinal Microbiome in Helicobacter Pylori Pathogenesis. Gut Microbes 2013, 4, 505–531. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, M.; Tokarz-Deptuła, B.; Deptuła, W. The Human Microbiome. Postepy Mikrobiol. 2017, 56, 33–42. [Google Scholar] [CrossRef]
- Crnčević, N.; Hukić, M.; Deumić, S.; Selimagić, A.; Dozić, A.; Gavrankapetanović, I.; Klepo, D.; Avdić, M. Gastrointestinal Tract Microbiome Effect and Role in Disease Development. Diseases 2022, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Avelar Rodriguez, D.; Ryan, P.M.; Toro Monjaraz, E.M.; Ramirez Mayans, J.A.; Quigley, E.M. Small Intestinal Bacterial Overgrowth in Children: A State-Of-The-Art Review. Front. Pediatr. 2019, 7, 363. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. WJG 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Minot, S.; Sinha, R.; Chen, J.; Li, H.; Keilbaugh, S.A.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. The Human Gut Virome: Inter-Individual Variation and Dynamic Response to Diet. Genome Res. 2011, 21, 1616–1625. [Google Scholar] [CrossRef]
- Szóstak, N.; Handschuh, L.; Samelak-Czajka, A.; Tomela, K.; Schmidt, M.; Pruss, Ł.; Milanowska-Zabel, K.; Kozlowski, P.; Philips, A. Host Factors Associated with Gut Mycobiome Structure. mSystems 2023, e0098622. [Google Scholar] [CrossRef]
- Hoffmann, C.; Dollive, S.; Grunberg, S.; Chen, J.; Li, H.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Archaea and Fungi of the Human Gut Microbiome: Correlations with Diet and Bacterial Residents. PLoS ONE 2013, 8, e66019. [Google Scholar] [CrossRef]
- Rodrigues Hoffmann, A.; Proctor, L.M.; Surette, M.G.; Suchodolski, J.S. The Microbiome: The Trillions of Microorganisms That Maintain Health and Cause Disease in Humans and Companion Animals. Vet. Pathol. 2016, 53, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project. Available online: https://hmpdacc.org (accessed on 16 February 2023).
- Gałecka, M.; Basińska, A.M.; Bartnicka, A. The importance of intestinal microbiota in shaping human health—implications in the practice of the family physician. Forum Med. Rodz. 2018, 12, 50–59. [Google Scholar]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, D.; Cichy, W.; Przysławski, J.; Drzymała-Czyż, S. The Role of Microbiota and Enteroendocrine Cells in Maintaining Homeostasis in the Human Digestive Tract. Adv. Med. Sci. 2021, 66, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, N.; Kiersztan, A. The Role of Gut Microbiota in the Pathogenesis of Obesity and Diabetes. Postępy Hig. Med. Dośw. 2013, 67, 288–303. [Google Scholar] [CrossRef]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A Critical Assessment of the “Sterile Womb” and “in Utero Colonization” Hypotheses: Implications for Research on the Pioneer Infant Microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 2019, 10, 1124. [Google Scholar] [CrossRef]
- de Goffau, M.C.; Lager, S.; Sovio, U.; Gaccioli, F.; Cook, E.; Peacock, S.J.; Parkhill, J.; Charnock-Jones, D.S.; Smith, G.C.S. Human Placenta Has No Microbiome but Can Contain Potential Pathogens. Nature 2019, 572, 329–334. [Google Scholar] [CrossRef]
