Effects of Sodium Nitrate and Coated Methionine on Lactation Performance, Rumen Fermentation Characteristics, Amino Acid Metabolism, and Microbial Communities in Lactating Buffaloes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diet and Animal Management
2.2. Determination of Dry Matter Intake (DMI), Milk Yield, and Milk Composition
2.3. Determination of Rumen Fermentation Parameters
2.4. Determination of Amino Acid Concentration
2.5. 16S rDNA Gene Sequencing and Bioinformatic Analysis
2.6. Statistical Analysis
3. Results
3.1. Milk Yield and Composition
3.2. Rumen Fermentation Characteristics
3.3. Ruminal Amino Acids
3.4. Ruminal Bacterial Communities
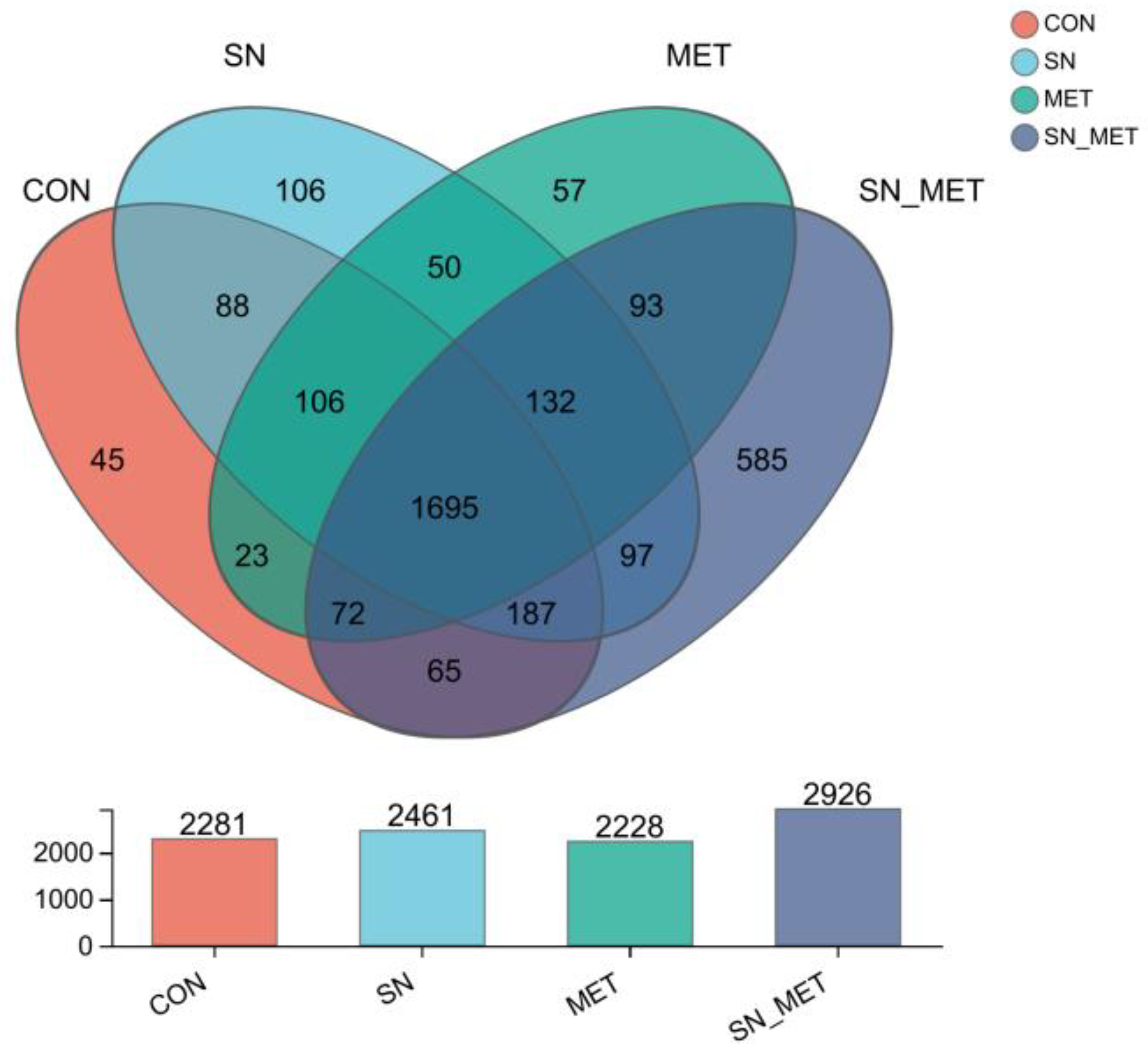
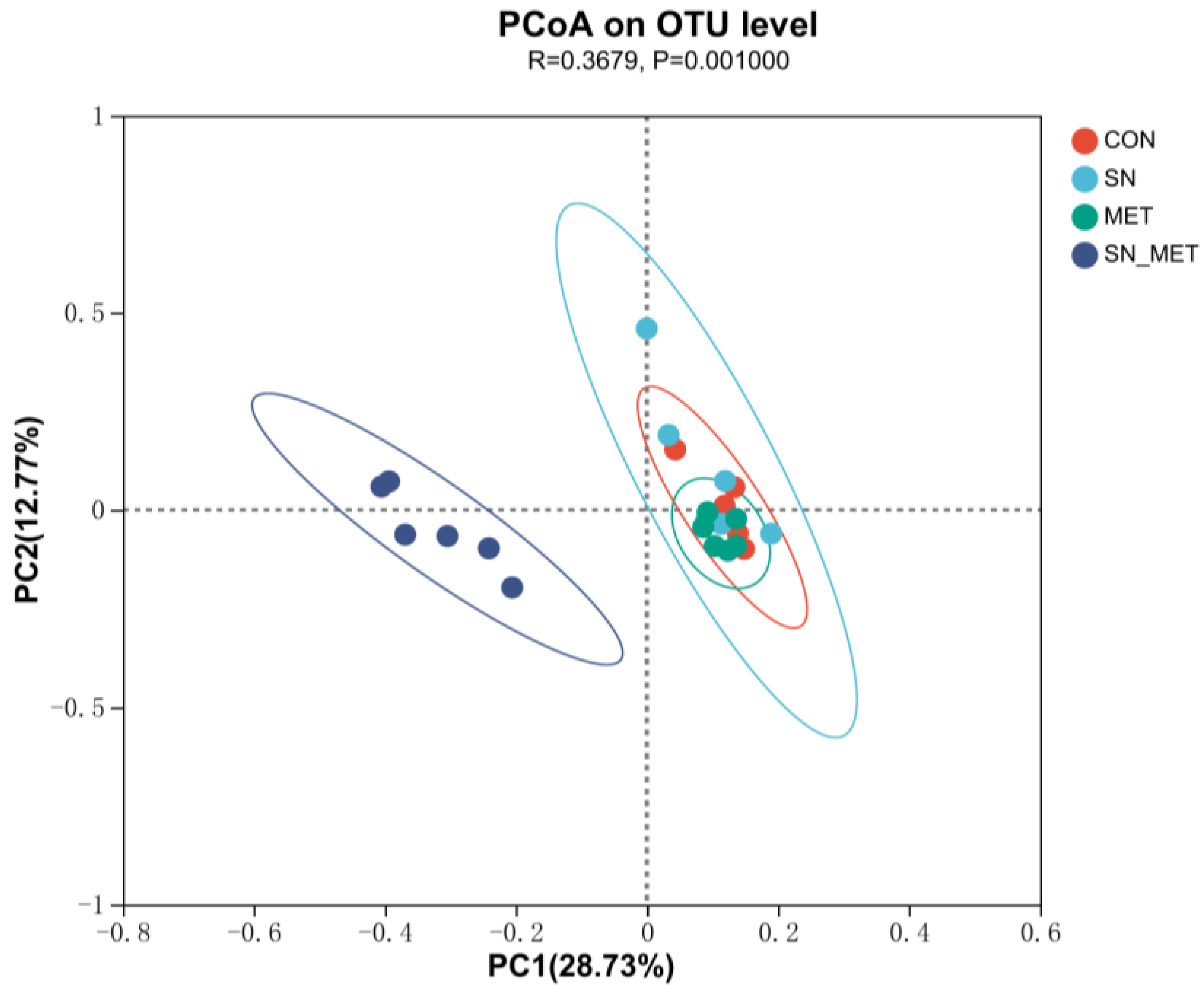
3.4.1. Rumen Bacterial Diversity
3.4.2. Relative Abundance of Bacterial Populations
3.4.3. Biomarker Bacteria Taxa and Metagenomic Functional Profile
