Lactobacilli and Their Probiotic Effects in the Vagina of Reproductive Age Women
Abstract
:1. Introduction
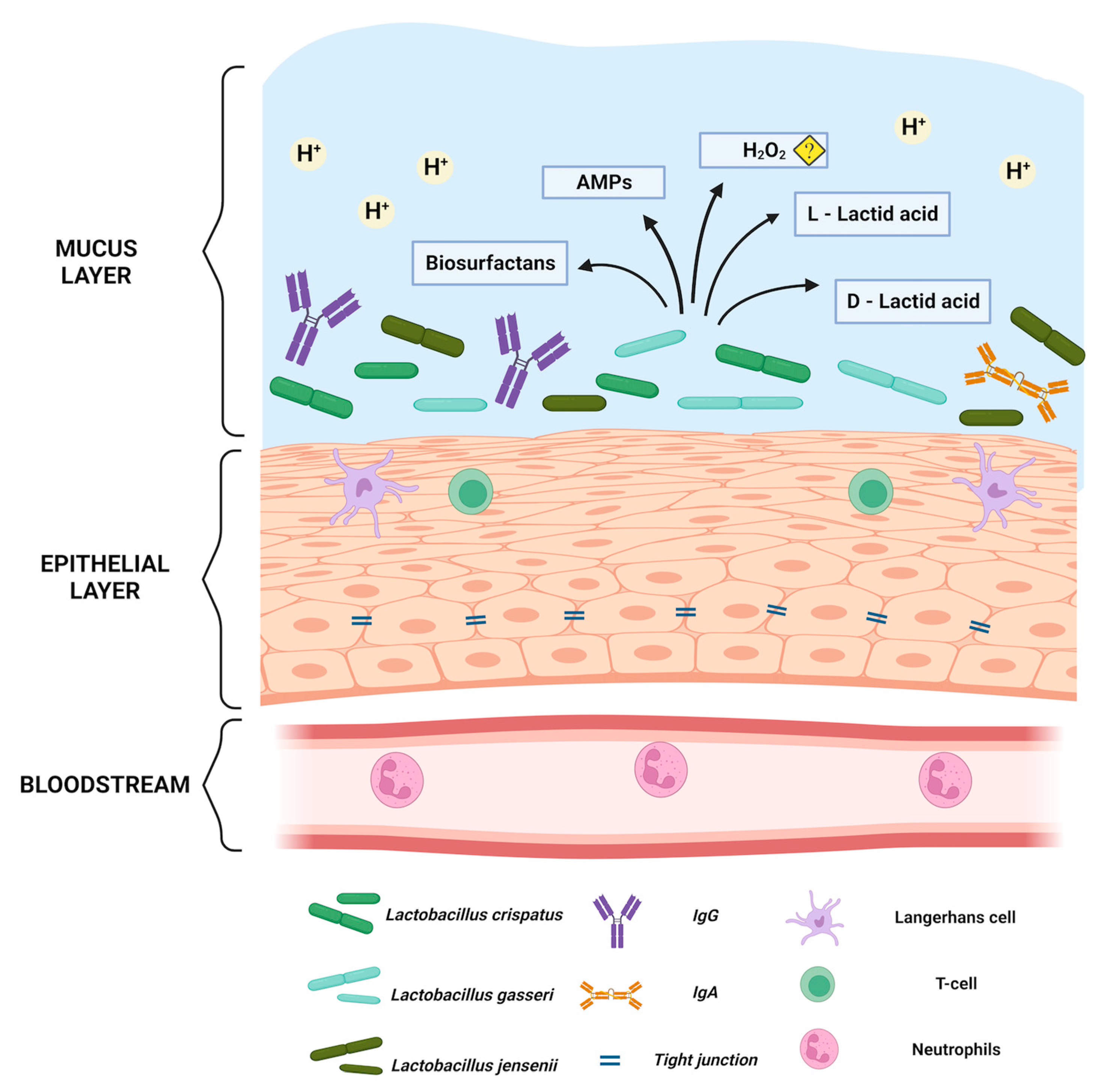
2. Physiological Role of Lactobacillus spp. in Maintaining Eubiosis in a Healthy Vagina
2.1. Vaginal Lactobacillus spp. and Lactic Acid
2.2. Lactobacilli and Hydrogen Peroxide
2.3. Lactobacilli, Bacteriocins and Core Proteins
2.4. L. iners and Its Differential Role
3. Natural Non-Lactobacillus-Based Defence Mechanisms of the Vagina
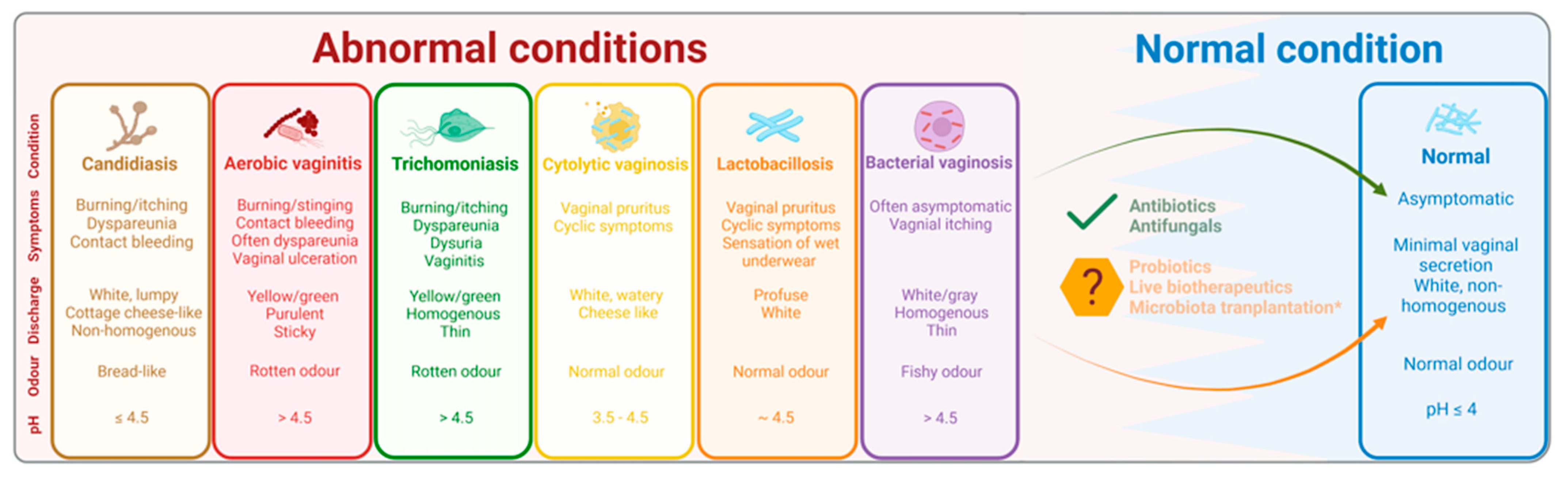
4. Vaginal Dysbiosis: Understanding the Abnormal Microbial Conditions of the Vagina
5. Vaginal Probiotic Supplements and Live Biotherapeutic Drugs: Success and Challenges
6. Regulatory Processes and Changes Required for Making Advancements
7. Future Perspectives on Vaginal Lactobacilli and Their Probiotic Usage
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thomas, S. Döderlein’s Bacillus: Lactobacillus Acidophilus. J. Infect. Dis. 1928, 43, 218–227. [Google Scholar] [CrossRef]
- Spear, G.T.; French, A.L.; Gilbert, D.; Zariffard, M.R.; Mirmonsef, P.; Sullivan, T.H.; Spear, W.W.; Landay, A.; Micci, S.; Lee, B.-H.; et al. Human α-Amylase Present in Lower-Genital-Tract Mucosal Fluid Processes Glycogen to Support Vaginal Colonization by Lactobacillus. J. Infect. Dis. 2014, 210, 1019–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hanlon, D.E.; Come, R.A.; Moench, T.R. Vaginal PH Measured in Vivo: Lactobacilli Determine PH and Lactic Acid Concentration. BMC Microbiol. 2019, 19, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, E.; Beasley, D.; Dunn, R.; Archie, E. Lactobacilli Dominance and Vaginal PH: Why Is the Human Vaginal Microbiome Unique? Front. Microbiol. 2016, 7, 1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. In Vaginal Fluid, Bacteria Associated with Bacterial Vaginosis Can Be Suppressed with Lactic Acid but Not Hydrogen Peroxide. BMC Infect. Dis. 2011, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef] [Green Version]
- France, M.T.; Ma, B.; Gajer, P.; Brown, S.; Humphrys, M.S.; Holm, J.B.; Waetjen, L.E.; Brotman, R.M.; Ravel, J. VALENCIA: A Nearest Centroid Classification Method for Vaginal Microbial Communities Based on Composition. Microbiome 2020, 8, 166. [Google Scholar] [CrossRef]
- Martin, H.L., Jr.; Richardson, B.A.; Nyange, P.M.; Lavreys, L.; Hillier, S.L.; Chohan, B.; Mandaliya, K.; Ndinya-Achola, J.O.; Bwayo, J.; Kreiss, J. Vaginal Lactobacilli, Microbial Flora, and Risk of Human Immunodeficiency Virus Type 1 and Sexually Transmitted Disease Acquisition. J. Infect. Dis. 1999, 180, 1863–1868. [Google Scholar] [CrossRef]
- Wiesenfeld, H.C.; Hillier, S.L.; Krohn, M.A.; Landers, D.V.; Sweet, R.L. Bacterial Vaginosis Is a Strong Predictor of Neisseria Gonorrhoeae and Chlamydia Trachomatis Infection. Clin. Infect. Dis. 2003, 36, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Cherpes, T.L.; Meyn, L.A.; Krohn, M.A.; Lurie, J.G.; Hillier, S.L. Association between Acquisition of Herpes Simplex Virus Type 2 in Women and Bacterial Vaginosis. Clin. Infect. Dis. 2003, 37, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Lokken, E.M.; Manhart, L.E.; Kinuthia, J.; Hughes, J.P.; Jisuvei, C.; Mwinyikai, K.; Muller, C.H.; Mandaliya, K.; Jaoko, W.; McClelland, R.S. Association between Bacterial Vaginosis and Fecundability in Kenyan Women Planning Pregnancies: A Prospective Preconception Cohort Study. Hum. Reprod. 