Angiotensin-Converting Enzyme 2 Expression and Severity of SARS-CoV-2 Infection
Abstract
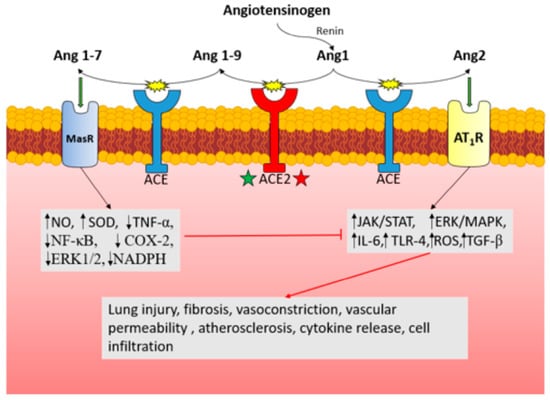
:1. Introduction
2. Methods
3. Demographic Characteristics
4. Disease States
4.1. Cardiovascular Disease
4.2. Hypertension
4.3. Diabetes
4.4. Kidney Disease
4.5. Pulmonary Diseases
5. Medications
5.1. Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers
5.2. Mineralocorticoid Receptor Antagonists
5.3. HMG CoA Reductase Inhibitors
5.4. Metformin
5.5. Glucagon-like Peptide-1 Receptor Agonists
5.6. Dipeptidyl Peptidase-4 Inhibitors
5.7. NSAIDs
6. Limitations
7. Summary
 Factors with increased expression: Female sex, estrogen levels, younger age*, nasal and bronchial epithelial cells with age, mechanical ventilation with age, post-MI x 4 weeks, heart failure, renal proximal tubule cells, renal glomerular podocytes, pancreatic islet cells, controlled diabetes (1/2), Type 2 pneumocytes, COPD, chronic tobacco exposure, e-cigarettes, alcohol consumption, atopic asthma, ACEi, ARBs, MRAs, statins, GLP-1 receptor agonists, DPP4, NSAIDs*.
Factors with increased expression: Female sex, estrogen levels, younger age*, nasal and bronchial epithelial cells with age, mechanical ventilation with age, post-MI x 4 weeks, heart failure, renal proximal tubule cells, renal glomerular podocytes, pancreatic islet cells, controlled diabetes (1/2), Type 2 pneumocytes, COPD, chronic tobacco exposure, e-cigarettes, alcohol consumption, atopic asthma, ACEi, ARBs, MRAs, statins, GLP-1 receptor agonists, DPP4, NSAIDs*.  Factors with reduced expression: Male sex, older age*, SARS-CoV-2 infection of cardiac myocytes, hypertension, uncontrolled diabetes (1/2), glomerulus of CKD patients, se-vere ARDS*, NSAIDs*, *Inconsisent evidence.
Factors with reduced expression: Male sex, older age*, SARS-CoV-2 infection of cardiac myocytes, hypertension, uncontrolled diabetes (1/2), glomerulus of CKD patients, se-vere ARDS*, NSAIDs*, *Inconsisent evidence.Author Contributions
Funding
Conflicts of Interest
References
- Golcuk, M.; Yildiz, A.; Gur, M. Omicron BA.1 and BA.2 Variants Increase the Interactions of SARS-CoV-2 Spike Glycoprotein with ACE2. J. Mol. Graph. Model. 2022, 117, 108286. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-NCoV Spike in the Prefusion Conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Tipnis, S.R.; Hooper, N.M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A.J. A Human Homolog of Angiotensin-Converting Enzyme: CLONING AND FUNCTIONAL EXPRESSION AS A CAPTOPRIL-INSENSITIVE CARBOXYPEPTIDASE*. J. Biol. Chem. 2000, 275, 33238–33243. [Google Scholar] [CrossRef] [Green Version]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K. A Novel Angiotensin-Converting Enzyme–Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9. Circ. Res. 2000, 87, e1–e9. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1–7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1–7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef] [Green Version]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-Converting Enzyme 2 (ACE2) Expression and Tissue Susceptibility to SARS-CoV-2 Infection. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Peiris, S.; Mesa, H.; Aysola, A.; Manivel, J.; Toledo, J.; Borges-Sa, M.; Aldighieri, S.; Reveiz, L. Pathological Findings in Organs and Tissues of Patients with COVID-19: A Systematic Review. PLoS ONE 2021, 16, e0250708. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.R.; Ausiello, J. Functional ACE2 Deficiency Leading to Angiotensin Imbalance in the Pathophysiology of COVID-19. Rev. Endocr. Metab. Disord. 2022, 23, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Tukiainen, T.; Villani, A.-C.; Yen, A.; Rivas, M.A.; Marshall, J.L.; Satija, R.; Aguirre, M.; Gauthier, L.; Fleharty, M.; Kirby, A.; et al. Landscape of X Chromosome Inactivation across Human Tissues. Nature 2017, 550, 244–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumbers, E.R.; Head, R.; Smith, G.R.; Delforce, S.J.; Jarrott, B.; Martin, J.H.; Pringle, K.G. The Interacting Physiology of COVID-19 and the Renin-Angiotensin-Aldosterone System: Key Agents for Treatment. Pharmacol. Res. Perspect. 2022, 10, e00917. [Google Scholar] [CrossRef] [PubMed]
