Microcystin-LR, a Cyanobacterial Toxin, Induces Changes in the Organization of Membrane Compartments in Arabidopsis
Abstract
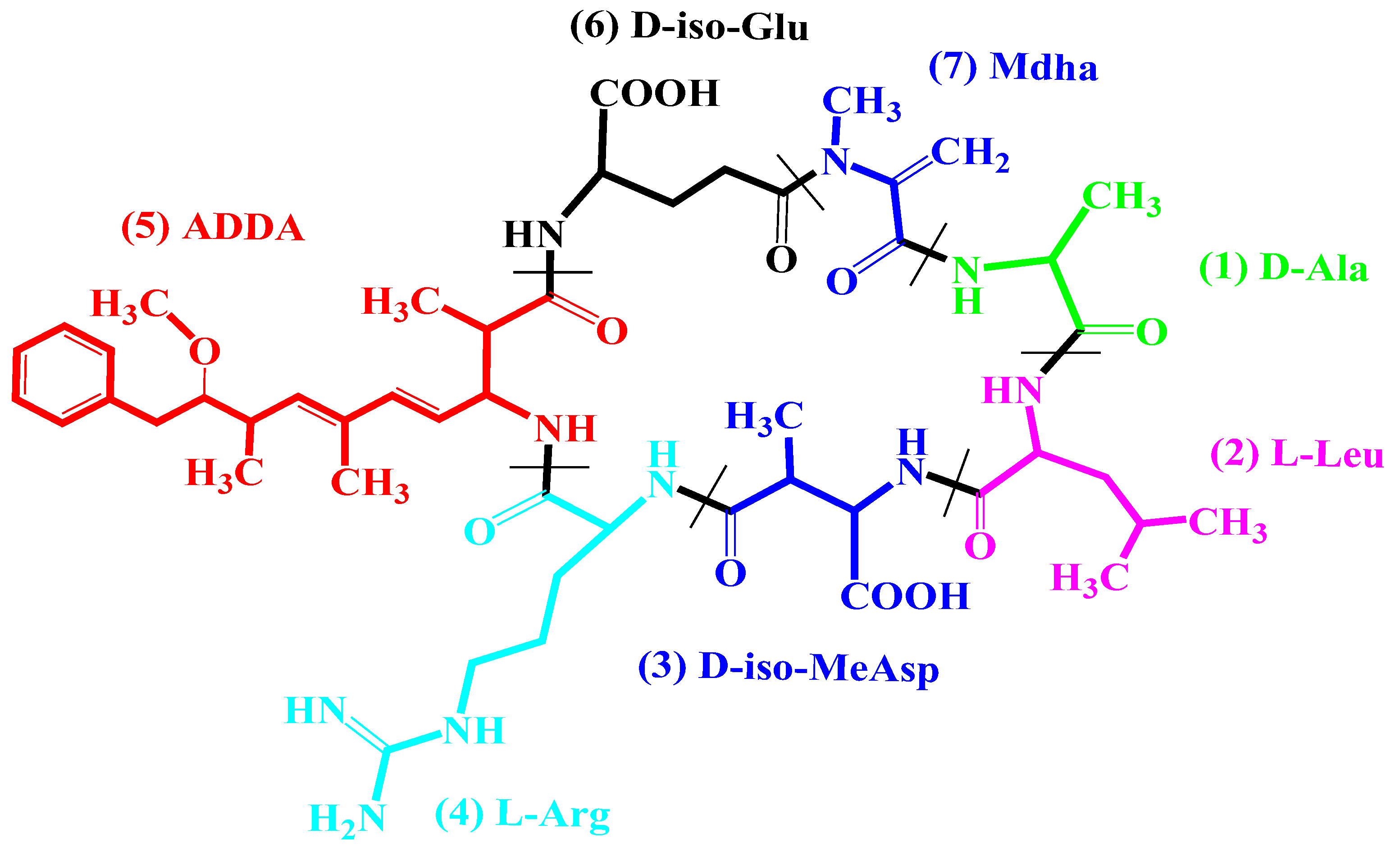
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Treatments
2.3. Visualization of Chloroplast Stromules and Mitochondria
2.4. ACAIN Staining and Microscopy
3. Results
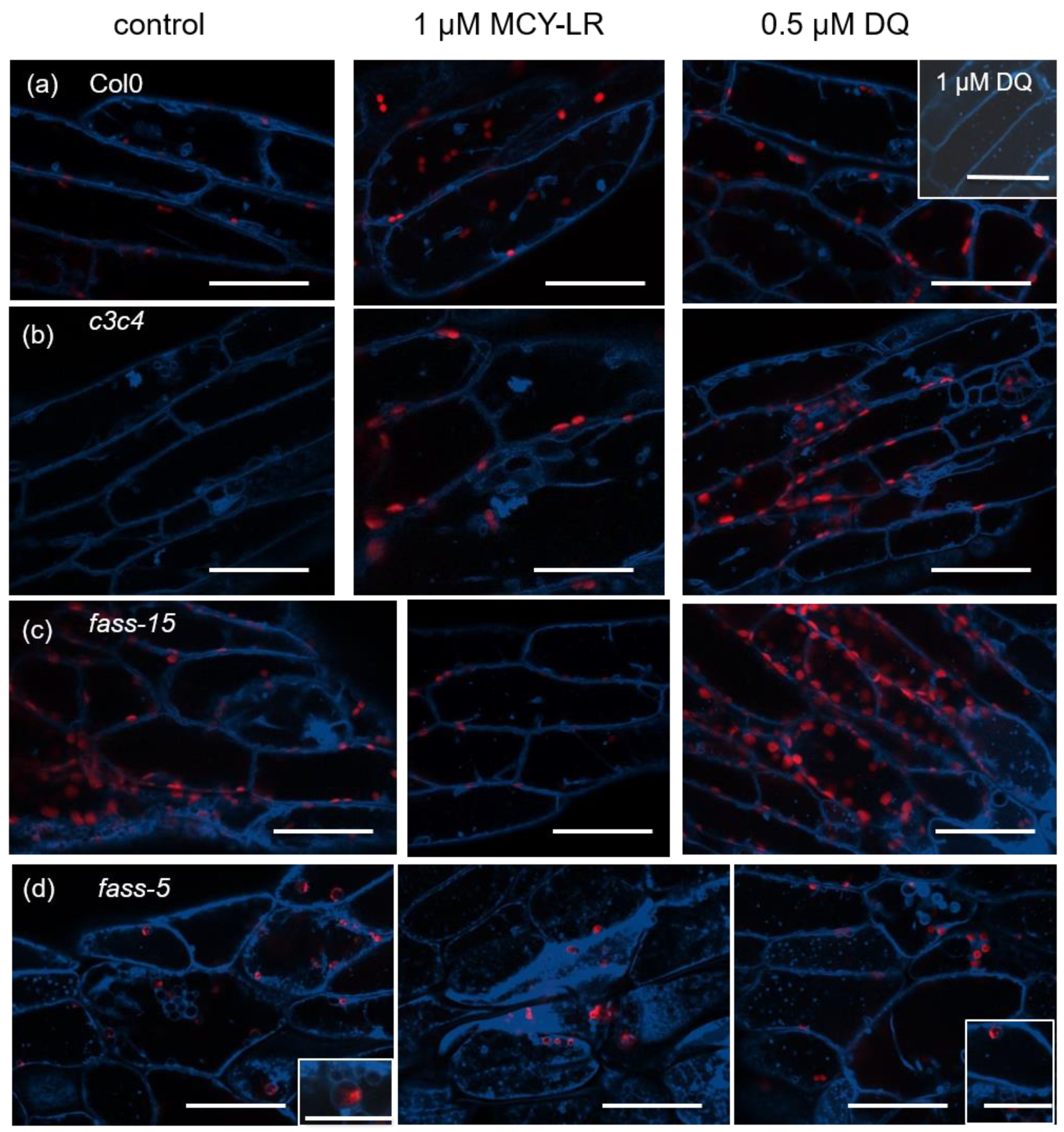
3.1. Changes in Tonoplast Organization Induced by MCY-LR and DQ in Wild-Type, c3c4, Fass-5 and Fass-15 Mutant Arabidopsis Thaliana (Col-0) Seedlings
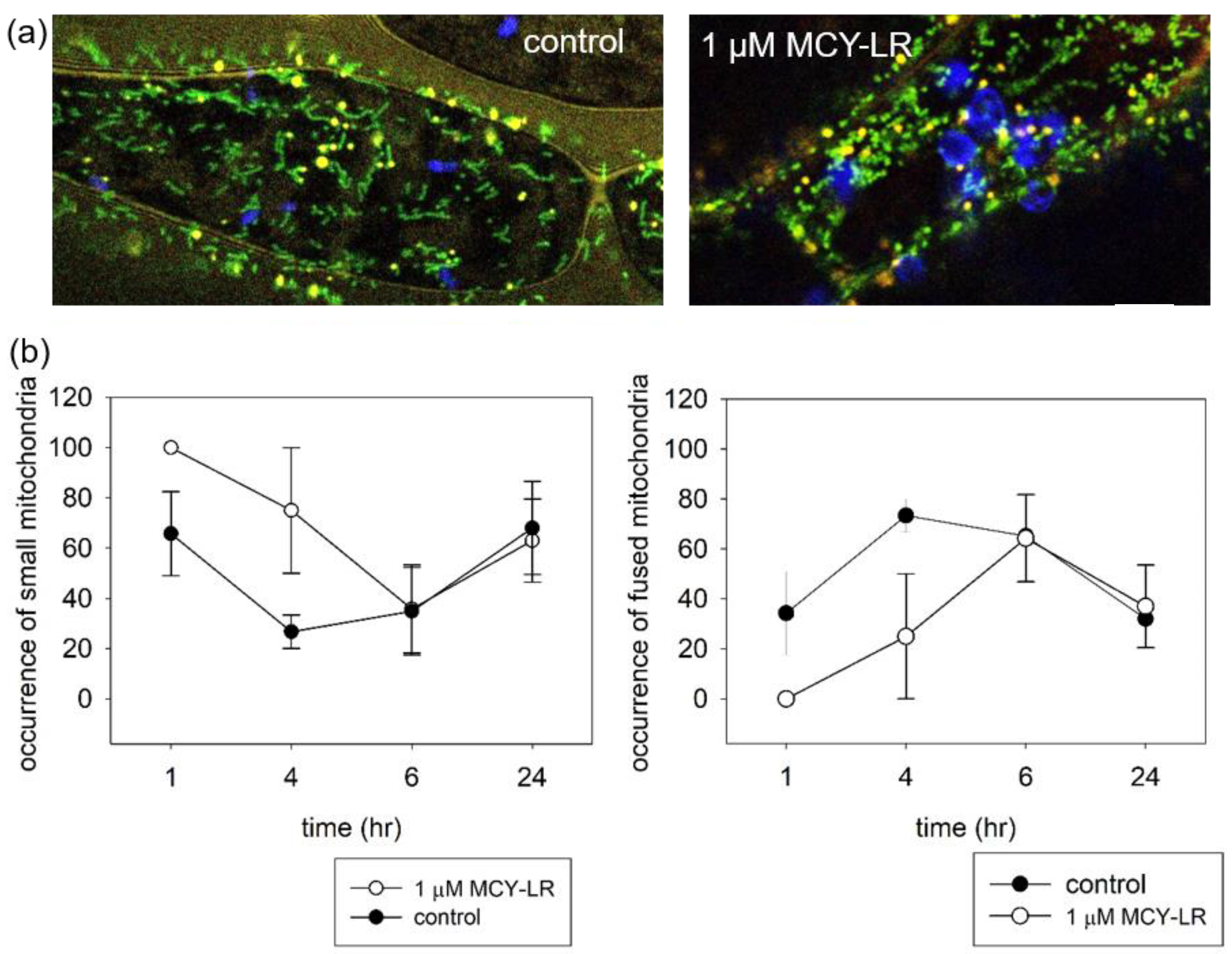
3.2. Effect of MCY-LR on Mitochondrial Morphology
3.3. Effect of MCY-LR on Chloroplast Stromule Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Joint Research Centre. In Algal Bloom and Its Economic Impact; Publications Office: Luxembourg, 2016. [Google Scholar]
- Paerl, H.W. Mitigating Harmful Cyanobacterial Blooms in a Human- and Climatically-Impacted World. Life 2014, 4, 988–1012. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W. Controlling Eutrophication along the Freshwater–Marine Continuum: Dual Nutrient (N and P) Reductions are Essential. Estuaries Coasts 2009, 32, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Vasas, G.; Bácsi, I.; Surányi, G.; Hamvas, M.M.; Máthé, C.; Nagy, S.A.; Borbély, G. Isolation of viable cell mass from frozen Microcystis viridis bloom containing microcystin-RR. Hydrobiologia 2010, 639, 147–151. [Google Scholar] [CrossRef]
- Nishiwaki-Matsushima, R.; Ohta, T.; Nishiwaki, S.; Suganuma, M.; Kohyama, K.; Ishikawa, T.; Carmichael, W.W.; Fujiki, H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J. Cancer Res. Clin. Oncol. 1992, 118, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Corbel, S.; Mougin, C.; Bouaïcha, N. Cyanobacterial toxins: Modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 2014, 96, 1–15. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular Mechanisms of Microcystin Toxicity in Animal Cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Miles, C.; Beach, D.; Labidi, Z.; Djabri, A.; Benayache, N.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef] [Green Version]