2021, 36, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Skafte-Holm, A.; Humaidan, P.; Bernabeu, A.; Lledo, B.; Jensen, J.S.; Haahr, T. The Association between Vaginal Dysbiosis and Reproductive Outcomes in Sub-Fertile Women Undergoing IVF-Treatment: A Systematic PRISMA Review and Meta-Analysis. Pathogens 2021, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Haahr, T.; Zacho, J.; Bräuner, M.; Shathmigha, K.; Skov Jensen, J.; Humaidan, P. Reproductive Outcome of Patients Undergoing in Vitro Fertilisation Treatment and Diagnosed with Bacterial Vaginosis or Abnormal Vaginal Microbiota: A Systematic PRISMA Review and Meta-Analysis. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitich, H.; Kiss, H. Asymptomatic Bacterial Vaginosis and Intermediate Flora as Risk Factors for Adverse Pregnancy Outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Floch, M.H. Probiotic Safety and Risk Factors. J. Clin. Gastroenterol. 2013, 47, 375–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli Inactivate Chlamydia Trachomatis through Lactic Acid but Not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef] [Green Version]
- Conti, C.; Malacrino, C.; Mastromarino, P. Inhibition of Herpes Simplex Virus Type 2 by Vaginal Lactobacilli. J. Physiol. Pharmacol. 2009, 60 (Suppl. 6), 19–26. [Google Scholar] [PubMed]
- Shukair, S.A.; Allen, S.A.; Cianci, G.C.; Stieh, D.J.; Anderson, M.R.; Baig, S.M.; Gioia, C.J.; Spongberg, E.J.; Kauffman, S.M.; McRaven, M.D.; et al. Human Cervicovaginal Mucus Contains an Activity That Hinders HIV-1 Movement. Mucosal. Immunol. 2013, 6, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [Green Version]
- Witkin, S.S.; Mendes-Soares, H.; Linhares, I.M.; Jayaram, A.; Ledger, W.J.; Forney, L.J. Influence of Vaginal Bacteria and D- and L-Lactic Acid Isomers on Vaginal Extracellular Matrix Metalloproteinase Inducer: Implications for Protection against Upper Genital Tract Infections. mBio 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Alakomi, H.L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [Green Version]
- Pacha-Herrera, D.; Erazo-Garcia, M.P.; Cueva, D.F.; Orellana, M.; Borja-Serrano, P.; Arboleda, C.; Tejera, E.; Machado, A. Clustering Analysis of the Multi-Microbial Consortium by Lactobacillus Species Against Vaginal Dysbiosis Among Ecuadorian Women. Front. Cell. Infect. Microbiol. 2022, 12, 863208. [Google Scholar] [CrossRef] [PubMed]
- Atassi, F.; Pho Viet Ahn, D.L.; Lievin-Le Moal, V. Diverse Expression of Antimicrobial Activities Against Bacterial Vaginosis and Urinary Tract Infection Pathogens by Cervicovaginal Microbiota Strains of Lactobacillus Gasseri and Lactobacillus Crispatus. Front. Microbiol. 2019, 10, 2900. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus Iners to Vaginal Health and Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2021, 11, 1177. [Google Scholar] [CrossRef]