- Sofronova, S.I.; Borzykh, A.A.; Gaynullina, D.K.; Kuzmin, I.V.; Shvetsova, A.A.; Lukoshkova, E.V.; Tarasova, O.S. Endothelial Nitric Oxide Weakens Arterial Contractile Responses and Reduces Blood Pressure during Early Postnatal Development in Rats. Nitric Oxide 2016, 55–56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Pahan, K. Is COVID-19 Gender-Sensitive? J. Neuroimmune Pharmacol. 2021, 16, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Yanez, N.D.; Weiss, N.S.; Romand, J.-A.; Treggiari, M.M. COVID-19 Mortality Risk for Older Men and Women. BMC Public Health 2020, 20, 1742. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-CoV-2 Cell Receptor Gene ACE2 in a Wide Variety of Human Tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cong, M.; Wang, N.; Li, X.; Zhang, H.; Zhang, K.; Jin, M.; Wu, N.; Qiu, C.; Li, J. Association of Angiotensin-Converting Enzyme 2 Gene Polymorphism and Enzymatic Activity with Essential Hypertension in Different Gender: A Case-Control Study. Medicine 2018, 97, e12917. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.C.; Tieri, P.; Ortona, E.; Ruggieri, A. ACE2 Expression and Sex Disparity in COVID-19. Cell Death Discov. 2020, 6, 37. [Google Scholar] [CrossRef]
- Bukowska, A.; Spiller, L.; Wolke, C.; Lendeckel, U.; Weinert, S.; Hoffmann, J.; Bornfleth, P.; Kutschka, I.; Gardemann, A.; Isermann, B.; et al. Protective Regulation of the ACE2/ACE Gene Expression by Estrogen in Human Atrial Tissue from Elderly Men. Exp. Biol. Med. 2017, 242, 1412–1423. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Xudong, X.; Chen, J.; Junzhu, C.; Wang, X.; Xingxiang, W.; Zhang, F.; Furong, Z.; Liu, Y.; Yanrong, L. Age- and Gender-Related Difference of ACE2 Expression in Rat Lung. Life Sci. 2006, 78, 2166–2171. [Google Scholar] [CrossRef]
- Salah, H.M.; Mehta, J.L. Hypothesis: Sex-Related Differences in ACE2 Activity May Contribute to Higher Mortality in Men Versus Women With COVID-19. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 114–118. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.; Lin, R.; Han, K. Clinical Features of COVID-19 in Elderly Patients: A Comparison with Young and Middle-Aged Patients. J. Infect. 2020, 80, e14–e18. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D. The Impact of COVID-19 Pandemic on Elderly Mental Health. Int. J. Geriatr. Psychiatry 2020, 35, 1466–1467. [Google Scholar] [CrossRef] [PubMed]
- Caramelo, F.; Ferreira, N.; Oliveiros, B. Estimation of Risk Factors for COVID-19 Mortality—Preliminary Results. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Nikolich-Zugich, J.; Knox, K.S.; Rios, C.T.; Natt, B.; Bhattacharya, D.; Fain, M.J. SARS-CoV-2 and COVID-19 in Older Adults: What We May Expect Regarding Pathogenesis, Immune Responses, and Outcomes. GeroScience 2020, 42, 505–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Jiang, Q.; Xia, X.; Liu, K.; Yu, Z.; Tao, W.; Gong, W.; Han, J.-D.J. Individual Variation of the SARS-CoV-2 Receptor ACE2 Gene Expression and Regulation. Aging Cell 2020, 19, e13168. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 2020, 323, 2427–2429. [Google Scholar] [CrossRef]
- Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Alabed, M.; Temsah, M.-H.; Al Heialy, S.; Hamid, Q.; Halwani, R. Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD. Mol. Ther.—Methods Clin. Dev. 2020, 18, 1–6. [Google Scholar] [CrossRef]
- Baker, S.A.; Kwok, S.; Berry, G.J.; Montine, T.J. Angiotensin-Converting Enzyme 2 (ACE2) Expression Increases with Age in Patients Requiring Mechanical Ventilation. PLoS ONE 2021, 16, e0247060. [Google Scholar] [CrossRef]
- Silva, M.G.; Falcoff, N.L.; Corradi, G.R.; Di Camillo, N.; Seguel, R.F.; Tabaj, G.C.; Guman, G.R.; de Matteo, E.; Nuñez, M.; Gironacci, M.M. Effect of Age on Human ACE2 and ACE2-Expressing Alveolar Type II Cells Levels. Pediatr. Res. 2022, 1–5. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-Cell RNA-Seq Data Analysis on the Receptor ACE2 Expression Reveals the Potential Risk of Different Human Organs Vulnerable to 2019-NCoV Infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crackower, M.A.; Sarao, R.; Oudit, G.Y.; Yagil, C.; Kozieradzki, I.; Scanga, S.E.; Oliveira-dos-Santos, A.J.; da Costa, J.; Zhang, L.; Pei, Y.; et al. Angiotensin-Converting Enzyme 2 Is an Essential Regulator of Heart Function. Nature 2002, 417, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.-M.; Burrell, L.M.; Kladis, A.; Campbell, D.J. Angiotensin and Bradykinin Peptides in Rats with Myocardial Infarction. J. Card. Fail. 1997, 3, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Domanski, M.J.; Exner, D.V.; Borkowf, C.B.; Geller, N.L.; Rosenberg, Y.; Pfeffer, M.A. Effect of Angiotensin Converting Enzyme Inhibition on Sudden Cardiac Death in Patients Following Acute Myocardial Infarction: A Meta-Analysis of Randomized Clinical Trials. J. Am. Coll. Cardiol. 1999, 33, 598–604. [Google Scholar] [CrossRef] [Green Version]