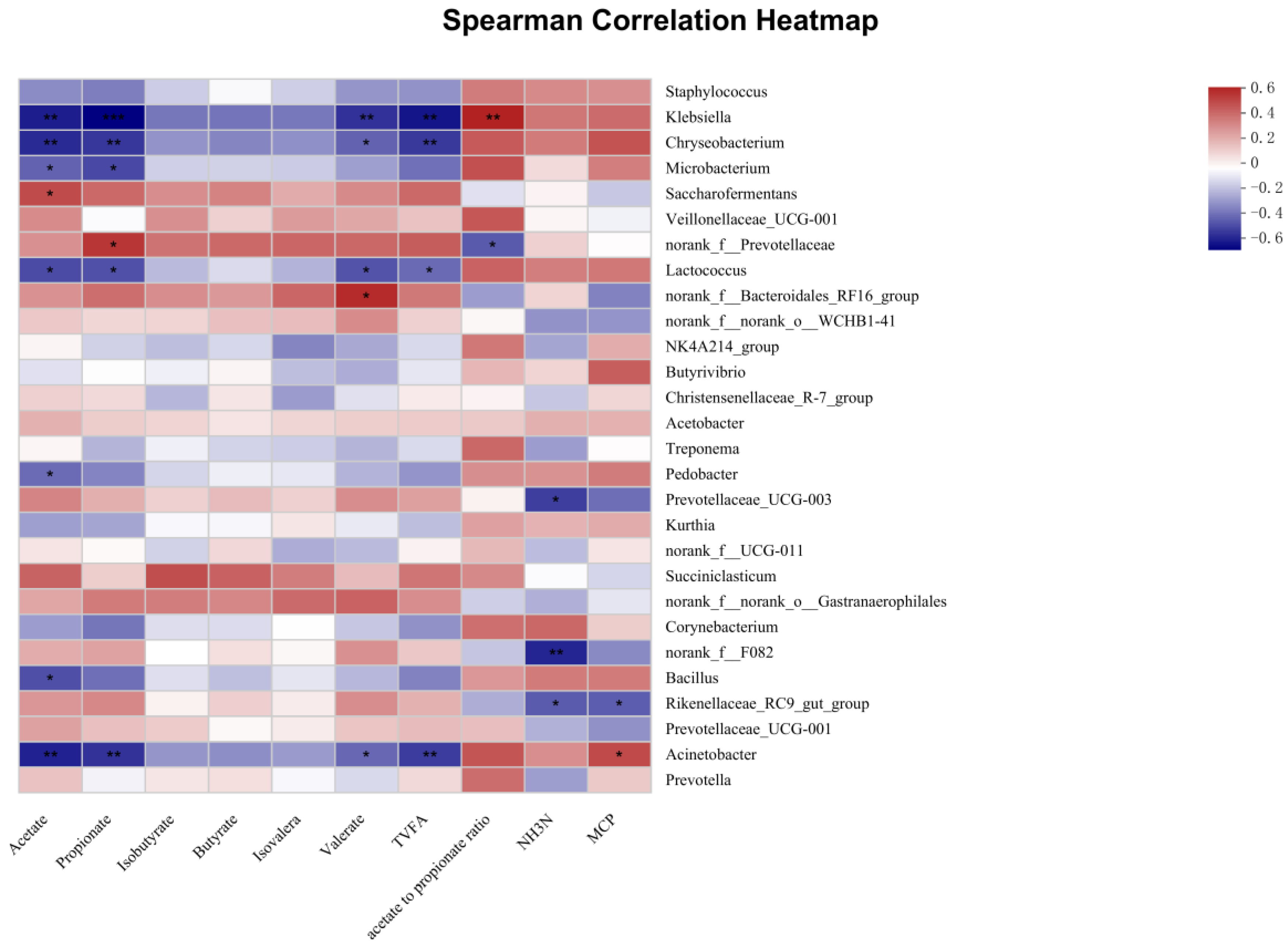
3.4.4. Association of Rumen Bacteria with Ruminal Fermentation Parameters and Amino Acid Contents
4. Discussion
4.1. Effect of Treatment on Milk Performance of Buffalo
4.2. Effect of Treatment on Rumen Fermentation Parameters
4.3. Effect of Treatment on Ruminal Amino Acids
4.4. Effect of Treatment on Rumen Microbial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Ni, Z.; Chen, Y.; Zhang, E.; Wang, Y. Study on distribution of free amino acid and the small peptides and the expression of related transporter genes in the gastrointestinal tract of sheep. Acta Vet. Et Zootech. Sin. 2017, 48, 462–473. [Google Scholar]
- Zhong, K.; Liu, Z.; Wang, H. Effects of unconventional protein ingredients on nitrogen metabolism and microbial protein synthesis in lactating goats. China Feed. 2021, 672, 64–67. [Google Scholar]