- Beghini, J.; Linhares, I.M.; Giraldo, P.C.; Ledger, W.J.; Witkin, S.S. Differential Expression of Lactic Acid Isomers, Extracellular Matrix Metalloproteinase Inducer, and Matrix Metalloproteinase-8 in Vaginal Fluid from Women with Vaginal Disorders. BJOG 2015, 122, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal PH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef]
- Yarbrough, V.L.; Winkle, S.; Herbst-Kralovetz, M.M. Antimicrobial Peptides in the Female Reproductive Tract: A Critical Component of the Mucosal Immune Barrier with Physiological and Clinical Implications. Hum. Reprod. Update 2015, 21, 353–377. [Google Scholar] [CrossRef] [Green Version]
- Patton, D.L.; Thwin, S.S.; Meier, A.; Hooton, T.M.; Stapleton, A.E.; Eschenbach, D.A. Epithelial Cell Layer Thickness and Immune Cell Populations in the Normal Human Vagina at Different Stages of the Menstrual Cycle. Am. J. Obstet. Gynecol. 2000, 183, 967–973. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.H.; Stanley, P.; Bertozzi, C.R.; Hart, G.W.; Etzler, M.E. (Eds.) Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009; ISBN 978-0-87969-770-9. [Google Scholar]
- Blaskewicz, C.D.; Pudney, J.; Anderson, D.J. Structure and Function of Intercellular Junctions in Human Cervical and Vaginal Mucosal Epithelia1. Biol. Reprod. 2011, 85, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Fazeli, A.; Bruce, C.; Anumba, D.O. Characterization of Toll-like Receptors in the Female Reproductive Tract in Humans. Hum. Reprod. 2005, 20, 1372–1378. [Google Scholar] [CrossRef] [Green Version]
- Bardan, A.; Nizet, V.; Gallo, R.L. Antimicrobial Peptides and the Skin. Expert. Opin. Biol. 2004, 4, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Izadpanah, A.; Gallo, R.L. Antimicrobial Peptides. J. Am. Acad. Dermatol. 2005, 52, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Siman-Tov, G.; Hall, G.; Bhalla, N.; Narayanan, A. Human Antimicrobial Peptides as Therapeutics for Viral Infections. Viruses 2019, 11, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacNeill, C.; Umstead, T.M.; Phelps, D.S.; Lin, Z.; Floros, J.; Shearer, D.A.; Weisz, J. Surfactant Protein A, an Innate Immune Factor, Is Expressed in the Vaginal Mucosa and Is Present in Vaginal Lavage Fluid. Immunology 2004, 111, 91–99. [Google Scholar] [CrossRef]
- Pandit, H.; Kale, K.; Yamamoto, H.; Thakur, G.; Rokade, S.; Chakraborty, P.; Vasudevan, M.; Kishore, U.; Madan, T.; Fichorova, R.N. Surfactant Protein D Reverses the Gene Signature of Transepithelial HIV-1 Passage and Restricts the Viral Transfer Across the Vaginal Barrier. Front. Immunol. 2019, 10, 264. [Google Scholar] [CrossRef]
- De Gregorio, P.R.; Parolin, C.; Abruzzo, A.; Luppi, B.; Protti, M.; Mercolini, L.; Silva, J.A.; Giordani, B.; Marangoni, A.; Nader-Macías, M.E.F.; et al. Biosurfactant from Vaginal Lactobacillus Crispatus BC1 as a Promising Agent to Interfere with Candida Adhesion. Microb. Cell Factories 2020, 19, 133. [Google Scholar] [CrossRef]
