House Flies Are Underappreciated Yet Important Reservoirs and Vectors of Microbial Threats to Animal and Human Health
Abstract
:1. Introduction
2. Adult House Fly Associations with Bacteria and Vector Potential
3. Wild House Flies Are Potential Threats to Animal and Human Health
4. House Flies as Reservoirs and Disseminators of Antimicrobial Resistance
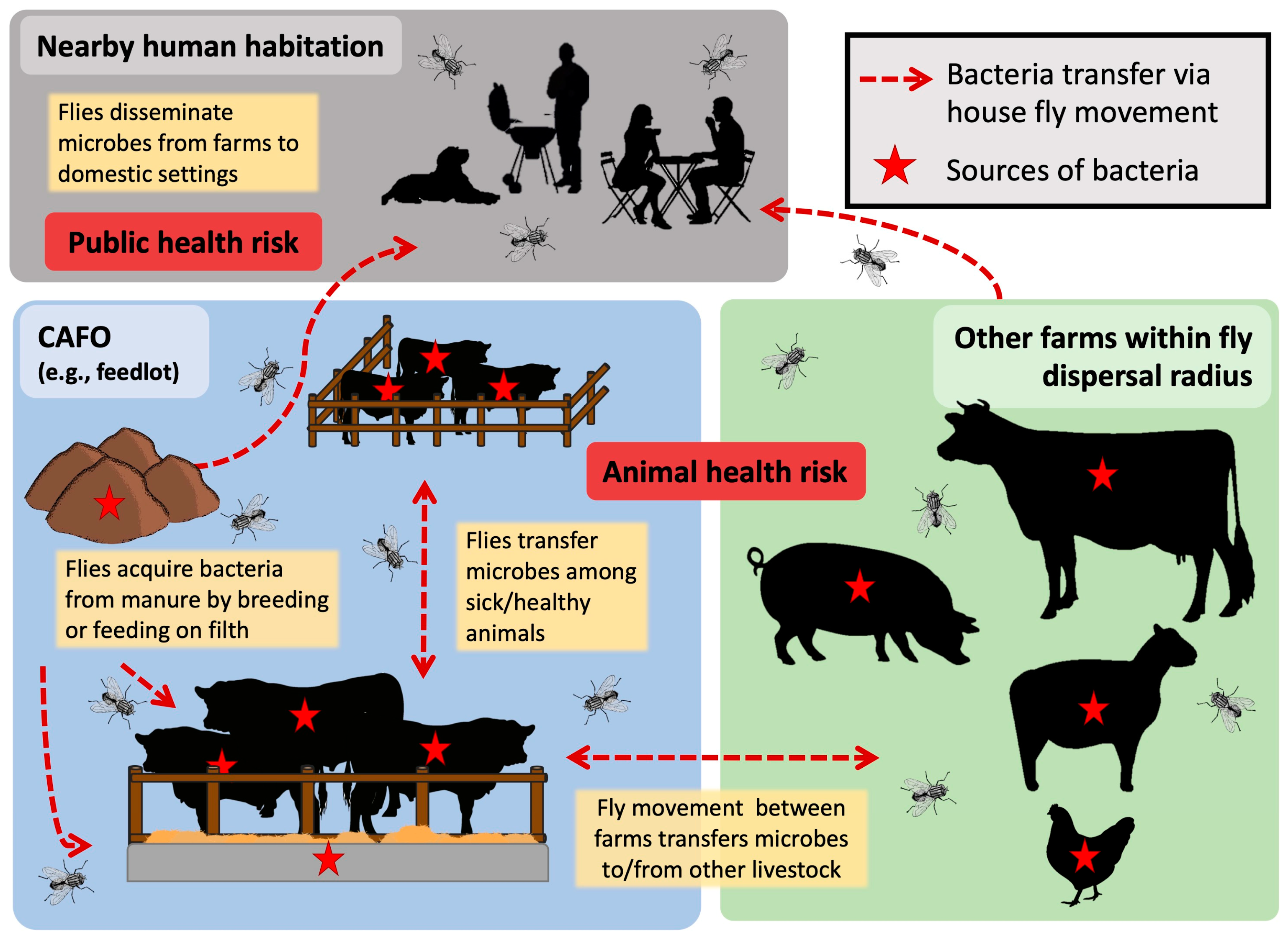
5. Unique Modes of Bacteria Dispersal from House Flies
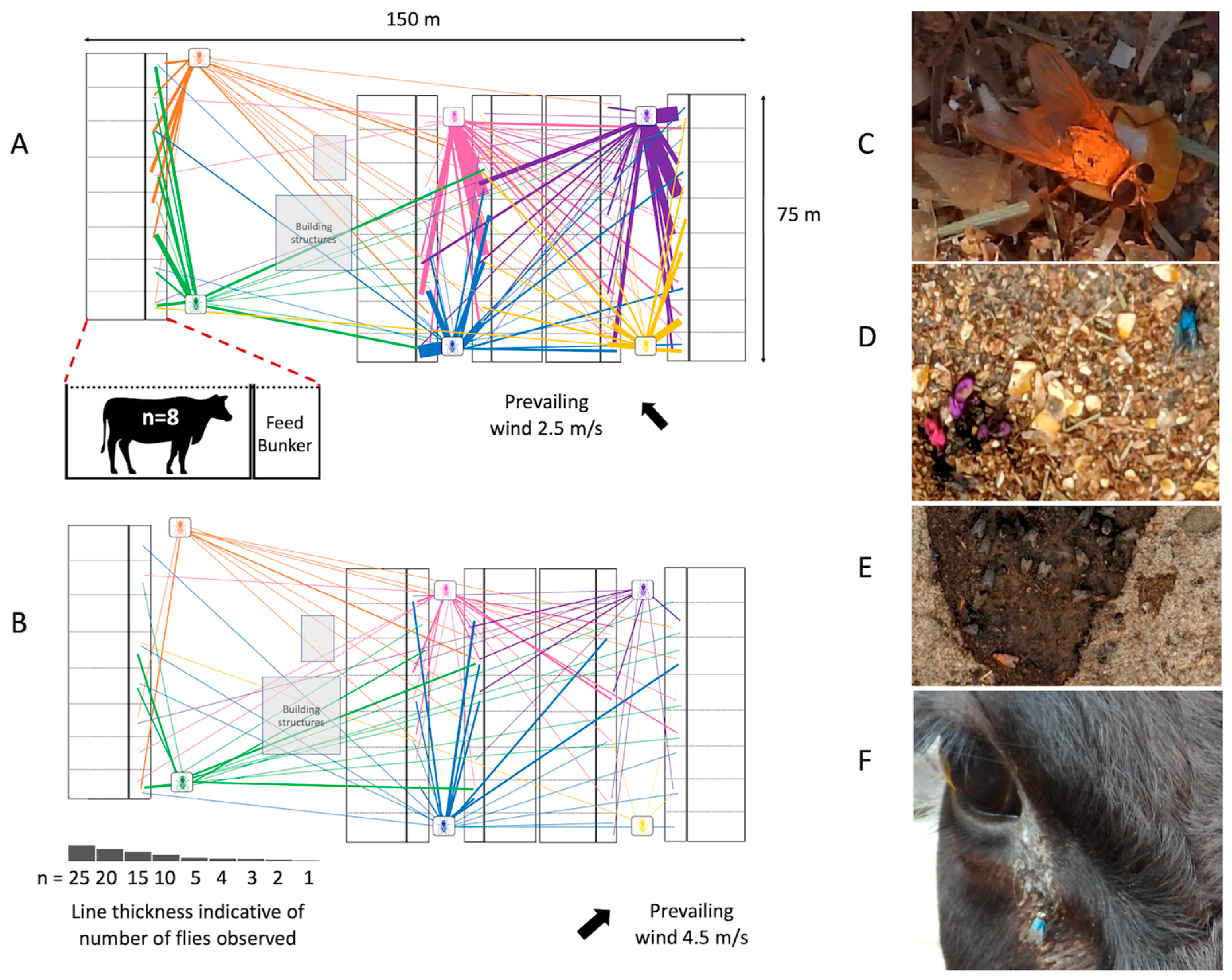
6. House Fly Movement and the Implication for Pathogen Transmission
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidtmann, E.T.; Martin, P.A.W. Relationship Between Selected Bacteria and the Growth of Immature House Flies, Musca domestica, in an Axenic Test System. J. Med. Entomol. 1992, 29, 232–235. [Google Scholar] [CrossRef]
- Zurek, L.; Schal, C.; Watson, D.W. Diversity and Contribution of the Intestinal Bacterial Community to the Development of Musca domestica (Diptera: Muscidae) Larvae. J. Med. Entomol. 2000, 37, 924–928. [Google Scholar] [CrossRef] [Green Version]
- Larsen, J.R.; Pfadt, R.E.; Peterson, L.G. Olfactory and Oviposition Responses of the House Fly to Domestic Manures, with Notes on an Autogenous Strain. J. Econ. Entomol. 1966, 59, 610–615. [Google Scholar] [CrossRef]
- Murvosh, C.M.; Fye, R.L.; Labrecque, G.C. Studies on the Mating Behavior of the House Fly. Musca domestica L. Ohio J. Sci. 1964, 64, 264. [Google Scholar]