- Ambrosioni, E.; Borghi, C.; Magnani, B. The Effect of the Angiotensin-Converting–Enzyme Inhibitor Zofenopril on Mortality and Morbidity after Anterior Myocardial Infarction. N. Engl. J. Med. 1995, 332, 80–85. [Google Scholar] [CrossRef]
- Køber, L.; Torp-Pedersen, C.; Carlsen, J.E.; Bagger, H.; Eliasen, P.; Lyngborg, K.; Videbæk, J.; Cole, D.S.; Auclert, L.; Pauly, N.C.; et al. A Clinical Trial of the Angiotensin-Converting–Enzyme Inhibitor Trandolapril in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N. Engl. J. Med. 1995, 333, 1670–1676. [Google Scholar] [CrossRef]
- Burrell, L.M.; Risvanis, J.; Kubota, E.; Dean, R.G.; MacDonald, P.S.; Lu, S.; Tikellis, C.; Grant, S.L.; Lew, R.A.; Smith, A.I.; et al. Myocardial Infarction Increases ACE2 Expression in Rat and Humans. Eur. Heart J. 2005, 26, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Dutta, P.; Nahrendorf, M. Monocytes in Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1066–1070. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, S.; Xiao, Z.; Gao, K.; Mao, L.; Chen, J.; Ping, W.; Hong, W.; Zhang, Z. Role and Interaction Between ACE1, ACE2 and Their Related Genes in Cardiovascular Disorders. Curr. Probl. Cardiol. 2022, 101162. [Google Scholar] [CrossRef]
- Kim, M.-A.; Yang, D.; Kida, K.; Molotkova, N.; Yeo, S.J.; Varki, N.; Iwata, M.; Dalton, N.D.; Peterson, K.L.; Siems, W.-E.; et al. Effects of ACE2 Inhibition in the Post-Myocardial Infarction Heart. J. Card. Fail. 2010, 16, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Weir, R.A.P.; McMurray, J.J.V.; Velazquez, E.J. Epidemiology of Heart Failure and Left Ventricular Systolic Dysfunction after Acute Myocardial Infarction: Prevalence, Clinical Characteristics, and Prognostic Importance. Am. J. Cardiol. 2006, 97, 13F–25F. [Google Scholar] [CrossRef]
- Mehra, M.R.; Ruschitzka, F. COVID-19 Illness and Heart Failure: A Missing Link? JACC Heart Fail. 2020, 8, 512–514. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of Angiotensin-Converting Enzyme 2 (ACE2) in COVID-19. Crit. Care Lond. Engl. 2020, 24, 422. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The Pivotal Link between ACE2 Deficiency and SARS-CoV-2 Infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mizuiri, S.; Ohashi, Y. ACE and ACE2 in Kidney Disease. World J. Nephrol. 2015, 4, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Velkoska, E.; Freeman, M.; Wai, B.; Lancefield, T.F.; Burrell, L.M. From Gene to Protein—Experimental and Clinical Studies of ACE2 in Blood Pressure Control and Arterial Hypertension. Front. Physiol. 2014, 5, 227. [Google Scholar] [CrossRef]
- Wysocki, J.; Ye, M.; Rodriguez, E.; González-Pacheco, F.R.; Barrios, C.; Evora, K.; Schuster, M.; Loibner, H.; Brosnihan, K.B.; Ferrario, C.M.; et al. Targeting the Degradation of Angiotensin II with Recombinant Angiotensin-Converting Enzyme 2: Prevention of Angiotensin II-Dependent Hypertension. Hypertens. Dallas Tex 1979 2010, 55, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez-Freire, C.; Vázquez, J.; Correa de Adjounian, M.F.; Ferrari, M.F.R.; Yuan, L.; Silver, X.; Torres, R.; Raizada, M.K. ACE2 Gene Transfer Attenuates Hypertension-Linked Pathophysiological Changes in the SHR. Physiol. Genomics 2006, 27, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikellis, C.; Cooper, M.E.; Bialkowski, K.; Johnston, C.I.; Burns, W.C.; Lew, R.A.; Smith, A.I.; Thomas, M.C. Developmental Expression of ACE2 in the SHR Kidney: A Role in Hypertension? Kidney Int. 2006, 70, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, R.D.; Macedo, A.V.S.; de Barros E Silva, P.G.M.; Moll-Bernardes, R.J.; dos Santos, T.M.; Mazza, L.; Feldman, A.; D’Andréa Saba Arruda, G.; de Albuquerque, D.C.; Camiletti, A.S.; et al. Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Insel, P.A. Risks of ACE Inhibitor and ARB Usage in COVID-19: Evaluating the Evidence. Clin. Pharmacol. Ther. 2020, 108, 236–241. [Google Scholar] [CrossRef]