- Butel, M.-J.; Suau, A.; Campeotto, F.; Magne, F.; Aires, J.; Ferraris, L.; Kalach, N.; Leroux, B.; Dupont, C. Conditions of Bifidobacterial Colonization in Preterm Infants: A Prospective Analysis. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 577. [Google Scholar] [CrossRef]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Hernández-Barranco, A.; Margolles, A.; de Los Reyes-Gavilán, C.G.; Gueimonde, M. Establishment and Development of Intestinal Microbiota in Preterm Neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, G.; Benenati, B.; Morelli, L.; Bessi, E.; Boehm, G. Cesarean Delivery May Affect the Early Biodiversity of Intestinal Bacteria. J. Nutr. 2008, 138, 1796S–1800S. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L. Gut Microbiota of Healthy Canadian Infants: Profiles by Mode of Delivery and Infant Diet at 4 Months. CMAJ 2013, 185, 385–394. [Google Scholar] [CrossRef]
- Chen, G.; Chiang, W.-L.; Shu, B.-C.; Guo, Y.L.; Chiou, S.-T.; Chiang, T. Associations of Caesarean Delivery and the Occurrence of Neurodevelopmental Disorders, Asthma or Obesity in Childhood Based on Taiwan Birth Cohort Study. BMJ Open 2017, 7, e017086. [Google Scholar] [CrossRef] [PubMed]
- Sevelsted, A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Cesarean Section and Chronic Immune Disorders. Pediatrics 2015, 135, e92–e98. [Google Scholar] [CrossRef]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.; Abe, F.; Osawa, R. Age-Related Changes in Gut Microbiota Composition from Newborn to Centenarian: A Cross-Sectional Study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Doré, J.; Schiffrin, E.J. The Inflammatory Status of Old Age Can Be Nurtured from the Intestinal Environment. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 13–20. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Levi Mortera, S.; Marzano, V.; Vernocchi, P.; Matteoli, M.C.; Guarrasi, V.; Gardini, S.; Del Chierico, F.; Rapini, N.; Deodati, A.; Fierabracci, A.; et al. Functional and Taxonomic Traits of the Gut Microbiota in Type 1 Diabetes Children at the Onset: A Metaproteomic Study. Int. J. Mol. Sci. 2022, 23, 15982. [Google Scholar] [CrossRef]
- Liu, J.; Qin, X.; Lin, B.; Cui, J.; Liao, J.; Zhang, F.; Lin, Q. Analysis of Gut Microbiota Diversity in Hashimoto’s Thyroiditis Patients. BMC Microbiol. 2022, 22, 318. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, Y.; Zhao, L.; Zhang, X. Modulating Gut Microbiota in Autoimmune Diseases: A Cutting-Edge Strategy from Prophylaxis to Therapeutics. Sci. Bull. 2022, 67, 771–773. [Google Scholar] [CrossRef]
- Maurice, C.F.; Haiser, H.J.; Turnbaugh, P.J. Xenobiotics Shape the Physiology and Gene Expression of the Active Human Gut Microbiome. Cell 2013, 152, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human Gut Microbiome Viewed across Age and Geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Sroka, N.; Rydzewska-Rosołowska, A.; Kakareko, K.; Rosołowski, M.; Głowińska, I.; Hryszko, T. Show Me What You Have Inside—The Complex Interplay between SIBO and Multiple Medical Conditions—A Systematic Review. Nutrients 2023, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Adike, A.; DiBaise, J.K. Small Intestinal Bacterial Overgrowth: Nutritional Implications, Diagnosis, and Management. Gastroenterol. Clin. 2018, 47, 193–208. [Google Scholar] [CrossRef]
- Roland, B.C.; Ciarleglio, M.M.; Clarke, J.O.; Semler, J.R.; Tomakin, E.; Mullin, G.E.; Pasricha, P.J. Low Ileocecal Valve Pressure Is Significantly Associated with Small Intestinal Bacterial Overgrowth (SIBO). Dig. Dis. Sci. 2014, 59, 1269–1277. [Google Scholar] [CrossRef]
- Pimentel, M.; Saad, R.J.; Long, M.D.; Rao, S.S.C. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am. J. Gastroenterol. 2020, 115, 165–178. [Google Scholar] [CrossRef]