- Khan, H.A.A.; Shad, S.A.; Akram, W. Combination of Phagostimulant and Visual Lure as an Effective Tool in Designing House Fly Toxic Baits: A Laboratory Evaluation. PLoS ONE 2013, 8, e77225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.G.; Leichter, C.A.; Rinkevihc, F.D.; Harris, S.A.; Su, C.; Aberegg, L.C.; Moon, R.; Geden, C.J.; Gerry, A.C.; Taylor, D.B.; et al. Insecticide Resistance in House Flies from the United States: Resistance Levels and Frequency of Pyrethroid Resistance Alleles. Pestic. Biochem. Physiol. 2013, 107, 377–384. [Google Scholar] [CrossRef]
- Thomson, J.L.; Yeater, K.M.; Zurek, L.; Nayduch, D. Abundance and Accumulation of Escherichia coli and Salmonella typhimurium Procured by Male and Female House Flies (Diptera: Muscidae) Exposed to Cattle Manure. Ann. Entomol. Soc. Am. 2017, 110, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Neupane, S.; Hall, B.; Brooke, G.; Nayduch, D. Sex-Specific Feeding Behavior of Adult House Flies, Musca domestica L. (Diptera: Muscidae). J. Med. Entomol. 2023, 60, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.L. The Procurement, Transmission, and Abundance of Bacteria in and among House Flies (Musca domestica L.). Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2019. Available online: https://krex.k-state.edu/dspace/handle/2097/40202 (accessed on 31 January 2023).