- Flacco, M.E.; Martellucci, C.A.; Bravi, F.; Parruti, G.; Cappadona, R.; Mascitelli, A.; Manfredini, R.; Mantovani, L.G.; Manzoli, L. Treatment with ACE Inhibitors or ARBs and Risk of Severe/Lethal COVID-19: A Meta-Analysis. Heart 2020, 106, 1519–1524. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Sun, X.; Xue, H.; Shao, J.; Cai, W.; Jing, Y.; Yue, M.; Dong, C. Association of Hypertension with the Severity and Fatality of SARS-CoV-2 Infection: A Meta-Analysis. Epidemiol. Infect. 2020, 148, e106. [Google Scholar] [CrossRef]
- Ma, K.; Gao, W.; Xu, H.; Liang, W.; Ma, G. Role and Mechanism of the Renin-Angiotensin-Aldosterone System in the Onset and Development of Cardiorenal Syndrome. J. Renin-Angiotensin-Aldosterone Syst. JRAAS 2022, 2022, 3239057. [Google Scholar] [CrossRef]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, Pathophysiology, Prognosis and Practical Considerations. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 303–310. [Google Scholar] [CrossRef]
- Kumar, A.; Arora, A.; Sharma, P.; Anikhindi, S.A.; Bansal, N.; Singla, V.; Khare, S.; Srivastava, A. Is Diabetes Mellitus Associated with Mortality and Severity of COVID-19? A Meta-Analysis. Diabetes Metab. Syndr. 2020, 14, 535–545. [Google Scholar] [CrossRef]
- Maksimowski, N.; Williams, V.R.; Scholey, J.W. Kidney ACE2 Expression: Implications for Chronic Kidney Disease. PLoS ONE 2020, 15, e0241534. [Google Scholar] [CrossRef]
- Reich, H.N.; Oudit, G.Y.; Penninger, J.M.; Scholey, J.W.; Herzenberg, A.M. Decreased Glomerular and Tubular Expression of ACE2 in Patients with Type 2 Diabetes and Kidney Disease. Kidney Int. 2008, 74, 1610–1616. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Lau, A.; So, H.-C. Exploring Diseases/Traits and Blood Proteins Causally Related to Expression of ACE2, the Putative Receptor of SARS-CoV-2: A Mendelian Randomization Analysis Highlights Tentative Relevance of Diabetes-Related Traits. Diabetes Care 2020, 43, 1416–1426. [Google Scholar] [CrossRef]
- Singh, A.K.; Khunti, K. Assessment of Risk, Severity, Mortality, Glycemic Control and Antidiabetic Agents in Patients with Diabetes and COVID-19: A Narrative Review. Diabetes Res. Clin. Pract. 2020, 165, 108266. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Banerjee, M. Are People with Uncontrolled Diabetes Mellitus at High Risk of Reinfections with COVID-19? Prim. Care Diabetes 2021, 15, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Badhwar, S.; Chandran, D.S.; Jaryal, A.K.; Jyotsna, V.P.; Deepak, K.K. Imbalance between Angiotensin II—Angiotensin (1-7) System Is Associated with Vascular Endothelial Dysfunction and Inflammation in Type 2 Diabetes with Newly Diagnosed Hypertension. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.A.; Beaubien-Souligny, W.; Shah, P.S.; Harel, S.; Blum, D.; Kishibe, T.; Meraz-Munoz, A.; Wald, R.; Harel, Z. The Prevalence of Acute Kidney Injury in Patients Hospitalized With COVID-19 Infection: A Systematic Review and Meta-Analysis. Kidney Med. 2021, 3, 83–98.e1. [Google Scholar] [CrossRef]
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-Associated Acute Kidney Injury. Nat. Rev. Nephrol. 2021, 17, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A.B.; Nie, X.; et al. Renal Histopathological Analysis of 26 Postmortem Findings of Patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Gok, M.; Cetinkaya, H.; Kandemir, T.; Karahan, E.; Tuncer, İ.B.; Bukrek, C.; Sahin, G. Chronic Kidney Disease Predicts Poor Outcomes of COVID-19 Patients. Int. Urol. Nephrol. 2021, 53, 1891–1898. [Google Scholar] [CrossRef]
- Ozturk, S.; Turgutalp, K.; Arici, M.; Odabas, A.R.; Altiparmak, M.R.; Aydin, Z.; Cebeci, E.; Basturk, T.; Soypacaci, Z.; Sahin, G.; et al. Mortality Analysis of COVID-19 Infection in Chronic Kidney Disease, Haemodialysis and Renal Transplant Patients Compared with Patients without Kidney Disease: A Nationwide Analysis from Turkey. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2020, 35, 2083–2095. [Google Scholar] [CrossRef]
- Kazama, I. Targeting ACE2 as a Potential Prophylactic Strategy against COVID-19-Induced Exacerbation of Chronic Kidney Disease. Inflamm. Res. 2022, 71, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Neugarten, J.; Bellin, E.; Yunes, M.; Stahl, L.; Johns, T.S.; Abramowitz, M.K.; Levy, R.; Kumar, N.; Mokrzycki, M.H.; et al. AKI in Hospitalized Patients with and without COVID-19: A Comparison Study. J. Am. Soc. Nephrol. JASN 2020, 31, 2145–2157. [Google Scholar] [CrossRef]