- Bratten, J.R.; Spanier, J.; Jones, M.P. Lactulose Breath Testing Does Not Discriminate Patients with Irritable Bowel Syndrome from Healthy Controls. Am. J. Gastroenterol. 2008, 103, 958–963. [Google Scholar] [CrossRef]
- Abbasi, M.H.; Zahedi, M.; Darvish Moghadam, S.; Shafieipour, S.; HayatBakhsh Abbasi, M. Small Bowel Bacterial Overgrowth in Patients with Irritable Bowel Syndrome: The First Study in Iran. Middle East J. Dig. Dis. 2015, 7, 36–40. [Google Scholar]
- Posserud, I.; Stotzer, P.-O.; Björnsson, E.S.; Abrahamsson, H.; Simrén, M. Small Intestinal Bacterial Overgrowth in Patients with Irritable Bowel Syndrome. Gut 2007, 56, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Kumar, S.; Mehrotra, M.; Lakshmi, C.; Misra, A. Frequency of Small Intestinal Bacterial Overgrowth in Patients with Irritable Bowel Syndrome and Chronic Non-Specific Diarrhea. J. Neurogastroenterol. Motil. 2010, 16, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Srivastava, D.; Ghoshal, U.; Misra, A. Breath Tests in the Diagnosis of Small Intestinal Bacterial Overgrowth in Patients with Irritable Bowel Syndrome in Comparison with Quantitative Upper Gut Aspirate Culture. Eur. J. Gastroenterol. Hepatol. 2014, 26, 753. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Caviglia, G.P.; Brignolo, P.; Ribaldone, D.G.; Reggiani, S.; Sguazzini, C.; Smedile, A.; Pellicano, R.; Resegotti, A.; Astegiano, M.; et al. Glucose Breath Test and Crohn’s Disease: Diagnosis of Small Intestinal Bacterial Overgrowth and Evaluation of Therapeutic Response. Scand. J. Gastroenterol. 2015, 50, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V.; Sharma, S.; Malik, A.; Kaur, J.; Prasad, K.K.; Sinha, S.K.; Singh, K. Small Intestinal Bacterial Overgrowth and Orocecal Transit Time in Patients of Inflammatory Bowel Disease. Dig. Dis. Sci. 2013, 58, 2594–2598. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Yadav, A.; Fatima, B.; Agrahari, A.P.; Misra, A. Small Intestinal Bacterial Overgrowth in Patients with Inflammatory Bowel Disease: A Case-Control Study. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2022, 41, 96–103. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, K.-M.; Chung, Y.Y.; Lee, Y.W.; Kim, D.B.; Sung, H.J.; Chung, W.C.; Paik, C.-N. Clinical Significance of the Glucose Breath Test in Patients with Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. 2015, 30, 990–994. [Google Scholar] [CrossRef]
- Klaus, J.; Spaniol, U.; Adler, G.; Mason, R.A.; Reinshagen, M.; von Tirpitz C, C. Small Intestinal Bacterial Overgrowth Mimicking Acute Flare as a Pitfall in Patients with Crohn’s Disease. BMC Gastroenterol. 2009, 9, 61. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Giorgetti, G. High Prevalence of Small Intestinal Bacterial Overgrowth in Celiac Patients with Persistence of Gastrointestinal Symptoms after Gluten Withdrawal. Am. J. Gastroenterol. 2003, 98, 839–843. [Google Scholar] [CrossRef]
- Lasa, J.S.; Zubiaurre, I.; Fanjul, I.; Olivera, P.; Soifer, L. Small Intestinal Bacterial Overgrowth Prevalence in Celiac Disease Patients Is Similar in Healthy Subjects and Lower in Irritable Bowel Syndrome Patients. Rev. Gastroenterol. México Engl. Ed. 2015, 80, 171–174. [Google Scholar] [CrossRef]