- Neupane, S.; White, K.; Thomson, J.L.; Zurek, L.; Nayduch, D. Environmental and Sex Effects on Bacterial Carriage by Adult House Flies (Musca domestica L.). Insects 2020, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.L.; Kalpana, M.; Lee, H.L. Wings of the Common House Fly (Musca domestica L.): Importance in Mechanical Transmission of Vibrio cholerae. Trop. Biomed. 2008, 25, 1–8. [Google Scholar]
- Jacques, B.J.; Bourret, T.J.; Shaffer, J.J. Role of Fly Cleaning Behavior on Carriage of Escherichia coli and Pseudomonas aeruginosa. J. Med. Entomol. 2017, 54, 1712–1717. [Google Scholar] [CrossRef] [Green Version]
- Wasala, L.; Talley, J.L.; Desilva, U.; Fletcher, J.; Wayadande, A. Transfer of Escherichia coli O157:H7 to Spinach by House Flies, Musca domestica (Diptera: Muscidae). Phytopathology 2013, 103, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Nayduch, D.; Burrus, R.G. Flourishing in Filth: House Fly–Microbe Interactions across Life History. Ann. Entomol. Soc. Am. 2017, 110, 6–18. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Nayduch, D.; Verma, P.; Shah, B.; Ghate, H.V.; Patole, M.S.; Shouche, Y.S. Phylogenetic Characterization of Bacteria in the Gut of House Flies (Musca domestica L.). FEMS Microbiol. Ecol. 2012, 79, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Dzialo, M.C.; Spaepen, S.; Nsabimana, D.; Gielens, K.; Devriese, H.; Crauwels, S.; Tito, R.Y.; Raes, J.; Lievens, B.; et al. Microbial Communities of the House Fly Musca domestica Vary with Geographical Location and Habitat. Microbiome 2019, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Nayduch, D. Effects of Habitat and Sampling Time on Bacterial Community Composition and Diversity in the Gut of the Female House Fly, Musca domestica Linnaeus (Diptera: Muscidae). Med. Vet. Entomol. 2022, 36, 435–443. [Google Scholar] [CrossRef]
- Bahrndorff, S.; de Jonge, N.; Skovgård, H.; Nielsen, J.L. Bacterial Communities Associated with Houseflies (Musca domestica L.) Sampled within and between Farms. PLoS ONE 2017, 12, e0169753. [Google Scholar] [CrossRef] [Green Version]
- Bahrndorff, S.; Ruiz-González, A.; de Jonge, N.; Nielsen, J.L.; Skovgård, H.; Pertoldi, C. Integrated Genome-Wide Investigations of the Housefly, a Global Vector of Diseases Reveal Unique Dispersal Patterns and Bacterial Communities across Farms. BMC Genom. 2020, 21, 66. [Google Scholar] [CrossRef] [Green Version]
- Poudel, A.; Kang, Y.; Mandal, R.K.; Kalalah, A.; Butaye, P.; Hathcock, T.; Kelly, P.; Walz, P.; Macklin, K.; Cattley, R.; et al. Comparison of Microbiota, Antimicrobial Resistance Genes and Mobile Genetic Elements in Flies and the Feces of Sympatric Animals. FEMS Microbiol. Ecol. 2020, 96, fiaa027. [Google Scholar] [CrossRef]
- Ghosh, A.; Zurek, L. Fresh Steam-Flaked Corn in Cattle Feedlots Is an Important Site for Fecal Coliform Contamination by House Flies. J. Food Prot. 2015, 78, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.J.; Ludek, Z. Association of Escherichia coli O157:H7 with Houseflies on a Cattle Farm. Appl. Environ. Microbiol. 2004, 70, 7578–7580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doud, C.W.; Scott, H.M.; Zurek, L. Role of House Flies in the Ecology of Enterococcus faecalis from Wastewater Treatment Facilities. Microb. Ecol. 2014, 67, 380–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurek, L.; Ghosh, A. Insects Represent a Link between Food Animal Farms and the Urban Environment for Antibiotic Resistance Traits. Appl. Environ. Microbiol. 2014, 80, 3562–3567. [Google Scholar] [CrossRef] [Green Version]
- Thomson, J.L.; Cernicchiaro, N.; Zurek, L.; Nayduch, D. Cantaloupe Facilitates Salmonella typhimurium Survival within and Transmission among Adult House Flies (Musca domestica L.). Foodborne Pathog. Dis. 2021, 18, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Nagaraja, T.G.; Zurek, L. Transmission of Escherichia coli O157:H7 to Cattle by House Flies. Prev. Vet. Med. 2007, 80, 74–81. [Google Scholar] [CrossRef]
- Moriya, K.; Fujibayashi, T.; Yoshihara, T.; Matsuda, A.; Sumi, N.; Umezaki, N.; Kurahashi, H.; Agui, N.; Wada, A.; Watanabe, H. Verotoxin-Producing Escherichia coli O157:H7 Carried by the Housefly in Japan. Med.Vet. Entomol. 1999, 13, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sasaki, T.; Saito, N.; Tamura, K.; Suzuki, K.; Watanabe, H.; Agui, N. Houseflies: Not Simple Mechanical Vectors of Enterohemorrhagic Escherichia coli O157:H7. Am. J. Trop. Med. Hyg. 1999, 61, 625–629. [Google Scholar] [CrossRef]
- Nayduch, D.; Cho, H.; Joyner, C. Staphylococcus Aureus in the House Fly: Temporospatial Fate of Bacteria and Expression of the Antimicrobial Peptide Defensin. J. Med. Entomol. 2013, 50, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Chifanzwa, R. House Fly (Musca Domestica L.) Temporal and Spatial Immune Response to Streptococcus pyogenes and Salmonella typhimurium: Role of Pathogen Density in Bacterial Fate, Persistence and Transmission. Master’s Thesis, Georgia Southern University, Statesboro, GA, USA, 2011. Available online: https://digitalcommons.georgiasouthern.edu/etd/749/ (accessed on 31 January 2023).