- Brogan, M.; Ross, M.J. The Impact of Chronic Kidney Disease on Outcomes of Patients with COVID-19 Admitted to the Intensive Care Unit. Nephron 2022, 146, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Pieters, T.T.; Verhaar, M.C.; Berger, S.P.; Bakker, S.J.L.; van Zuilen, A.D.; Joles, J.A.; Vernooij, R.W.M.; van Balkom, B.W.M. A Systematic Review and Meta-Analysis of COVID-19 in Kidney Transplant Recipients: Lessons to Be Learned. Am. J. Transplant. 2021, 21, 3936–3945. [Google Scholar] [CrossRef] [PubMed]
- Marinaki, S.; Tsiakas, S.; Korogiannou, M.; Grigorakos, K.; Papalois, V.; Boletis, I. A Systematic Review of COVID-19 Infection in Kidney Transplant Recipients: A Universal Effort to Preserve Patients’ Lives and Allografts. J. Clin. Med. 2020, 9, 2986. [Google Scholar] [CrossRef] [PubMed]
- Cahova, M.; Kveton, M.; Petr, V.; Funda, D.; Dankova, H.; Viklicky, O.; Hruba, P. Local Angiotensin-Converting Enzyme 2 Gene Expression in Kidney Allografts Is Not Affected by Renin-Angiotensin-Aldosterone Inhibitors. Kidney Blood Press. Res. 2021, 46, 245–249. [Google Scholar] [CrossRef]
- Jonigk, D.; Werlein, C.; Lee, P.D.; Kauczor, H.-U.; Länger, F.; Ackermann, M. Pulmonary and Systemic Pathology in COVID-19. Dtsch. Arzteblatt Int. 2022, 119, 429–435. [Google Scholar] [CrossRef]
- Marshall, R.P.; Webb, S.; Bellingan, G.J.; Montgomery, H.E.; Chaudhari, B.; McAnulty, R.J.; Humphries, S.E.; Hill, M.R.; Laurent, G.J. Angiotensin Converting Enzyme Insertion/Deletion Polymorphism Is Associated with Susceptibility and Outcome in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2002, 166, 646–650. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-Converting Enzyme 2 Protects from Severe Acute Lung Failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Hoshizaki, M.; Minato, T.; Nirasawa, S.; Asaka, M.N.; Niiyama, M.; Imai, M.; Uda, A.; Chan, J.F.-W.; Takahashi, S.; et al. ACE2-like Carboxypeptidase B38-CAP Protects from SARS-CoV-2-Induced Lung Injury. Nat. Commun. 2021, 12, 6791. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.S.; Chen, R.E.; Alam, F.; Yildiz, S.; Case, J.B.; Uccellini, M.B.; Holtzman, M.J.; Garcia-Sastre, A.; Schotsaert, M.; Diamond, M.S. SARS-CoV-2 Causes Lung Infection without Severe Disease in Human ACE2 Knock-In Mice. J. Virol. 2022, 96, e01511-21. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, F.L.; Netea, M.G.; van Deuren, M.; van der Meer, J.W.; de Mast, Q.; Brüggemann, R.J.; van der Hoeven, H. Kallikrein-Kinin Blockade in Patients with COVID-19 to Prevent Acute Respiratory Distress Syndrome. eLife 2020, 9, e57555. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, V.; Ferreira, A.J.; Qi, Y.; Fraga-Silva, R.A.; Díez-Freire, C.; Dooies, A.; Jun, J.Y.; Sriramula, S.; Mariappan, N.; Pourang, D.; et al. The Angiotensin-Converting Enzyme 2/Angiogenesis-(1–7)/Mas Axis Confers Cardiopulmonary Protection against Lung Fibrosis and Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2010, 182, 1065–1072. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Yu, C.-H.; Li, W.; Li, T.; Luo, W.; Huang, S.; Wu, P.-S.; Cai, S.-X.; Li, X. Angiotensin-Converting Enzyme 2/Angiotensin-(1-7)/Mas Axis Protects against Lung Fibrosis by Inhibiting the MAPK/NF-ΚB Pathway. Am. J. Respir. Cell Mol. Biol. 2014, 50, 723–736. [Google Scholar] [CrossRef]
- Jacobs, M.; Van Eeckhoutte, H.P.; Wijnant, S.R.A.; Janssens, W.; Joos, G.F.; Brusselle, G.G.; Bracke, K.R. Increased Expression of ACE2, the SARS-CoV-2 Entry Receptor, in Alveolar and Bronchial Epithelium of Smokers and COPD Subjects. Eur. Respir. J. 2020, 56, 2002378. [Google Scholar] [CrossRef]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.-L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 Expression in the Small Airway Epithelia of Smokers and COPD Patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef] [Green Version]
- Meza, D.; Khuder, B.; Bailey, J.I.; Rosenberg, S.R.; Kalhan, R.; Reyfman, P.A. Mortality from COVID-19 in Patients with COPD: A US Study in the N3C Data Enclave. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 2323–2326. [Google Scholar] [CrossRef]
- Huang, B.Z.; Chen, Z.; Sidell, M.A.; Eckel, S.P.; Martinez, M.P.; Lurmann, F.; Thomas, D.C.; Gilliland, F.D.; Xiang, A.H. Asthma Disease Status, COPD, and COVID-19 Severity in a Large Multiethnic Population. J. Allergy Clin. Immunol. Pract. 2021, 9, 3621–3628.e2. [Google Scholar] [CrossRef]