- Madrid, A.M.; Poniachik, J.; Quera, R.; Defilippi, C. Small Intestinal Clustered Contractions and Bacterial Overgrowth: A Frequent Finding in Obese Patients. Dig. Dis. Sci. 2011, 56, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Fialho, A.; Thota, P.; McCullough, A.; Shen, B. Higher Visceral to Subcutaneous Fat Ratio Is Associated with Small Intestinal Bacterial Overgrowth. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Pavin, E.J.; Parisi, M.C.R.; Lorena, S.L.S.; Brunetto, S.Q.; Ramos, C.D.; Pavan, C.R.; Mesquita, M.A. Delayed Small Intestinal Transit in Patients with Long-Standing Type 1 Diabetes Mellitus: Investigation of the Relationships with Clinical Features, Gastric Emptying, Psychological Distress, and Nutritional Parameters. Diabetes Technol. Ther. 2013, 15, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Cani, P.D.; Everard, A.; Neyrinck, A.M.; Bindels, L.B. Gut Microorganisms as Promising Targets for the Management of Type 2 Diabetes. Diabetologia 2015, 58, 2206–2217. [Google Scholar] [CrossRef]
- Malik, A.; Morya, R.K.; Bhadada, S.K.; Rana, S. Type 1 Diabetes Mellitus: Complex Interplay of Oxidative Stress, Cytokines, Gastrointestinal Motility and Small Intestinal Bacterial Overgrowth. Eur. J. Clin. Investig. 2018, 48, e13021. [Google Scholar] [CrossRef]
- Rana, S.; Bhansali, A.; Bhadada, S.; Sharma, S.; Kaur, J.; Singh, K. Orocecal Transit Time and Small Intestinal Bacterial Overgrowth in Type 2 Diabetes Patients from North India. Diabetes Technol. Ther. 2011, 13, 1115–1120. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.-Q. The Prevalence of Small Intestinal Bacterial Overgrowth in Diabetes Mellitus: A Systematic Review and Meta-Analysis. Aging 2022, 14, 975–988. [Google Scholar] [CrossRef]
- Fialho, A.; Fialho, A.; Thota, P.; McCullough, A.J.; Shen, B. Small Intestinal Bacterial Overgrowth Is Associated with Non-Alcoholic Fatty Liver Disease. J. Gastrointest. Liver Dis. JGLD 2016, 25, 159–165. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Baba, C.S.; Ghoshal, U.; Alexander, G.; Misra, A.; Saraswat, V.A.; Choudhuri, G. Low-Grade Small Intestinal Bacterial Overgrowth Is Common in Patients with Non-Alcoholic Steatohepatitis on Quantitative Jejunal Aspirate Culture. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2017, 36, 390–399. [Google Scholar] [CrossRef]
- Shi, H.; Mao, L.; Wang, L.; Quan, X.; Xu, X.; Cheng, Y.; Zhu, S.; Dai, F. Small Intestinal Bacterial Overgrowth and Orocecal Transit Time in Patients of Non-alcoholic Fatty Liver Disease. Eur. J. Gastroenterol. Hepatol. 2021, 33, e535. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Cao, B.; Tian, Q. The Effect of Small Intestinal Bacterial Overgrowth on Minimal Hepatic Encephalopathy in Patients with Cirrhosis. Arch. Med. Sci. 2016, 12, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Ghoshal, U.C.; Srivastava, D.; Misra, A.; Mohindra, S. Small Intestinal Bacterial Overgrowth Is Common Both among Patients with Alcoholic and Idiopathic Chronic Pancreatitis. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al 2014, 14, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Novljan, U.; Pintar, T. Small Intestinal Bacterial Overgrowth in Patients with Roux-En-Y Gastric Bypass and One-Anastomosis Gastric Bypass. Obes. Surg. 2022, 32, 4102–4109. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, J.; Gonska, T. Bacterial Overgrowth, Dysbiosis, Inflammation, and Dysmotility in the Cystic Fibrosis Intestine. J. Cyst. Fibros. 2017, 16, S14–S23. [Google Scholar] [CrossRef] [PubMed]