- Botes, D.P.; Wessels, P.L.; Runnegar, M.T.C.; Santikarn, S.; Smith, R.J.; Barna, J.C.J.; Williams, D.H. Structural studies on cyanoginosins-LR, -YR, -YA, and -YM, peptide toxins from Microcystis aeruginosa. J. Chem. Soc. Perkin Trans. 1985, 1, 2747–2748. [Google Scholar] [CrossRef]
- Boaru, D.A.; Dragoş, N.; Schirmer, K. Microcystin-LR induced cellular effects in mammalian and fish primary hepatocyte cultures and cell lines: A comparative study. Toxicology 2006, 218, 134–148. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, J.; Huang, H.-B.; Kwon, Y.-G.; Greengard, P.; Nairn, A.C.; Kuriyan, J. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature 1995, 376, 745–753. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, R.W.; Dalby, K.N.; Campbell, D.G.; Cohen, P.T.; Cohen, P.; MacKintosh, C. The cyanobacterial toxin microcystin binds covalently to cysteine-273 on protein phosphatase 1. FEBS Lett. 1995, 371, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Tsukitani, Y.; John, P.C.; Zhang, K. Mitotic Arrest in Tobacco Caused by the Phosphoprotein Phosphatase Inhibitor Okadaic Acid. Plant Cell Physiol. 1992, 33, 677–688. [Google Scholar] [CrossRef]
- Chen, L.; Xie, P. Mechanisms of Microcystin-induced Cytotoxicity and Apoptosis. Mini Rev. Med. Chem. 2016, 16, 1018–1031. [Google Scholar] [CrossRef]
- Oda, Y.; Higaki, T.; Hasezawa, S.; Kutsuna, N. Chapter 3 New Insights into Plant Vacuolar Structure and Dynamics. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2009; Volume 277, pp. 103–135. [Google Scholar]
- Higaki, T.; Kutsuna, N.; Okubo, E.; Sano, T.; Hasezawa, S. Actin Microfilaments Regulate Vacuolar Structures and Dynamics: Dual Observation of Actin Microfilaments and Vacuolar Membrane in Living Tobacco BY-2 Cells. Plant Cell Physiol. 2006, 47, 839–852. [Google Scholar] [CrossRef] [Green Version]