- Doud, C.W.; Zurek, L. Enterococcus faecalis OG1RF:PMV158 Survives and Proliferates in the House Fly Digestive Tract. J. Med. Entomol. 2012, 49, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Nayduch, D.; Pittman Noblet, G.; Stutzenberger, F.J. Vector Potential of Houseflies for the Bacterium Aeromonas caviae. Med. Vet. Entomol. 2002, 16, 193–198. [Google Scholar] [CrossRef]
- Joyner, C.; Mills, M.K.; Nayduch, D. Pseudomonas aeruginosa in Musca domestica L.: Temporospatial Examination of Bacteria Population Dynamics and House Fly Antimicrobial Responses. PLoS ONE 2013, 8, e79224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chifanzwa, R.; Nayduch, D. Dose-Dependent Effects on Replication and Persistence of Salmonella enterica Serovar Typhimurium in House Flies (Diptera: Muscidae). J. Med. Entomol. 2018, 55, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kobayashi, M.; Agui, N. Epidemiological Potential of Excretion and Regurgitation by Musca domestica (Diptera: Muscidae) in the Dissemination of Escherichia coli O157: H7 to Food. J. Med. Entomol. 2000, 37, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Kumar, H.V.; Joyner, C.; Reynolds, A.; Nayduch, D. Temporospatial Fate of Bacteria and Immune Effector Expression in House Flies Fed GFP-Escherichia coli O157:H7. Med. Vet. Entomol. 2014, 28, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.H.V.; Nayduch, D. Dose-Dependent Fate of GFP-Expressing Escherichia coli in the Alimentary Canal of Adult House Flies. Med. Vet. Entomol. 2016, 30, 218–228. [Google Scholar] [CrossRef] [Green Version]
- Gill, C.; Bahrndorff, S.; Lowenberger, C. Campylobacter jejuni in Musca domestica: An Examination of Survival and Transmission Potential in Light of the Innate Immune Responses of the House Flies. Insect Sci. 2017, 24, 584–598. [Google Scholar] [CrossRef] [Green Version]
- Mcgaughey, J.; Nayduch, D. Temporal and Spatial Fate of GFP-Expressing Motile and Nonmotile Aeromonas hydrophila in the House Fly Digestive Tract. J. Med. Entomol. 2009, 46, 123–130. [Google Scholar] [CrossRef]
- Oo, K.N.; Sebastian, A.A.; Aye, T. Carriage of Enteric Bacterial Pathogens by House Fly in Yangon, Myanmar. J. Diarrheal Dis. Res. 1989, 7, 81–84. [Google Scholar]
- Olsen, A.R.; Hammack, T.S. Isolation of Salmonella spp. from the Housefly, Musca domestica L., and the Dump Fly, Hydrotaea aenescens (Wiedemann) (Diptera: Muscidae), at Caged-Layer Houses. J. Food Prot. 2000, 63, 958–960. [Google Scholar] [CrossRef]
- Mian, L.S.; Maag, H.; Tacal, J.V. Isolation of Salmonella from Muscoid Flies at Commercial Animal Establishments in San Bernardino County, California. J. Vector Ecol. 2002, 27, 82–85. [Google Scholar]
- Wang, Y.-C.; Chang, Y.-C.; Chuang, H.-L.; Chiu, C.-C.; Yeh, K.-S.; Chang, C.-C.; Hsuan, S.-L.; Lin, W.-H.; Chen, T.-H. Transmission of Salmonella between Swine Farms by the Housefly (Musca domestica). J. Food Prot. 2011, 74, 1012–1016. [Google Scholar] [CrossRef]
- Xu, Y.; Tao, S.; Hinkle, N.; Harrison, M.; Chen, J. Salmonella, Including Antibiotic-Resistant Salmonella, from Flies Captured from Cattle Farms in Georgia, U.S.A. Sci. Total Environ. 2018, 616–617, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Sobur, A.; Hasan, M.; Haque, E.; Mridul, A.I.; Noreddin, A.; El Zowalaty, M.E.; Rahman, T. Molecular Detection and Antibiotyping of Multidrug-Resistant Salmonella Isolated from Houseflies in a Fish Market. Pathogens 2019, 8, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, A.M.; Paulsen, D.J.; Trout-Fryxell, R.T.; Orta, V.E.; Gorman, S.J.; Smith, D.M.; Buchanan, J.R.; Wszelaki, A.L.; Critzer, F.J. Prevalence of Salmonella enterica in Flies on a Diversified Cattle and Fresh Produce Farm across Two Growing Seasons. J. Food Prot. 2021, 84, 1009–1015. [Google Scholar] [CrossRef]
- Buma, R.; Sanada, H.; Maeda, T.; Kamei, M.; Kourai, H. Isolation and Characterization of Pathogenic Bacteria, Including Escherichia coli O157: H7, from Flies Collected at a Dairy Farm Field. Med. Entomol. Zool. 1999, 50, 313–321. [Google Scholar] [CrossRef]
- Iwasa, M.; Makino, S.-I.; Asakura, H.; Kobori, H.; Morimoto, Y. Detection of Escherichia coli O157:H7 from Musca domestica (Diptera: Muscidae) at a Cattle Farm in Japan. J. Med. Entomol. 1999, 36, 108–112. [Google Scholar] [CrossRef]