- Furnari, M.; De Alessandri, A.; Cresta, F.; Haupt, M.; Bassi, M.; Calvi, A.; Haupt, R.; Bodini, G.; Ahmed, I.; Bagnasco, F.; et al. The Role of Small Intestinal Bacterial Overgrowth in Cystic Fibrosis: A Randomised Case-Controlled Clinical Trial with Rifaximin. J. Gastroenterol. 2019, 54, 261–270. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail. 2016, 4, 220–227. [Google Scholar] [CrossRef]
- Katsimichas, T.; Ohtani, T.; Motooka, D.; Tsukamoto, Y.; Kioka, H.; Nakamoto, K.; Konishi, S.; Chimura, M.; Sengoku, K.; Miyawaki, H.; et al. Non-Ischemic Heart Failure With Reduced Ejection Fraction Is Associated With Altered Intestinal Microbiota. Circ. J. 2018, 82, 1640–1650. [Google Scholar] [CrossRef]
- Konrad, P.; Chojnacki, J.; Kaczka, A.; Pawłowicz, M.; Rudnicki, C.; Chojnacki, C. Thyroid dysfunction in patients with small intestinal bacterial overgrowth. Pol. Merkuriusz Lek. 2018, 44, 15–18. [Google Scholar]
- Hasuike, Y.; Endo, T.; Koroyasu, M.; Matsui, M.; Mori, C.; Yamadera, M.; Fujimura, H.; Sakoda, S. Bile Acid Abnormality Induced by Intestinal Dysbiosis Might Explain Lipid Metabolism in Parkinson’s Disease. Med. Hypotheses 2020, 134, 109436. [Google Scholar] [CrossRef]
- Barboza, J.L.; Okun, M.S.; Moshiree, B. The Treatment of Gastroparesis, Constipation and Small Intestinal Bacterial Overgrowth Syndrome in Patients with Parkinson’s Disease. Expert Opin. Pharmacother. 2015, 16, 2449–2464. [Google Scholar] [CrossRef]
- Niu, X.-L.; Liu, L.; Song, Z.-X.; Li, Q.; Wang, Z.-H.; Zhang, J.-L.; Li, H.-H. Prevalence of Small Intestinal Bacterial Overgrowth in Chinese Patients with Parkinson’s Disease. J. Neural Transm. 2016, 123, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhou, L.; Wang, Q.; Zheng, G.; Su, S. Effect of Compound Lactic Acid Bacteria Capsules on the Small Intestinal Bacterial Overgrowth in Patients with Depression and Diabetes: A Blinded Randomised Controlled Clinical Trial. Dis. Markers 2022, 2022, 6721695. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Konrad, P.; Błońska, A.; Medrek-Socha, M.; Przybylowska-Sygut, K.; Chojnacki, J.; Poplawski, T. Altered Tryptophan Metabolism on the Kynurenine Pathway in Depressive Patients with Small Intestinal Bacterial Overgrowth. Nutrients 2022, 14, 3217. [Google Scholar] [CrossRef] [PubMed]
- Kossewska, J.; Bierlit, K.; Trajkovski, V. Personality, Anxiety, and Stress in Patients with Small Intestine Bacterial Overgrowth Syndrome. The Polish Preliminary Study. Int. J. Environ. Res. Public. Health 2022, 20, 93. [Google Scholar] [CrossRef]
- Gyger, G.; Baron, M. Gastrointestinal Manifestations of Scleroderma: Recent Progress in Evaluation, Pathogenesis, and Management. Curr. Rheumatol. Rep. 2012, 14, 22–29. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Tandon, P.; Gohel, T.; Corrigan, M.L.; Coughlin, K.L.; Shatnawei, A.; Chatterjee, S.; Kirby, D.F. Gastrointestinal Manifestations, Malnutrition, and Role of Enteral and Parenteral Nutrition in Patients With Scleroderma. J. Clin. Gastroenterol. 2015, 49, 559. [Google Scholar] [CrossRef]
- Rukavina Mikusic, N.L.; Kouyoumdzian, N.M.; Choi, M.R. Gut Microbiota and Chronic Kidney Disease: Evidences and Mechanisms That Mediate a New Communication in the Gastrointestinal-Renal Axis. Pflüg. Arch. Eur. J. Physiol. 2020, 472, 303–320. [Google Scholar] [CrossRef]
- Barrios, C.; Beaumont, M.; Pallister, T.; Villar, J.; Goodrich, J.K.; Clark, A.; Pascual, J.; Ley, R.E.; Spector, T.D.; Bell, J.T.; et al. Gut-Microbiota-Metabolite Axis in Early Renal Function Decline. PLoS ONE 2015, 10, e0134311. [Google Scholar] [CrossRef]