- Giordani, B.; Costantini, P.E.; Fedi, S.; Cappelletti, M.; Abruzzo, A.; Parolin, C.; Foschi, C.; Frisco, G.; Calonghi, N.; Cerchiara, T.; et al. Liposomes Containing Biosurfactants Isolated from Lactobacillus Gasseri Exert Antibiofilm Activity against Methicillin Resistant Staphylococcus Aureus Strains. Eur. J. Pharm. Biopharm. 2019, 139, 246–252. [Google Scholar] [CrossRef]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus Iners: Friend or Foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef]
- Rampersaud, R.; Planet, P.J.; Randis, T.M.; Kulkarni, R.; Aguilar, J.L.; Lehrer, R.I.; Ratner, A.J. Inerolysin, a Cholesterol-Dependent Cytolysin Produced by Lactobacillus Iners. J. Bacteriol. 2011, 193, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. (Eds.) Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2015. [Google Scholar]
- Gipson, I.K.; Inatomi, T. Mucin Genes Expressed by the Ocular Surface Epithelium. Prog. Retin. Eye Res. 1997, 16, 81–98. [Google Scholar] [CrossRef]
- Jalanti, R.; Isliker, H. Immunoglobulins in Human Cervico-Vaginal Secretions. Int. Arch. Allergy Appl. Immunol. 1977, 53, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Kannan, A.; Nunn, K.L.; Murphy, M.A.; Subramani, D.B.; Moench, T.; Cone, R.; Lai, S.K. IgG in Cervicovaginal Mucus Traps HSV and Prevents Vaginal Herpes Infections. Mucosal. Immunol. 2014, 7, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Cone, R.A. Chapter 4—Mucus. In Mucosal Immunology, 3rd ed.; Mestecky, J., Lamm, M.E., McGhee, J.R., Bienenstock, J., Mayer, L., Strober, W., Eds.; Academic Press: Burlington, ON, Canada, 2005; pp. 49–72. ISBN 978-0-12-491543-5. [Google Scholar]
- Ballweber, L.; Robinson, B.; Kreger, A.; Fialkow, M.; Lentz, G.; McElrath, M.J.; Hladik, F. Vaginal Langerhans Cells Nonproductively Transporting HIV-1 Mediate Infection of T Cells. J. Virol. 2011, 85, 13443–13447. [Google Scholar] [CrossRef] [Green Version]
- Linhares, I.M.; Sisti, G.; Minis, E.; de Freitas, G.B.; Moron, A.F.; Witkin, S.S. Contribution of Epithelial Cells to Defense Mechanisms in the Human Vagina. Curr. Infect. Dis. Rep. 2019, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Wira, C.R.; Rossoll, R.M.; Kaushic, C. Antigen-Presenting Cells in the Female Reproductive Tract: Influence of Estradiol on Antigen Presentation by Vaginal Cells. Endocrinology 2000, 141, 2877–2885. [Google Scholar] [CrossRef]
- Iijima, N.; Thompson, J.M.; Iwasaki, A. Dendritic Cells and Macrophages in the Genitourinary Tract. Mucosal. Immunol. 2008, 1, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Pudney, J.; Quayle, A.J.; Anderson, D.J. Immunological Microenvironments in the Human Vagina and Cervix: Mediators of Cellular Immunity Are Concentrated in the Cervical Transformation Zone. Biol. Reprod. 2005, 73, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, T.; Kurtz, S.E.; Blauvelt, A.; Shimada, S. The Role of Langerhans Cells in the Sexual Transmission of HIV. J. Dermatol. Sci. 2005, 40, 147–155. [Google Scholar] [CrossRef]
- Eschenbach, D.A.; Thwin, S.S.; Patton, D.L.; Hooton, T.M.; Stapleton, A.E.; Agnew, K.; Winter, C.; Meier, A.; Stamm, W.E. Influence of the Normal Menstrual Cycle on Vaginal Tissue, Discharge, and Microflora. Clin. Infect. Dis. 2000, 30, 901–907. [Google Scholar] [CrossRef]