- Máthé, C.; M-Hamvas, M.; Vasas, G. Microcystin-LR and Cylindrospermopsin Induced Alterations in Chromatin Organization of Plant Cells. Mar. Drugs 2013, 11, 3689–3717. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Xing, W.; Li, D.; Liu, Y. Microcystin-RR induced apoptosis in tobacco BY-2 suspension cells is mediated by reactive oxygen species and mitochondrial permeability transition pore status. Toxicol. Vitr. 2008, 22, 328–337. [Google Scholar] [CrossRef]
- Freytag, C.; Máthé, C.; Rigó, G.; Nodzyński, T.; Kónya, Z.; Erdődi, F.; Cséplő, Á.; Pózer, E.; Szabados, L.; Kelemen, A.; et al. Microcystin-LR, a cyanobacterial toxin affects root development by changing levels of PIN proteins and auxin response in Arabidopsis roots. Chemosphere 2021, 276, 130183. [Google Scholar] [CrossRef]
- Garda, T.; Kónya, Z.; Tándor, I.; Beyer, D.; Vasas, G.; Erdődi, F.; Vereb, G.; Papp, G.; Riba, M.; M-Hamvas, M.; et al. Microcystin-LR induces mitotic spindle assembly disorders in Vicia faba by protein phosphatase inhibition and not reactive oxygen species induction. J. Plant Physiol. 2016, 199, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pappas, D.; Gkelis, S.; Panteris, E. The effects of microcystin-LR in Oryza sativa root cells: F-actin as a new target of cyanobacterial toxicity. Plant Biol. 2020, 22, 839–849. [Google Scholar] [CrossRef]
- Nagy, M.; Kéki, S.; Rácz, D.; Mathur, J.; Vereb, G.; Garda, T.; M-Hamvas, M.; Chaumont, F.; Bóka, K.; Böddi, B.; et al. Novel fluorochromes label tonoplast in living plant cells and reveal changes in vacuolar organization after treatment with protein phosphatase inhibitors. Protoplasma 2018, 255, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Arimura, S.-I.; Tsutsumi, N. A dynamin-like protein (ADL2b), rather than FtsZ, is involved in Arabidopsis mitochondrial division. Proc. Natl. Acad. Sci. USA 2002, 99, 5727–5731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arimura, S.