- O’Sullivan, B.P.; Freedman, S.D. Cystic Fibrosis. The Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef]
- Fanen, P.; Wohlhuter-Haddad, A.; Hinzpeter, A. Genetics of Cystic Fibrosis: CFTR Mutation Classifications toward Genotype-Based CF Therapies. Int. J. Biochem. Cell Biol. 2014, 52, 94–102. [Google Scholar] [CrossRef]
- Van Biervliet, S.; de Clercq, C.; Declercq, D.; Van Braeckel, E.; Van Daele, S.; De Baets, F.; De Looze, D. Gastro-Intestinal Manifestations in Cystic Fibrosis Patients. Acta Gastro-Enterol. Belg. 2016, 79, 481–486. [Google Scholar]
- Dhooghe, B.; Noël, S.; Huaux, F.; Leal, T. Lung Inflammation in Cystic Fibrosis: Pathogenesis and Novel Therapies. Clin. Biochem. 2014, 47, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Durie, P.; Baillargeon, J.-D.; Bouchard, S.; Donnellan, F.; Zepeda-Gomez, S.; Teshima, C. Diagnosis and Management of Pancreatic Exocrine Insufficiency (PEI) in Primary Care: Consensus Guidance of a Canadian Expert Panel. Curr. Med. Res. Opin. 2018, 34, 25–33. [Google Scholar] [CrossRef]
- Walkowiak, J.; Lisowska, A.; Blaszczyński, M. The Changing Face of the Exocrine Pancreas in Cystic Fibrosis: Pancreatic Sufficiency, Pancreatitis and Genotype. Eur. J. Gastroenterol. Hepatol. 2008, 20, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.H.; Narkewicz, M.R. Cystic Fibrosis-Related Cirrhosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2017, 16, S50–S61. [Google Scholar] [CrossRef]
- Singh, V.K.; Schwarzenberg, S.J. Pancreatic Insufficiency in Cystic Fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2017, 16, S70–S78. [Google Scholar] [CrossRef]
- Assis, D.N.; Debray, D. Gallbladder and Bile Duct Disease in Cystic Fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2017, 16, S62–S69. [Google Scholar] [CrossRef]
- Lewindon, P.J.; Robb, T.A.; Moore, D.J.; Davidson, G.P.; Martin, A.J. Bowel Dysfunction in Cystic Fibrosis: Importance of Breath Testing. J. Paediatr. Child Health 1998, 34, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, A.; Wójtowicz, J.; Walkowiak, J. Small Intestine Bacterial Overgrowth Is Frequent in Cystic Fibrosis: Combined Hydrogen and Methane Measurements Are Required for Its Detection. Acta Biochim. Pol. 2009, 56, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, A.; Pogorzelski, A.; Oracz, G.; Skorupa, W.; Cofta, S.; Szydłowski, J.; Socha, J.; Walkowiak, J. Antibiotic Therapy and Fat Digestion and Absorption in Cystic Fibrosis. Acta Biochim. Pol. 2011, 58, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, E.C.; Gabrielli, M.; Scarpellini, E.; Lupascu, A.; Novi, M.; Sottili, S.; Vitale, G.; Cesario, V.; Serricchio, M.; Cammarota, G.; et al. Small Intestinal Bacterial Overgrowth Recurrence after Antibiotic Therapy. Am. J. Gastroenterol. 2008, 103, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs Alter Symptoms and the Metabolome of Patients with IBS: A Randomised Controlled Trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Toscano, M.; De Grandi, R.; Casini, V.; Pace, F. Persisting Changes of Intestinal Microbiota after Bowel Lavage and Colonoscopy. Eur. J. Gastroenterol. Hepatol. 2016, 28, 532–537. [Google Scholar] [CrossRef]