- Brotman, R.M.; Klebanoff, M.A.; Nansel, T.R.; Andrews, W.W.; Schwebke, J.R.; Zhang, J.; Yu, K.F.; Zenilman, J.M.; Scharfstein, D.O. A Longitudinal Study of Vaginal Douching and Bacterial Vaginosis—A Marginal Structural Modeling Analysis. Am. J. Epidemiol. 2008, 168, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fethers, K.A.; Fairley, C.K.; Hocking, J.S.; Gurrin, L.C.; Bradshaw, C.S. Sexual Risk Factors and Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2008, 47, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Nouioui, I.; Carro, L.; García-López, M.; Meier-Kolthoff, J.P.; Woyke, T.; Kyrpides, N.C.; Pukall, R.; Klenk, H.-P.; Goodfellow, M.; Göker, M. Genome-Based Taxonomic Classification of the Phylum Actinobacteria. Front. Microbiol. 2018, 9, 2007. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, C.A. Bacterial Vaginosis. Clin. Microbiol. Rev. 1991, 4, 485–502. [Google Scholar] [CrossRef]
- Demba, E.; Morison, L.; van der Loeff, M.S.; Awasana, A.A.; Gooding, E.; Bailey, R.; Mayaud, P.; West, B. Bacterial Vaginosis, Vaginal Flora Patterns and Vaginal Hygiene Practices in Patients Presenting with Vaginal Discharge Syndrome in The Gambia, West Africa. BMC Infect. Dis. 2005, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donders, G.G.G.; Vereecken, A.; Bosmans, E.; Dekeersmaecker, A.; Salembier, G.; Spitz, B. Definition of a Type of Abnormal Vaginal Flora That Is Distinct from Bacterial Vaginosis: Aerobic Vaginitis. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 34–43. [Google Scholar] [CrossRef]
- Ncib, K.; Bahia, W.; Leban, N.; Mahdhi, A.; Trifa, F.; Mzoughi, R.; Haddad, A.; Jabeur, C.; Donders, G. Microbial Diversity and Pathogenic Properties of Microbiota Associated with Aerobic Vaginitis in Women with Recurrent Pregnancy Loss. Diagnostics 2022, 12, 2444. [Google Scholar] [CrossRef]
- Oerlemans, E.F.M.; Wuyts, S.; Bellen, G.; Wittouck, S.; De Boeck, I.; Ruban, K.; Allonsius, C.N.; van den Broek, M.F.L.; Donders, G.G.G.; Lebeer, S. The Dwindling Microbiota of Aerobic Vaginitis, an Inflammatory State Enriched in Pathobionts with Limited TLR Stimulation. Diagnostics 2020, 10, 879. [Google Scholar] [CrossRef]
- Koumans, E.H.; Sternberg, M.; Bruce, C.; McQuillan, G.; Kendrick, J.; Sutton, M.; Markowitz, L.E. The Prevalence of Bacterial Vaginosis in the United States, 2001–2004; Associations with Symptoms, Sexual Behaviors, and Reproductive Health. Sex. Transm. Dis. 2007, 34, 864–869. [Google Scholar] [CrossRef]
- Fredricks, D.N.; Fiedler, T.L.; Marrazzo, J.M. Molecular Identification of Bacteria Associated with Bacterial Vaginosis. N. Engl. J. Med. 2005, 353, 1899–1911. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, S.; Hoffman, N.G.; Morgan, M.T.; Matsen, F.A.; Fiedler, T.L.; Hall, R.W.; Ross, F.J.; McCoy, C.O.; Bumgarner, R.; Marrazzo, J.M.; et al. Bacterial Communities in Women with Bacterial Vaginosis: High Resolution Phylogenetic Analyses Reveal Relationships of Microbiota to Clinical Criteria. PLoS ONE 2012, 7, e37818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.; Earl, J.; Retchless, A.; Hillier, S.L.; Rabe, L.K.; Cherpes, T.L.; Powell, E.; Janto, B.; Eutsey, R.; Hiller, N.L.; et al. Comparative Genomic Analyses of 17 Clinical Isolates of Gardnerella Vaginalis Provide Evidence of Multiple Genetically Isolated Clades Consistent with Subspeciation into Genovars. J. Bacteriol. 