- Szalanski, A.L.; Owens, C.B.; Mckay, T.; Steelman, C.D. Detection of Campylobacter and Escherichia coli O157:H7 from Filth Flies by Polymerase Chain Reaction. Med. Vet. Entomol. 2004, 18, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Förster, M.; Klimpel, S.; Sievert, K. The House Fly (Musca domestica) as a Potential Vector of Metazoan Parasites Caught in a Pig-Pen in Germany. Vet. Parasitol. 2009, 160, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Burrus, R.G.; Hogsette, J.A.; Kaufman, P.E.; Maruniak, J.E.; Simonne, A.H.; Mai, V. Prevalence of Escherichia coli O157:H7 From House Flies (Diptera: Muscidae) and Dairy Samples in North Central Florida. J. Med. Entomol. 2017, 54, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Puri-Giri, R.; Ghosh, A.; Thomson, J.L.; Zurek, L. House Flies in the Confined Cattle Environment Carry Non-O157 Shiga Toxin-Producing Escherichia coli. J. Med. Entomol. 2017, 54, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Rosef, O.; Kapperud, G. House Flies (Musca Domestica) as Possible Vectors of Campylobacter Fetus Subsp. jejuni. Appl. Environ. Microbiol. 1983, 45, 381–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, L.C.; Saleha, A.A.; Wai, S.S.; Fauziah, N. Isolation of Campylobacter and Salmonella from Houseflies (Musca domestica) in a University Campus and a Poultry Farm in Selangor, Malaysia. Trop. Biomed. 2011, 28, 16–20. [Google Scholar]
- Royden, A.; Wedley, A.; Merga, J.Y.; Rushton, S.; Hald, B.; Humphrey, T.; Williams, N.J. A Role for Flies (Diptera) in the Transmission of Campylobacter to Broilers? Epidemiol. Infect. 2016, 144, 3326–3334. [Google Scholar] [CrossRef] [Green Version]
- Ommi, D.; Hemmatinezhad, B.; Hafshejani, T.T.; Khamesipour, F. Incidence and Antimicrobial Resistance of Campylobacter and Salmonella from Houseflies (Musca domestica) in Kitchens, Farms, Hospitals and Slaughter Houses. Proc. Natl. Acad. Sci. India B—Biol. Sci. 2017, 87, 1285–1291. [Google Scholar] [CrossRef]
- Nayduch, D.; Honko, A.; Noblet, G.P.; Stutzenberger, F. Detection of Aeromonas caviae in the Common Housefly (Musca domestica) by Culture and Polymerase Chain Reaction. Epidemiol. Infect. 2001, 127, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Nayduch, D.; Zurek, L. House Flies (Musca domestica) Pose a Risk of Carriage and Transmission of Bacterial Pathogens Associated with Bovine Respiratory Disease (BRD). Insects 2019, 10, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, J.F.; Garcia-Maruniak, A.; Meek, F.; Maruniak, J.E. Wild Florida House Flies (Musca domestica) as Carriers of Pathogenic Bacteria. Fla. Entomol. 2010, 93, 218–223. [Google Scholar] [CrossRef]
- Khamesipour, F.; Lankarani, K.B.; Honarvar, B.; Kwenti, T.E. A Systematic Review of Human Pathogens Carried by the Housefly (Musca domestica L.). BMC Public Health 2018, 18, 1049. [Google Scholar] [CrossRef] [PubMed]
- Onwugamba, F.C.; Fitzgerald, J.R.; Rochon, K.; Guardabassi, L.; Alabi, A.; Kühne, S.; Grobusch, M.P.; Schaumburg, F. The Role of ‘Filth Flies’ in the Spread of Antimicrobial Resistance. Travel Med. Infect. Dis. 2018, 22, 8–17. [Google Scholar] [CrossRef]
- Geden, C.J.; Nayduch, D.; Scott, J.G.; Burgess, E.R., IV; Gerry, A.C.; Kaufman, P.E.; Thomson, J.; Pickens, V.; Machtinger, E.T. House Fly (Diptera: Muscidae): Biology, Pest Status, Current Management Prospects, and Research Needs. J. Integr. Pest Manag. 2021, 12, 39. [Google Scholar] [CrossRef]
- Sudagidan, M.; Ozalp, V.C.; Can, Ö.; Eligül, H.; Yurt, M.N.Z.; Tasbasi, B.B.; Acar, E.E.; Kavruk, M.; Koçak, O. Surface Microbiota and Associated Staphylococci of Houseflies (Musca domestica) Collected from Different Environmental Sources. Microb. Pathog. 2022, 164, 105439. [Google Scholar] [CrossRef] [PubMed]