- Pop, M.; Walker, A.W.; Paulson, J.; Lindsay, B.; Antonio, M.; Hossain, M.A.; Oundo, J.; Tamboura, B.; Mai, V.; Astrovskaya, I.; et al. Diarrhea in Young Children from Low-Income Countries Leads to Large-Scale Alterations in Intestinal Microbiota Composition. Genome Biol. 2014, 15, R76. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Valentina, C.; Fabio, P. Gut Microbiota, Dysbiosis and Colon Lavage. Dig. Liver Dis. 2019, 51, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Selmin, O.I.; Papoutsis, A.J.; Hazan, S.; Smith, C.; Greenfield, N.; Donovan, M.G.; Wren, S.N.; Doetschman, T.C.; Snider, J.M.; Snider, A.J.; et al. N-6 High Fat Diet Induces Gut Microbiome Dysbiosis and Colonic Inflammation. Int. J. Mol. Sci. 2021, 22, 6919. [Google Scholar] [CrossRef]
- Meriwether, D.; Sulaiman, D.; Volpe, C.; Dorfman, A.; Grijalva, V.; Dorreh, N.; Solorzano-Vargas, R.S.; Wang, J.; O’Connor, E.; Papesh, J.; et al. Apolipoprotein A-I Mimetics Mitigate Intestinal Inflammation in a COX2-Dependent Inflammatory Disease Model. J. Clin. Investig. 2019, 129, 3670–3685. [Google Scholar] [CrossRef]
- Cinel, I.; Opal, S.M. Molecular Biology of Inflammation and Sepsis: A Primer. Crit. Care Med. 2009, 37, 291–304. [Google Scholar] [CrossRef]
- Antonopoulos, D.A.; Huse, S.M.; Morrison, H.G.; Schmidt, T.M.; Sogin, M.L.; Young, V.B. Reproducible Community Dynamics of the Gastrointestinal Microbiota Following Antibiotic Perturbation. Infect. Immun. 2009, 77, 2367–2375. [Google Scholar] [CrossRef]
- Croswell, A.; Amir, E.; Teggatz, P.; Barman, M.; Salzman, N.H. Prolonged Impact of Antibiotics on Intestinal Microbial Ecology and Susceptibility to Enteric Salmonella Infection. Infect. Immun. 2009, 77, 2741–2753. [Google Scholar] [CrossRef]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-Term Impacts of Antibiotic Exposure on the Human Intestinal Microbiota. Microbiol. Read. Engl. 2010, 156, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-Term Ecological Impacts of Antibiotic Administration on the Human Intestinal Microbiota. ISME J. 2007, 1, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Kong, C.; Li, H.; Huang, L.; Qu, X.; Qin, N.; Qin, H. Dysbiosis Signature of Mycobiota in Colon Polyp and Colorectal Cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.C.K.W.; Peacock, S.J.; van der Poll, T.; Wiersinga, W.J. The Impact of Diabetes on the Pathogenesis of Sepsis. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2012, 31, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, A.; Rao, S.S.C. Small Intestinal Fungal Overgrowth. Curr. Gastroenterol. Rep. 2015, 17, 16. [Google Scholar] [CrossRef]
- Singh, R.; Mullin, G.E. A Wasting Syndrome and Malnutrition Caused by Small Intestine Fungal Overgrowth: Case Report and Review of the Literature. Integr. Med. Clin. J. 2017, 16, 48–51. [Google Scholar]
- Jacobs, C.; Coss Adame, E.; Attaluri, A.; Valestin, J.; Rao, S.S.C. Dysmotility and Proton Pump Inhibitor Use Are Independent Risk Factors for Small Intestinal Bacterial and/or Fungal Overgrowth. Aliment. Pharmacol. Ther. 2013, 37, 1103–1111. [Google Scholar] [CrossRef]
- Schulze, J.; Sonnenborn, U. Yeasts in the Gut: From Commensals to Infectious Agents. Dtsch. Arzteblatt Int. 2009, 106, 837–842. [Google Scholar] [CrossRef]
- Erdoğan, A.; Lee, Y.Y.; Sifuentes, H.; Rao, S. Small Intestinal Fungal Overgrowth (SIFO): A Cause of Gastrointestinal Symptoms. Gastroenterology 2014, 146, S-358. [Google Scholar] [CrossRef]
- Bhagatwala, J.; Rao, S.S.C. Chapter 24—Small Intestinal Bacterial and Fungal Overgrowth. In Clinical and Basic Neurogastroenterology and Motility; Rao, S.S.C., Lee, Y.Y., Ghoshal, U.C., Eds.; Academic Press: Cambridge, MA, USA, 2020; ISBN 978-0-12-813037-7. [Google Scholar]