2012, 194, 3922–3937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaneechoutte, M.; Guschin, A.; Van Simaey, L.; Gansemans, Y.; Van Nieuwerburgh, F.; Cools, P. Emended Description of Gardnerella Vaginalis and Description of Gardnerella Leopoldii Sp. Nov., Gardnerella Piotii Sp. Nov. and Gardnerella Swidsinskii Sp. Nov., with Delineation of 13 Genomic Species within the Genus Gardnerella. Int. J. Syst. Evol. Microbiol. 2019, 69, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Janulaitiene, M.; Gegzna, V.; Baranauskiene, L.; Bulavaitė, A.; Simanavicius, M.; Pleckaityte, M. Phenotypic Characterization of Gardnerella Vaginalis Subgroups Suggests Differences in Their Virulence Potential. PLoS ONE 2018, 13, e0200625. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.L.; Girerd, P.H.; Karjane, N.W.; Jefferson, K.K. Effect of Biofilm Phenotype on Resistance of Gardnerella Vaginalis to Hydrogen Peroxide and Lactic Acid. Am. J. Obstet. Gynecol. 2007, 197, 170.e1–170.e7. [Google Scholar] [CrossRef] [Green Version]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Swidsinski, S.; Dörffel, Y.; Scholze, J.; Lochs, H.; Verstraelen, H. An Adherent Gardnerella Vaginalis Biofilm Persists on the Vaginal Epithelium after Standard Therapy with Oral Metronidazole. Am. J. Obstet. Gynecol. 2008, 198, 97.e1–97.e6. [Google Scholar] [CrossRef]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef] [Green Version]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The Vaginal Microbiota, Human Papillomavirus and Cervical Dysplasia: A Systematic Review and Network Meta-Analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 171–180. [Google Scholar] [CrossRef]
- Ventolini, G.; Gandhi, K.; Manales, N.J.; Garza, J.; Sanchez, A.; Martinez, B. Challenging Vaginal Discharge, Lactobacillosis and Cytolytic Vaginitis. J. Fam. Reprod. Health 2022, 16, 102–105. [Google Scholar] [CrossRef]
- Horowitz, B.J.; Mårdh, P.A.; Nagy, E.; Rank, E.L. Vaginal Lactobacillosis. Am. J. Obstet. Gynecol. 1994, 170, 857–861. [Google Scholar] [CrossRef]
- Datcu, R. Characterization of the Vaginal Microflora in Health and Disease. Dan Med. J. 2014, 61, B4830. [Google Scholar] [PubMed]
- Cibley, L.J.; Cibley, L.J. Cytolytic Vaginosis. Am. J. Obstet. Gynecol. 1991, 165, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Mallappa, R.H.; Grover, S. Comprehensive Approaches for Assessing the Safety of Probiotic Bacteria. Food Control 2020, 108, 106872. [Google Scholar] [CrossRef]
- Bruce, A.W.; Reid, G. Intravaginal Instillation of Lactobacilli for Prevention of Recurrent Urinary Tract Infections. Can. J. Microbiol. 1988, 34, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, G.E.; Heinemann, C.; Baroja, M.L.; Bruce, A.W.; Beuerman, D.; Madrenas, J.; Reid, G. Oral Administration of the Probiotic Combination Lactobacillus Rhamnosus GR-1 and L. Fermentum RC-14 for Human Intestinal Applications. Int. Dairy J. 2002, 12, 191–196. [Google Scholar] [CrossRef]