- Wheaton, A.G.; Liu, Y.; Croft, J.B.; VanFrank, B.; Croxton, T.L.; Punturieri, A.; Postow, L.; Greenlund, K.J. Chronic Obstructive Pulmonary Disease and Smoking Status—United States, 2017. Morb. Mortal. Wkly. Rep. 2019, 68, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.C.; Sausville, E.L.; Girish, V.; Yuan, M.L.; Vasudevan, A.; John, K.M.; Sheltzer, J.M. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev. Cell 2020, 53, 514–529.e3. [Google Scholar] [CrossRef] [PubMed]
- Lallai, V.; Manca, L.; Fowler, C.D. E-Cigarette Vape and Lung ACE2 Expression: Implications for Coronavirus Vulnerability. Environ. Toxicol. Pharmacol. 2021, 86, 103656. [Google Scholar] [CrossRef] [PubMed]
- Chhapola Shukla, S. ACE2 Expression in Allergic Airway Disease May Decrease the Risk and Severity of COVID-19. Eur. Arch. Otorhinolaryngol. 2021, 278, 2637–2640. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.L.; Sayles, H.; Campbell, J.; Khalid, N.; Anglim, M.; Ponce, J.; Wyatt, T.A.; McClay, J.C.; Burnham, E.L.; Anzalone, A.; et al. COVID-19 Patients with Documented Alcohol Use Disorder or Alcohol-Related Complications Are More Likely to Be Hospitalized and Have Higher All-Cause Mortality. Alcohol. Clin. Exp. Res. 2022, 46, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Simou, E.; Leonardi-Bee, J.; Britton, J. The Effect of Alcohol Consumption on the Risk of ARDS: A Systematic Review and Meta-Analysis. CHEST 2018, 154, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassen, M.C.H.; Skaarup, K.G.; Sengeløv, M.; Iversen, K.; Ulrik, C.S.; Jensen, J.U.S.; Biering-Sørensen, T. Alcohol Consumption and the Risk of Acute Respiratory Distress Syndrome in COVID-19. Ann. Am. Thorac. Soc. 2021, 18, 1074–1076. [Google Scholar] [CrossRef]
- Friske, M.M.; Giannone, F.; Senger, M.; Seitz, R.; Hansson, A.C.; Spanagel, R. Chronic Alcohol Intake Regulates Expression of SARS-CoV-2 Infection-Relevant Genes in an Organ-Specific Manner. Alcohol. Clin. Exp. Res. 2023, 47, 76–86. [Google Scholar] [CrossRef]
- Solopov, P.A.; Colunga Biancatelli, R.M.L.; Catravas, J.D. Alcohol Increases Lung Angiotensin-Converting Enzyme 2 Expression and Exacerbates Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein Subunit 1-Induced Acute Lung Injury in K18-HACE2 Transgenic Mice. Am. J. Pathol. 2022, 192, 990–1000. [Google Scholar] [CrossRef]
- Ferrario, C.M.; Jessup, J.; Chappell, M.C.; Averill, D.B.; Brosnihan, K.B.; Tallant, E.A.; Diz, D.I.; Gallagher, P.E. Effect of Angiotensin-Converting Enzyme Inhibition and Angiotensin II Receptor Blockers on Cardiac Angiotensin-Converting Enzyme 2. Circulation 2005, 111, 2605–2610. [Google Scholar] [CrossRef] [Green Version]
- Ishiyama, Y.; Gallagher, P.E.; Averill, D.B.; Tallant, E.A.; Brosnihan, K.B.; Ferrario, C.M. Upregulation of Angiotensin-Converting Enzyme 2 after Myocardial Infarction by Blockade of Angiotensin II Receptors. Hypertens. Dallas Tex 1979 2004, 43, 970–976. [Google Scholar] [CrossRef] [Green Version]
- Takeda, Y.; Zhu, A.; Yoneda, T.; Usukura, M.; Takata, H.; Yamagishi, M. Effects of Aldosterone and Angiotensin II Receptor Blockade on Cardiac Angiotensinogen and Angiotensin-Converting Enzyme 2 Expression in Dahl Salt-Sensitive Hypertensive Rats. Am. J. Hypertens. 2007, 20, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Karakiulakis, G.; Roth, M. Are Patients with Hypertension and Diabetes Mellitus at Increased Risk for COVID-19 Infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Kali, M.S.K. Association of Angiotensin-Converting Enzyme Inhibitors and Angiotensin-Receptor Blockers with Risk of Mortality, Severity or SARS-CoV-2 Test Positivity in COVID-19 Patients: Meta-Analysis. Sci. Rep. 2021, 11, 5012. [Google Scholar] [CrossRef] [PubMed]
- Sama, I.E.; Ravera, A.; Santema, B.T.; van Goor, H.; ter Maaten, J.M.; Cleland, J.G.F.; Rienstra, M.; Friedrich, A.W.; Samani, N.J.; Ng, L.L.; et al. Circulating Plasma Concentrations of Angiotensin-Converting Enzyme 2 in Men and Women with Heart Failure and Effects of Renin–Angiotensin–Aldosterone Inhibitors. Eur. Heart J. 2020, 41, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kovacs, R.; Harrington, B. Joint HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. J. Card. Fail. 2020, 26, 370. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, M.; Yoshimura, M.; Nakayama, M.; Abe, K.; Sumida, H.; Sugiyama, S.; Saito, Y.; Nakao, K.; Yasue, H.; Ogawa, H. Aldosterone, but Not Angiotensin II, Reduces Angiotensin Converting Enzyme 2 Gene Expression Levels in Cultured Neonatal Rat Cardiomyocytes. Circ. J. 2008, 72, 1346–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keidar, S.; Gamliel-Lazarovich, A.; Kaplan, M.; Pavlotzky, E.; Hamoud, S.; Hayek, T.; Karry, R.; Abassi, Z. Mineralocorticoid Receptor Blocker Increases Angiotensin-Converting Enzyme 2 Activity in Congestive Heart Failure Patients. Circ. Res. 2005, 97, 946–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardi, S.; Toffoli, B.; Zennaro, C.; Bossi, F.; Losurdo, P.; Michelli, A.; Carretta, R.; Mulatero, P.; Fallo, F.; Veglio, F.; et al. Aldosterone Effects on Glomerular Structure and Function. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 730–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Li, X.; Song, Q.; Hu, C.; Su, F.; Dai, J.; Ye, Y.; Huang, J.; Zhang, X. Assessment of Hypokalemia and Clinical Characteristics in Patients With Coronavirus Disease 2019 in Wenzhou, China. JAMA Netw. Open 2020, 3, e2011122. [Google Scholar] [CrossRef] [PubMed]
- Akin, S.; Schriek, P.; van Nieuwkoop, C.; Neuman, R.I.; Meynaar, I.; van Helden, E.J.; Bouazzaoui, H.E.; Baak, R.; Veuger, M.; Mairuhu, R.A.T.A.; et al. A Low Aldosterone/Renin Ratio and High Soluble ACE2 Associate with COVID-19 Severity. J. Hypertens. 2022, 40, 606–614. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Goren, A.; Wambier, C.G. Spironolactone May Provide Protection from SARS-CoV-2: Targeting Androgens, Angiotensin Converting Enzyme 2 (ACE2), and Renin-Angiotensin-Aldosterone System (RAAS). Med. Hypotheses 2020, 143, 110112. [Google Scholar] [CrossRef]
- Tikoo, K.; Patel, G.; Kumar, S.; Karpe, P.A.; Sanghavi, M.; Malek, V.; Srinivasan, K. Tissue Specific up Regulation of ACE2 in Rabbit Model of Atherosclerosis by Atorvastatin: Role of Epigenetic Histone Modifications. Biochem. Pharmacol. 2015, 93, 343–351. [Google Scholar] [CrossRef]
- Shin, Y.H.; Min, J.J.; Lee, J.-H.; Kim, E.-H.; Kim, G.E.; Kim, M.H.; Lee, J.J.; Ahn, H.J. The Effect of Fluvastatin on Cardiac Fibrosis and Angiotensin-Converting Enzyme-2 Expression in Glucose-Controlled Diabetic Rat Hearts. Heart Vessels 2017, 32, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.; Ventura, F.; Rodríguez-Delfín, L. El aumento de la expresión del ARNm de la enzima convertidora de angiotensina I homóloga (ECA-2) inducido por atorvastatina se asocia a menor fibrosis e hipertrofia ventricular izquierda en un modelo de cardiomiopatía diabética. Rev. Peru. Med. Exp. Salud Pública 2011, 28, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-H.; Wang, Q.-X.; Zhou, J.-W.; Chu, X.-M.; Man, Y.-L.; Liu, P.; Ren, B.-B.; Sun, T.-R.; An, Y. Effects of Rosuvastatin on Expression of Angiotensin-Converting Enzyme 2 after Vascular Balloon Injury in Rats. J. Geriatr. Cardiol. JGC 2013, 10, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, R.; Ahlqvist, V.H.; Lundberg, M.; Hergens, M.-P.; Sundström, J.; Bell, M.; Magnusson, C. HMG-CoA Reductase Inhibitors and COVID-19 Mortality in Stockholm, Sweden: A Registry-Based Cohort Study. PLoS Med. 2021, 18, e1003820. [Google Scholar] [CrossRef] [PubMed]
- Al Sulaiman, K.; Aljuhani, O.; Korayem, G.B.; Altebainawi, A.F.; Al Harbi, S.; Al Shaya, A.; Badreldin, H.A.; Kensara, R.; Alharthi, A.F.; Alghamdi, J.; et al. The Impact of HMG-CoA Reductase Inhibitors Use on the Clinical Outcomes in Critically Ill Patients with COVID-19: A Multicenter, Cohort Study. Front. Public Health 2022, 10, 2000688. [Google Scholar] [CrossRef]
- Bailey, C.J.; Gwilt, M. Diabetes, Metformin and the Clinical Course of COVID-19: Outcomes, Mechanisms and Suggestions on the Therapeutic Use of Metformin. Front. Pharmacol. 2022, 13, 784459. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, J.; Martin, M.; He, M.; Gongol, B.; Marin, T.L.; Chen, L.; Shi, X.; Yin, Y.; Shang, F.; et al. AMP-Activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 509–520. [Google Scholar] [CrossRef]
- Malhotra, A.; Hepokoski, M.; McCowen, K.C.; Y-J Shyy, J. ACE2, Metformin, and COVID-19. iScience 2020, 23, 101425. [Google Scholar] [CrossRef]
- Luo, P.; Qiu, L.; Liu, Y.; Liu, X.; Zheng, J.; Xue, H.; Liu, W.; Liu, D.; Li, J. Metformin Treatment Was Associated with Decreased Mortality in COVID-19 Patients with Diabetes in a Retrospective Analysis. Am. J. Trop. Med. Hyg. 2020, 103, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Patel, N.; Vemparala, P.; Krishnamurthy, M. Metformin Is Associated with Favorable Outcomes in Patients with COVID-19 and Type 2 Diabetes Mellitus. Sci. Rep. 2022, 12, 5553. [Google Scholar] [CrossRef]