-I.; Aida, G.P.; Fujimoto, M.; Nakazono, M.; Tsutsumi, N. Arabidopsis Dynamin-Like Protein 2a (ADL2a), Like ADL2b, is Involved in Plant Mitochondrial Division. Plant Cell Physiol. 2004, 45, 236–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, I.; Tobin, A.K.; Logan, D.C. BIGYIN, an orthologue of human and yeast FIS1 genes functions in the control of mitochondrial size and number in Arabidopsis thaliana. J. Exp. Bot. 2006, 57, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Hu, J. Two small protein families, DYNAMIN-RELATED PROTEIN3 and FISSION1, are required for peroxisome fission in Arabidopsis. Plant J. 2009, 57, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Arimura, S.-I. Fission and Fusion of Plant Mitochondria, and Genome Maintenance. Plant Physiol. 2018, 176, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Dagda, R.K.; Merrill, R.A.; Cribbs, J.T.; Chen, Y.; Hell, J.W.; Usachev, Y.M.; Strack, S. The Spinocerebellar Ataxia 12 Gene Product and Protein Phosphatase 2A Regulatory Subunit Bβ2 Antagonizes Neuronal Survival by Promoting Mitochondrial Fission. J. Biol. Chem. 2008, 283, 36241–36248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wang, J.; Zhu, J.; Chen, Y.; Han, X. Microcystin-leucine-arginine induced neurotoxicity by initiating mitochondrial fission in hippocampal neurons. Sci. Total. Environ. 2020, 703, 134702. [Google Scholar] [CrossRef]
- Natesan, S.K.A.; Sullivan, J.A.; Gray, J.C. Stromules: A characteristic cell-specific feature of plastid morphology. J. Exp. Bot. 2005, 56, 787–797. [Google Scholar] [CrossRef]
- Gray, J.C.; Sullivan, J.A.; Hibberd, J.M.; Hansen, M.R. Stromules: Mobile Protrusions and Interconnections Between Plastids. Plant Biol. 2001, 3, 223–233. [Google Scholar] [CrossRef]
- Schattat, M.H.; Barton, K.A.; Mathur, J. The myth of interconnected plastids and related phenomena. Protoplasma 2015, 252, 359–371. [Google Scholar] [CrossRef]