- Gioia, G.; Freeman, J.; Sipka, A.; Santisteban, C.; Wieland, M.; Gallardo, V.A.; Monistero, V.; Scott, J.G.; Moroni, P. Pathogens associated with houseflies from different areas within a New York State dairy. JDS Commun. 2022, 3, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Hald, B.; Skovgård, H.; Bang, D.D.; Pedersen, K.; Dybdahl, J.; Jespersen, J.B.; Madsen, M. Flies and Campylobacter Infection of Broiler Flocks. Emerg. Infect. Dis. 2004, 10, 1490–1492. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.S.; Geden, C.J.; Moore, R.W.; Gast, R.K. Isolation of Salmonella enterica Serovar Enteritidis from Houseflies (Musca domestica) Found in Rooms Containing Salmonella Serovar Enteritidis Challenged Hens. Appl. Environ. Microbiol. 2007, 73, 6030–6035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [Green Version]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial Resistance: A Global Emerging Threat to Public Health Systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Hoelzer, K.; Wong, N.; Thomas, J.; Talkington, K.; Jungman, E.; Coukell, A. Antimicrobial Drug Use in Food-Producing Animals and Associated Human Health Risks: What, and How Strong, Is the Evidence? BMC Vet. Res. 2017, 13, 211. [Google Scholar] [CrossRef]
- Baptiste, K.E.; Kyvsgaard, N.C. Do Antimicrobial Mass Medications Work? A Systematic Review and Meta-Analysis of Randomised Clinical Trials Investigating Antimicrobial Prophylaxis or Metaphylaxis against Naturally Occurring Bovine Respiratory Disease. Pathog. Dis. 2017, 75, ftx083. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, A.M.; Hu, D.; Totton, S.C.; Scott, N.; Winder, C.B.; Wang, B.; Wang, C.; Glanville, J.; Wood, H.; White, B.; et al. A Systematic Review and Network Meta-Analysis of Injectable Antibiotic Options for the Control of Bovine Respiratory Disease in the First 45 Days Post Arrival at the Feedlot. Anim. Health Res. Rev. 2019, 20, 163–181. [Google Scholar] [CrossRef]
- Wallinga, D.; Smit, L.A.M.; Davis, M.F.; Casey, J.A.; Nachman, K.E. A Review of the Effectiveness of Current US Policies on Antimicrobial Use in Meat and Poultry Production. Curr. Environ. Health Rep. 2022, 9, 339–354. [Google Scholar] [CrossRef]
- Graham, J.P.; Price, L.B.; Evans, S.L.; Graczyk, T.K.; Silbergeld, E.K. Antibiotic Resistant Enterococci and Staphylococci Isolated from Flies Collected near Confined Poultry Feeding Operations. Sci. Total Environ. 2009, 407, 2701–2710. [Google Scholar] [CrossRef] [PubMed]
- Literak, I.; Dolejska, M.; Rybarikova, J.; Cizek, A.; Strejckova, P.; Vyskocilova, M.; Friedman, M.; Klimes, J. Highly Variable Patterns of Antimicrobial Resistance in Commensal Escherichia coli Isolates from Pigs, Sympatric Rodents, and Flies. Microb. Drug Resist. 2009, 15, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Rybaříková, J.; Dolejská, M.; Materna, D.; Literák, I.; Čížek, A. Phenotypic and Genotypic Characteristics of Antimicrobial Resistant Escherichia coli Isolated from Symbovine Flies, Cattle and Sympatric Insectivorous House Martins from a Farm in the Czech Republic (2006–2007). Res. Vet. Sci. 2010, 89, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ghosh, A.; Schal, C.; Zurek, L. Insects in Confined Swine Operations Carry a Large Antibiotic Resistant and Potentially Virulent Enterococcal Community. BMC Microbiol. 2011, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Usui, M.; Iwasa, T.; Fukuda, A.; Sato, T.; Okubo, T.; Tamura, Y. The Role of Flies in Spreading the Extended-Spectrum β-Lactamase Gene from Cattle. Microb. Drug Resist. 2013, 19, 415–420. [Google Scholar] [CrossRef]
- Usui, M.; Shirakawa, T.; Fukuda, A.; Tamura, Y. The Role of Flies in Disseminating Plasmids with Antimicrobial-Resistance Genes Between Farms. Microb. Drug Resist. 2015, 21, 562–569. [Google Scholar] [CrossRef]
- Blaak, H.; Hamidjaja, R.A.; van Hoek, A.H.A.M.; de Heer, L.; de Roda, H.A.M.; Schets, F.M. Detection of Extended-Spectrum Beta-Lactamase (ESBL)-Producing Escherichia coli on Flies at Poultry Farms. Appl. Environ. Microbiol. 2014, 80, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Solà-Ginés, M.; González-López, J.J.; Cameron-Veas, K.; Piedra-Carrasco, N.; Cerdà-Cuéllar, M.; Migura-Garcia, L. Houseflies (Musca domestica) as Vectors for Extended-Spectrum β-Lactamase-Producing Escherichia coli on Spanish Broiler Farms. Appl. Environ. Microbiol. 2015, 81, 3604–3611. [Google Scholar] [CrossRef] [Green Version]
- Cervelin, V.; Fongaro, G.; Pastore, J.B.; Engel, F.; Reimers, M.A.; Viancelli, A. Enterobacteria Associated with Houseflies (Musca domestica) as an Infection Risk Indicator in Swine Production Farms. Acta Trop. 2018, 185, 13–17. [Google Scholar] [CrossRef]