- Bramante, C.T.; Ingraham, N.E.; Murray, T.A.; Marmor, S.; Hovertsen, S.; Gronski, J.; McNeil, C.; Feng, R.; Guzman, G.; Abdelwahab, N.; et al. Metformin and Risk of Mortality in Patients Hospitalised with COVID-19: A Retrospective Cohort Analysis. Lancet Healthy Longev. 2021, 2, e34–e41. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Yan, P.; Sun, T.; Zeng, Z.; Li, S. Metformin in Patients With COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 704666. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Huling, J.D.; Tignanelli, C.J.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Cohen, K.; Puskarich, M.A.; Belani, H.K.; Proper, J.L.; et al. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for COVID-19. N. Engl. J. Med. 2022, 387, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Liu, M. Letter to the Editor: Comment on GLP-1-Based Drugs and COVID-19 Treatment. Acta Pharm. Sin. B 2020, 10, 1249–1250. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Jun, H.-S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediators Inflamm. 2016, 2016, 3094642. [Google Scholar] [CrossRef] [Green Version]
- Romaní-Pérez, M.; Outeiriño-Iglesias, V.; Moya, C.M.; Santisteban, P.; González-Matías, L.C.; Vigo, E.; Mallo, F. Activation of the GLP-1 Receptor by Liraglutide Increases ACE2 Expression, Reversing Right Ventricle Hypertrophy, and Improving the Production of SP-A and SP-B in the Lungs of Type 1 Diabetes Rats. Endocrinology 2015, 156, 3559–3569. [Google Scholar] [CrossRef]
- Wright, J.R. Immunoregulatory Functions of Surfactant Proteins. Nat. Rev. Immunol. 2005, 5, 58–68. [Google Scholar] [CrossRef]
- Iacobellis, G. COVID-19 and Diabetes: Can DPP4 Inhibition Play a Role? Diabetes Res. Clin. Pract. 2020, 162, 108125. [Google Scholar] [CrossRef]
- Beraldo, J.I.; Benetti, A.; Borges-Júnior, F.A.; Arruda-Junior, D.F.; Martins, F.L.; Jensen, L.; Dariolli, R.; Shimizu, M.H.; Seguro, A.C.; Luchi, W.M.; et al. Cardioprotection Conferred by Sitagliptin Is Associated with Reduced Cardiac Angiotensin II/Angiotensin-(1-7) Balance in Experimental Chronic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 1940. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Cai, Z.; Zhang, J. DPP-4 Inhibitors May Improve the Mortality of Coronavirus Disease 2019: A Meta-Analysis. PLoS ONE 2021, 16, e0251916. [Google Scholar] [CrossRef]
- Pal, R.; Banerjee, M.; Mukherjee, S.; Bhogal, R.S.; Kaur, A.; Bhadada, S.K. Dipeptidyl Peptidase-4 Inhibitor Use and Mortality in COVID-19 Patients with Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018821996482. [Google Scholar] [CrossRef]
- Day, M. COVID-19: Ibuprofen Should Not Be Used for Managing Symptoms, Say Doctors and Scientists. BMJ 2020, 368, m1086. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, R.; Pedrosa, M.A.; Garrido-Gil, P.; Labandeira, C.M.; Navarro, G.; Franco, R.; Rodriguez-Perez, A.I.; Labandeira-Garcia, J.L. Interactions between Ibuprofen, ACE2, Renin-Angiotensin System, and Spike Protein in the Lung. Implications for COVID-19. Clin. Transl. Med. 2021, 11, e371. [Google Scholar] [CrossRef]
- de Bruin, N.; Schneider, A.-K.; Reus, P.; Talmon, S.; Ciesek, S.; Bojkova, D.; Cinatl, J.; Lodhi, I.; Charlesworth, B.; Sinclair, S.; et al. Ibuprofen, Flurbiprofen, Etoricoxib or Paracetamol Do Not Influence ACE2 Expression and Activity In Vitro or in Mice and Do Not Exacerbate In-Vitro SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 1049. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabsi, S.; Dhole, A.; Hozayen, S.; Chapman, S.A. Angiotensin-Converting Enzyme 2 Expression and Severity of SARS-CoV-2 Infection. Microorganisms 2023, 11, 612. https://doi.org/10.3390/microorganisms11030612
Alabsi S, Dhole A, Hozayen S, Chapman SA. Angiotensin-Converting Enzyme 2 Expression and Severity of SARS-CoV-2 Infection. Microorganisms. 2023; 11(3):612. https://doi.org/10.3390/microorganisms11030612
Chicago/Turabian StyleAlabsi, Sarah, Atharva Dhole, Sameh Hozayen, and Scott A. Chapman. 2023. "Angiotensin-Converting Enzyme 2 Expression and Severity of SARS-CoV-2 Infection" Microorganisms 11, no. 3: 612. https://doi.org/10.3390/microorganisms11030612
APA StyleAlabsi, S., Dhole, A., Hozayen, S., & Chapman, S. A. (2023). Angiotensin-Converting Enzyme 2 Expression and Severity of SARS-CoV-2 Infection. Microorganisms, 11(3), 612. https://doi.org/10.3390/microorganisms11030612