- Gunning, B.E.S. Plastid stromules: Video microscopy of their outgrowth, retraction, tensioning, anchoring, branching, bridging, and tip-shedding. Protoplasma 2005, 225, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kwok, E.Y.; Hanson, M.R. In vivo analysis of interactions between GFP-labeled microfilaments and plastid stromules. BMC Plant Biol. 2004, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, J.L.; Kumar, A.S.; Park, E.; Padmanabhan, M.S.; Hoban, K.; Modla, S.; Czymmek, K.; Dinesh-Kumar, S.P. Chloroplast Stromules Function during Innate Immunity. Dev. Cell 2015, 34, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Brunkard, J.O.; Runkel, A.M.; Zambryski, P.C. Chloroplasts extend stromules independently and in response to internal redox signals. Proc. Natl. Acad. Sci. USA 2015, 112, 10044–10049. [Google Scholar] [CrossRef] [Green Version]
- Vasas, G.; Gáspár, A.; Páger, C.; Surányi, G.; Máthé, C.; Hamvas, M.M.; Borbely, G. Analysis of cyanobacterial toxins (anatoxin-a, cylindrospermopsin, microcystin-LR) by capillary electrophoresis. Electrophoresis 2004, 25, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, C.; Azimzadeh, J.; Pastuglia, M.; Bellini, C.; Grandjean, O.; Bouchez, D. The Arabidopsis TONNEAU2 Gene Encodes a Putative Novel Protein Phosphatase 2A Regulatory Subunit Essential for the Control of the Cortical Cytoskeleton. Plant Cell 2002, 14, 833–845. [Google Scholar] [CrossRef] [Green Version]
- Kirik, A.; Ehrhardt, D.W.; Kirik, V. TONNEAU2/FASS Regulates the Geometry of Microtubule Nucleation and Cortical Array Organization in Interphase Arab. Cells. Plant Cell 2012, 24, 1158–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinner, L.; Gadeyne, A.; Belcram, K.; Goussot, M.; Moison, M.; Duroc, Y.; Eeckhout, D.; De Winne, N.; Schaefer, E.; Van De Slijke, E.; et al. A protein phosphatase 2A complex spatially controls plant cell division. Nat. Commun. 2013, 4, 1863. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Logan, D.C.; Leaver, C.J. Mitochondria-targeted GFP highlights the heterogeneity of mitochondrial shape, size and movement within living plant cells. J. Exp. Bot. 2000, 51, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Mathur, J.; Mathur, N.; Hulskamp, M. Simultaneous Visualization of Peroxisomes and Cytoskeletal Elements Reveals Actin and Not Microtubule-Based Peroxisome Motility in Plants. Plant Physiol. 2002, 128, 1031–1045. [Google Scholar] [CrossRef] [Green Version]
- Delfosse, K.; Wozny, M.R.; Jaipargas, E.-A.; Barton, K.A.; Anderson, C.; Mathur, J. Fluorescent Protein Aided Insights on Plastids and their Extensions: A Critical Appraisal. Front. Plant Sci. 2016, 6, 1253. [Google Scholar] [CrossRef] [Green Version]
- Welchen, E.; Garcia, L.; Mansilla, N.; Gonzalez, D.H. Coordination of plant mitochondrial biogenesis: Keeping pace with cellular requirements. Front. Plant Sci. 2014, 4, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesaki, H.; Jensen, R.E. Division versus Fusion: Dnm1p and Fzo1p Antagonistically Regulate Mitochondrial Shape. J. Cell Biol. 1999, 147, 699–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-C.; Hu, J.-P. FISSION1A and FISSION1B Proteins Mediate the Fission of Peroxisomes and Mitochondria in Arabidopsis. Mol. Plant 2008, 1, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Yoon, Y.; Bonekamp, N.A.; McNiven, M.A.; Schrader, M. A Role for Fis1 in Both Mitochondrial and Peroxisomal Fission in Mammalian Cells. Mol. Biol. Cell 2005, 16, 5077–5086. [Google Scholar] [CrossRef]
- Pan, R.; Kaur, N.; Hu, J. The Arabidopsis mitochondrial membrane-bound ubiquitin protease UBP27 contributes to mitochondrial morphogenesis. Plant J. 2014, 78, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Arimura, S.-I.; Niwa, Y.; Tsutsumi, N.; Uchimiya, H.; Kawai-Yamada, M. Mitochondrial Behaviour in the Early Stages of ROS Stress Leading to Cell Death in Arabidopsis thaliana. Ann. Bot. 2005, 96, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logan, D. Having a swell time—Mitochondrial morphology and plant cell death programmes. J. Microsc. 2008, 231, 215–224. [Google Scholar] [CrossRef]