- Fukuda, A.; Usui, M.; Okubo, T.; Tagaki, C.; Sukpanyatham, N.; Tamura, Y. Co-Harboring of Cephalosporin (bla)/Colistin (mcr) Resistance Genes among Enterobacteriaceae from Flies in Thailand. FEMS Microbiol. Lett. 2018, 365, fny178. [Google Scholar] [CrossRef]
- Poudel, A.; Hathcock, T.; Butaye, P.; Kang, Y.; Price, S.; Macklin, K.; Walz, P.; Cattley, R.; Kalalah, A.; Adekanmbi, F.; et al. Multidrug-Resistant Escherichia coli, Klebsiella pneumoniae and Staphylococcus spp. in Houseflies and Blowflies from Farms and Their Environmental Settings. Int. J. Environ. Res. Public Health. 2019, 16, 3583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akter, S.; Sabuj, A.A.M.; Haque, Z.F.; Kafi, M.A.; Rahman, M.T.; Saha, S. Detection of Antibiotic-Resistant Bacteria and Their Resistance Genes from Houseflies. Vet. World 2020, 13, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, M.T.; Chase, J.R.; Shu, R.; Teng, L.; Jeong, K.C.; Kaufman, P.E.; Wong, A.C.N. Prevalence of Field-Collected House Flies and Stable Flies With Bacteria Displaying Cefotaxime and Multidrug Resistance. J. Med. Entomol. 2021, 58, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Wadaskar, B.; Kolhe, R.; Waskar, V.; Budhe, M.; Kundu, K.; Chaudhari, S. Detection of Antimicrobial Resistance in Escherichia coli and Salmonella Isolated from Flies Trapped at Animal and Poultry Farm Premises. J. Anim. Res. 2021, 11, 341–350. [Google Scholar] [CrossRef]
- Macovei, L.; Miles, B.; Zurek, L. Potential of Houseflies to Contaminate Ready-to-Eat Food with Antibiotic-Resistant Enterococci. J. Food Prot. 2008, 71, 435–439. [Google Scholar] [CrossRef]
- Dolejska, M.; Duskova, E.; Rybarikova, J.; Janoszowska, D.; Roubalova, E.; Dibdakova, K.; Maceckova, G.; Kohoutaova, L.; Literak, I.; Smola, J.; et al. Plasmids Carrying blaCTX-M-1 and qnr Genes in Escherichia coli Isolates from an Equine Clinic and a Horseback Riding Centre. J. Antimicrob. Chemother. 2011, 66, 757–764. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, J.; Chen, L.; Yassin, A.K.; Kelly, P.; Butaye, P.; Li, J.; Gong, J.; Cattley, R.; Qi, K.; et al. Housefly (Musca domestica) and Blow Fly (Protophormia terraenovae) as Vectors of Bacteria Carrying Colistin Resistance Genes. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [Green Version]
- Petridis, M.; Bagdasarian, M.; Waldor, M.K.; Walker, E. Horizontal Transfer of Shiga Toxin and Antibiotic Resistance Genes among Escherichia coli Strains in House Fly (Diptera: Muscidae) Gut. J. Med. Entomol. 2006, 43, 288–295. [Google Scholar] [CrossRef]
- Akhtar, M.; Hirt, H.; Zurek, L. Horizontal Transfer of the Tetracycline Resistance Gene tetM Mediated by PCF10 among Enterococcus faecalis in the House Fly (Musca domestica L.) Alimentary Canal. Microb. Ecol. 2009, 58, 509–518. [Google Scholar] [CrossRef]
- Fukuda, A.; Usui, M.; Okubo, T.; Tamura, Y. Horizontal Transfer of Plasmid-Mediated Cephalosporin Resistance Genes in the Intestine of Houseflies (Musca domestica). Microb. Drug Resist. 2016, 22, 336–341. [Google Scholar] [CrossRef]
- Pickens, L.G. Factors Affecting the Distance of Scatter of House Flies (Diptera: Muscidae) from Electrocuting Traps. J. Econ. Entomol. 1989, 82, 149–151. [Google Scholar] [CrossRef]
- Ananth, G.P.; Bronson, D.C.; Brown, J.K. Generation of Airborne Fly Body Particles by Four Electrocution Fly Traps and an Electronic Fly Trap. Int. J. Environ. Health Res. 1992, 2, 106–113. [Google Scholar] [CrossRef]
- Broce, A.B. Electrocuting and Electronic Insect Traps: Trapping Efficiency and Production of Airborne Particles. Int. J. Environ. Health Res. 1993, 3, 47–58. [Google Scholar] [CrossRef]
- Tesch, M.J.; Goodman, W.G. Dissemination of Microbial Contaminants from House Flies Electrocuted by Five Insect Light Traps. Int. J. Environ. Health Res. 1995, 5, 303–309. [Google Scholar] [CrossRef]
- Cooke, E.A.; O’neill, G.; Anderson, M. The Survival of Ingested Serratia marcescens in Houseflies (Musca domestica L.) after Electrocution with Electric Fly Killers. Curr. Microbiol. 2003, 46, 0151–0153. [Google Scholar] [CrossRef]