- Reid, G.; Bruce, A.W.; Fraser, N.; Heinemann, C.; Owen, J.; Henning, B. Oral Probiotics Can Resolve Urogenital Infections. FEMS Immunol. Med. Microbiol. 2001, 30, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Yefet, E.; Colodner, R.; Strauss, M.; Gam Ze Letova, Y.; Nachum, Z. A Randomized Controlled Open Label Crossover Trial to Study Vaginal Colonization of Orally Administered Lactobacillus Reuteri RC-14 and Rhamnosus GR-1 in Pregnant Women at High Risk for Preterm Labor. Nutrients 2020, 12, 1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, S.; Allotey, J.; Drymoussi, Z.; Wilks, M.; Fernandez-Felix, B.; Whiley, A.; Dodds, J.; Thangaratinam, S.; McCourt, C.; Prosdocimi, E.; et al. Effects of Oral Probiotic Supplements on Vaginal Microbiota during Pregnancy: A Randomised, Double-Blind, Placebo-Controlled Trial with Microbiome Analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Anukam, K.C.; Osazuwa, E.; Osemene, G.I.; Ehigiagbe, F.; Bruce, A.W.; Reid, G. Clinical Study Comparing Probiotic Lactobacillus GR-1 and RC-14 with Metronidazole Vaginal Gel to Treat Symptomatic Bacterial Vaginosis. Microbes Infect 2006, 8, 2772–2776. [Google Scholar] [CrossRef]
- Mastromarino, P.; Macchia, S.; Meggiorini, L.; Trinchieri, V.; Mosca, L.; Perluigi, M.; Midulla, C. Effectiveness of Lactobacillus-Containing Vaginal Tablets in the Treatment of Symptomatic Bacterial Vaginosis. Clin. Microbiol. Infect. 2009, 15, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Oduyebo, O.O.; Anorlu, R.I.; Ogunsola, F.T. The Effects of Antimicrobial Therapy on Bacterial Vaginosis in Non-Pregnant Women. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Huang, H.; Song, L.; Zhao, W. Effects of Probiotics for the Treatment of Bacterial Vaginosis in Adult Women: A Meta-Analysis of Randomized Clinical Trials. Arch. Gynecol. Obstet. 2014, 289, 1225–1234. [Google Scholar] [CrossRef]
- Jeng, H.-S.; Yan, T.-R.; Chen, J.-Y. Treating Vaginitis with Probiotics in Non-Pregnant Females: A Systematic Review and Meta-Analysis. Exp. Med. 2020, 20, 3749–3765. [Google Scholar] [CrossRef]
- Muñoz-Barreno, A.; Cabezas-Mera, F.; Tejera, E.; Machado, A. Comparative Effectiveness of Treatments for Bacterial Vaginosis: A Network Meta-Analysis. Antibiotics 2021, 10, 978. [Google Scholar] [CrossRef]
- Larsson, P.-G.; Brandsborg, E.; Forsum, U.; Pendharkar, S.; Andersen, K.K.; Nasic, S.; Hammarström, L.; Marcotte, H. Extended Antimicrobial Treatment of Bacterial Vaginosis Combined with Human Lactobacilli to Find the Best Treatment and Minimize the Risk of Relapses. BMC Infect. Dis. 2011, 11, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendharkar, S.; Brandsborg, E.; Hammarström, L.; Marcotte, H.; Larsson, P.