- Madigan, K.E.; Bundy, R.; Weinberg, R.B. Distinctive Clinical Correlates of Small Intestinal Bacterial Overgrowth with Methanogens. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, 1598–1605.e2. [Google Scholar] [CrossRef]
- Kim, G.; Deepinder, F.; Morales, W.; Hwang, L.; Weitsman, S.; Chang, C.; Gunsalus, R.; Pimentel, M. Methanobrevibacter Smithii Is the Predominant Methanogen in Patients with Constipation-Predominant IBS and Methane on Breath. Dig. Dis. Sci. 2012, 57, 3213–3218. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, D. Homemade Elemental Diet to Treat Intestinal Methanogen Overgrowth: A Case Report. Integr. Med. Encinitas Calif 2021, 20, 32–41. [Google Scholar]
- Pimentel, M.; Chow, E.J.; Lin, H.C. Normalization of Lactulose Breath Testing Correlates with Symptom Improvement in Irritable Bowel Syndrome. a Double-Blind, Randomised, Placebo-Controlled Study. Am. J. Gastroenterol. 2003, 98, 412–419. [Google Scholar] [CrossRef]
- Low, K.; Hwang, L.; Hua, J.; Zhu, A.; Morales, W.; Pimentel, M. A Combination of Rifaximin and Neomycin Is Most Effective in Treating Irritable Bowel Syndrome Patients with Methane on Lactulose Breath Test. J. Clin. Gastroenterol. 2010, 44, 547–550. [Google Scholar] [CrossRef] [PubMed]
| Type of Antibiotic | Gut Microbiota | |||
|---|---|---|---|---|
| Actinobacteria | Bacteroidetes | Firmicutes | Proteobacteria | |
| Macrolide | Actinobacteria↓ | Bacteroides↑ | Firmicutes↓ | Proteobacteria↑ |
| Clarithromycin | Actinobacteria↓ | Bacteroides↑ | Firmicutes↓ | Proteobacteria↑ |
| Vancomycin | Lactobacillus↓ Clostridium↓ | |||
| Ciprofloxacin | Bifidobacterium↓ | Alistipes↓ Bacteroides↑ | Faecalibacterium↓ Oscillospira↓ Ruminococcus↓ Dialister↓ | |
| Clindamycin | Bifidobacteriaceae↓ Lactobacillus↓ | |||
| Antibiotic | Recommended Dose | Efficacy |
|---|---|---|
| Nonabsorbable antibiotic | ||
| Rifaximin | 550 mg t.i.d. | 61–78% |
| Systemic antibiotic | ||
| Amoxicillin-clavulanic acid | 875 mg b.i.d. | 50% |
| Ciprofloxacin | 500 mg b.i.d. | 43–100% |
| Doxycycline | 100 mg q.d. to b.i.d. | a |
| Metronidazole | 250 mg t.i.d. | 43–87% |
| Neomycin | 500 mg b.i.d. | 33–55% |
| Norfloxacin | 400 mg q.d. | 30–100% |
| Tetracycline | 250 mg q.i.d. | 87.5% |
| Trimethoprim-sulfamethoxazole | 160 mg/800 mg b.i.d. | 95% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banaszak, M.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Association between Gut Dysbiosis and the Occurrence of SIBO, LIBO, SIFO and IMO. Microorganisms 2023, 11, 573. https://doi.org/10.3390/microorganisms11030573
Banaszak M, Górna I, Woźniak D, Przysławski J, Drzymała-Czyż S. Association between Gut Dysbiosis and the Occurrence of SIBO, LIBO, SIFO and IMO. Microorganisms. 2023; 11(3):573. https://doi.org/10.3390/microorganisms11030573
Chicago/Turabian StyleBanaszak, Michalina, Ilona Górna, Dagmara Woźniak, Juliusz Przysławski, and Sławomira Drzymała-Czyż. 2023. "Association between Gut Dysbiosis and the Occurrence of SIBO, LIBO, SIFO and IMO" Microorganisms 11, no. 3: 573. https://doi.org/10.3390/microorganisms11030573
APA StyleBanaszak, M., Górna, I., Woźniak, D., Przysławski, J., & Drzymała-Czyż, S. (2023). Association between Gut Dysbiosis and the Occurrence of SIBO, LIBO, SIFO and IMO. Microorganisms, 11(3), 573. https://doi.org/10.3390/microorganisms11030573