- Medveczky, I.; Kovacs, L.; Sz, F.K.; Papp, L. The Role of the Housefly, Musca domestica, in the Spread of Aujeszky’s Disease (Pseudorabies). Med. Vet. Entomol. 1988, 2, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Krafsur, E.S.; Black, W.C., IV; Church, C.J.; Barnes, D.A. Age Structure and Reproductive Biology of a Natural House Fly (Diptera: Muscidae) Population. Environ. Entomol. 1985, 14, 159–164. [Google Scholar] [CrossRef]
- Zahn, L.K.; Gerry, A.C. Diurnal Flight Activity of House Flies (Musca domestica) Is Influenced by Sex, Time of Day, and Environmental Conditions. Insects 2020, 11, 391. [Google Scholar] [CrossRef]
- Renault, D. A Review of the Phenotypic Traits Associated with Insect Dispersal Polymorphism, and Experimental Designs for Sorting out Resident and Disperser Phenotypes. Insects 2020, 11, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, R. Dispersion of Musca domestica, Linnaeus, under City Conditions in Montana. J. Econ. Entomol. 1916, 9, 325–354. [Google Scholar] [CrossRef]
- Schoof, H.; Siverly, R.; Jensen, J. House Fly Dispersion Studies in Metropolitan Areas. J. Econ. Entomol. 1952, 45, 675–683. [Google Scholar] [CrossRef]
- Schoof, H.; Siverly, R. Multiple Release Studies on the Dispersion of Musca domestica at Phoenix, Arizona. J. Econ. Entomol. 1954, 47, 830–838. [Google Scholar] [CrossRef]
- Nazni, W.A.; Luke, H.; Wan Rozita, W.M.; Abdullah, A.G.; Sa’diyah, I.; Azahari, A.H.; Zamree, I.; Tan, S.B.; Lee, H.L.; Sofian, M.A. Determination of the Flight Range and Dispersal of the House Fly, Musca domestica (L.) Using Mark Release Recapture Technique. Trop. Biomed. 2005, 22, 53–61. [Google Scholar] [PubMed]
- Winpisinger, K.A.; Ferketich, A.K.; Berry, R.L.; Moeschberger, M.L. Spread of Musca domestica (Diptera: Muscidae), from Two Caged Layer Facilities to Neighboring Residences in Rural Ohio. J. Med. Entomol. 2005, 42, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Lasker, A.W. Suit Barred for Plaintiffs Who ‘Came to the Nuisance’ of Fly-Infested Cattle Farm. Ill. Bar J. 2013, 101, 170. [Google Scholar]
- Baise, G.; Smithfield Loses $50 Million Lawsuit. Farm Progress. 2018. Available online: https://www.farmprogress.com/commentary/smithfield-loses-50-million-lawsuit (accessed on 31 January 2023).
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the Bovine Rumen Bacterial Community from Birth to Adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Sun, H.; Wu, X.; Guan, L.L.; Liu, J. Assessment of Rumen Microbiota from a Large Dairy Cattle Cohort Reveals the Pan and Core Bacteriomes Contributing to Varied Phenotypes. Appl. Environ. Microbiol. 2018, 84, e00970-18. [Google Scholar] [CrossRef] [Green Version]
- Wharton, R.; Seow, C.; Ganapathipillai, A.; Jabaratnam, G. House Fly Populations and Their Dispersion in Malaya with Particular Reference to the Fly Problem in the Cameron Highlands. Med. J. Malays. 1962, 17, 115–131. [Google Scholar]
- WHO. World Health Organization Vector Control Series: The Housefly: Training and Information Guide; World Health Organization: Geneva, Switzerland, 1986; Available online: https://apps.who.int/iris/handle/10665/310997 (accessed on 31 January 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayduch, D.; Neupane, S.; Pickens, V.; Purvis, T.; Olds, C. House Flies Are Underappreciated Yet Important Reservoirs and Vectors of Microbial Threats to Animal and Human Health. Microorganisms 2023, 11, 583. https://doi.org/10.3390/microorganisms11030583
Nayduch D, Neupane S, Pickens V, Purvis T, Olds C. House Flies Are Underappreciated Yet Important Reservoirs and Vectors of Microbial Threats to Animal and Human Health. Microorganisms. 2023; 11(3):583. https://doi.org/10.3390/microorganisms11030583
Chicago/Turabian StyleNayduch, Dana, Saraswoti Neupane, Victoria Pickens, Tanya Purvis, and Cassandra Olds. 2023. "House Flies Are Underappreciated Yet Important Reservoirs and Vectors of Microbial Threats to Animal and Human Health" Microorganisms 11, no. 3: 583. https://doi.org/10.3390/microorganisms11030583
APA StyleNayduch, D., Neupane, S., Pickens, V., Purvis, T., & Olds, C. (2023). House Flies Are Underappreciated Yet Important Reservoirs and Vectors of Microbial Threats to Animal and Human Health. Microorganisms, 11(3), 583. https://doi.org/10.3390/microorganisms11030583