-G. Vaginal Colonisation by Probiotic Lactobacilli and Clinical Outcome in Women Conventionally Treated for Bacterial Vaginosis and Yeast Infection. BMC Infect. Dis. 2015, 15, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillier, S.L.; Krohn, M.A.; Klebanoff, S.J.; Eschenbach, D.A. The Relationship of Hydrogen Peroxide-Producing Lactobacilli to Bacterial Vaginosis and Genital Microflora in Pregnant Women. Obstet. Gynecol. 1992, 79, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Boahen, A.; Than, L.T.L.; Loke, Y.-L.; Chew, S.Y. The Antibiofilm Role of Biotics Family in Vaginal Fungal Infections. Front. Microbiol. 2022, 13, 787119. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.G.; Moreira, D.A.; Gullón, P.; Gullón, B.; Cardelle-Cobas, A.; Tavaria, F.K. Topical Application of Probiotics in Skin: Adhesion, Antimicrobial and Antibiofilm in Vitro Assays. J. Appl. Microbiol. 2017, 122, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Van de Wijgert, J.; Verwijs, M.C. Lactobacilli-Containing Vaginal Probiotics to Cure or Prevent Bacterial or Fungal Vaginal Dysbiosis: A Systematic Review and Recommendations for Future Trial Designs. BJOG 2020, 127, 287–299. [Google Scholar] [CrossRef] [PubMed]
- EcovagBalance® Vaginal Capsule. Available online: http://Ecovag.Com/Product-Solutions/Ecovagbalance-Vaginal-Capsule/ (accessed on 12 December 2022).
- CE Marking. Available online: https://Laegemiddelstyrelsen.Dk/En/Devices/Ce-Marking/# (accessed on 12 December 2022).
- Health: Medical Devices. Available online: https://www.Asa.Org.Uk/Advice-Online/Health-Medical-Devices.Html (accessed on 12 December 2022).
- Haahr, T.; Jensen, J.S.; Humaidan, P. Research and Business—The Yin and Yang in Modern Medicine. Reprod. Biomed. Online 2020, 40, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance. Available online: https://www.Who.Int/News-Room/Fact-Sheets/Detail/Antimicrobial-Resistance (accessed on 29 December 2022).
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal Microbiome Transplantation in Women with Intractable Bacterial Vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Guio, J.; Viveros-Carreño, D.A.; Sierra-Barrios, E.M.; Martinez-Velasquez, M.Y.; Grillo-Ardila, C.F. Antibiotic Treatment for the Sexual Partners of Women with Bacterial Vaginosis. Cochrane Database Syst. Rev. 2016, 10, CD011701. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pendharkar, S.; Skafte-Holm, A.; Simsek, G.; Haahr, T. Lactobacilli and Their Probiotic Effects in the Vagina of Reproductive Age Women. Microorganisms 2023, 11, 636. https://doi.org/10.3390/microorganisms11030636
Pendharkar S, Skafte-Holm A, Simsek G, Haahr T. Lactobacilli and Their Probiotic Effects in the Vagina of Reproductive Age Women. Microorganisms. 2023; 11(3):636. https://doi.org/10.3390/microorganisms11030636
Chicago/Turabian StylePendharkar, Sonal, Axel Skafte-Holm, Gizem Simsek, and Thor Haahr. 2023. "Lactobacilli and Their Probiotic Effects in the Vagina of Reproductive Age Women" Microorganisms 11, no. 3: 636. https://doi.org/10.3390/microorganisms11030636
APA StylePendharkar, S., Skafte-Holm, A., Simsek, G., & Haahr, T. (2023). Lactobacilli and Their Probiotic Effects in the Vagina of Reproductive Age Women. Microorganisms, 11(3), 636. https://doi.org/10.3390/microorganisms11030636