- Freytag, C.; Garda, T.; Kónya, Z.; M-Hamvas, M.; Tóth-Várady, B.; Juhász, G.P.; Ujlaky-Nagy, L.; Kelemen, A.; Vasas, G.; Máthé, C. B” and C subunits of PP2A regulate the levels of reactive oxygen species and superoxide dismutase activities in Arabidopsis. Plant Physiol. Biochem. 2023, 195, 182–192. [Google Scholar] [CrossRef]
- Kuběnová, L.; Takáč, T.; Šamaj, J.; Ovečka, M. Single Amino Acid Exchange in ACTIN2 Confers Increased Tolerance to Oxidative Stress in Arabidopsis der1–3 Mutant. Int. J. Mol. Sci. 2021, 22, 1879. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, G.P.; Kéki, S.; Dékány-Adamoczky, A.; Freytag, C.; Vasas, G.; Máthé, C.; Garda, T. Microcystin-LR, a Cyanobacterial Toxin, Induces Changes in the Organization of Membrane Compartments in Arabidopsis. Microorganisms 2023, 11, 586. https://doi.org/10.3390/microorganisms11030586
Juhász GP, Kéki S, Dékány-Adamoczky A, Freytag C, Vasas G, Máthé C, Garda T. Microcystin-LR, a Cyanobacterial Toxin, Induces Changes in the Organization of Membrane Compartments in Arabidopsis. Microorganisms. 2023; 11(3):586. https://doi.org/10.3390/microorganisms11030586
Chicago/Turabian StyleJuhász, Gabriella Petra, Sándor Kéki, Anita Dékány-Adamoczky, Csongor Freytag, Gábor Vasas, Csaba Máthé, and Tamás Garda. 2023. "Microcystin-LR, a Cyanobacterial Toxin, Induces Changes in the Organization of Membrane Compartments in Arabidopsis" Microorganisms 11, no. 3: 586. https://doi.org/10.3390/microorganisms11030586
APA StyleJuhász, G. P., Kéki, S., Dékány-Adamoczky, A., Freytag, C., Vasas, G., Máthé, C., & Garda, T. (2023). Microcystin-LR, a Cyanobacterial Toxin, Induces Changes in the Organization of Membrane Compartments in Arabidopsis. Microorganisms, 11(3), 586. https://doi.org/10.3390/microorganisms11030586