- Soltan, Y.A.; Natel, A.S.; Araujo, R.C.; Morsy, A.S.; Abdalla, A.L. Progressive adaptation of sheep to a microencapsulated blend of essential oils: Ruminal fermentation, methane emission, nutrient digestibility, and microbial protein synthesis. Anim. Feed Sci. Technol. 2018, 237, 8–18. [Google Scholar] [CrossRef]
- Guo, Y.; Hassan, F.; Li, M.; Tang, Z.; Peng, L.; Peng, K.; Yang, C. Effect of hydrogen-consuming compounds on in vitro ruminal fermentation, fatty acids profile, and microbial community in water buffalo. Curr. Microbiol. 2022, 79, 220. [Google Scholar] [CrossRef]
- Xie, T.; Xia, Y.; Zeng, Y.; Li, X.; Zhang, Y. Nitrate concentration-shift cultivation to enhance protein content of heterotrophic microalga Chlorella vulgaris: Over-compensation strategy. Bioresour. Technol. 2017, 233, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Asanuma, N.; Hino, T. Effects of pH and electron donors on nitrate and nitrite reduction in ruminal microbiota. Anim. Sci. J. 2001, 72, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Hassan, F.-u.; Li, M.; Xie, H.; Peng, L.; Tang, Z.; Yang, C. Effect of sodium nitrate and cysteamine on in vitro ruminal fermentation, amino acid metabolism and microbiota in buffalo. Microorganisms 2022, 10, 2038. [Google Scholar] [CrossRef]
- Guo, Y.; Li, M.; Tang, Z.; Peng, L.; Peng, K.; Xie, F.; Xie, H.; Yang, C. Effects of different dosages of sodium nitrate on fatty acid composition and microbial population of buffalo rumen fermentation in vitro under the condition of linoleic acid. Acta Prataculturae Sin. 2021, 30, 159–167. [Google Scholar]
- Guo, Y.; Li, M.; Peng, L.; Peng, K.; Tang, Z.; Liang, X.; Xie, F.; Yang, C. Effects of sodium nitrate on methane production and fatty acid hydrogenation process of buffalo in vitro fermentation. China Anim. Husb. Vet. Med. 2020, 47, 2071–2080. [Google Scholar]
- Han, Z.; Lin, Y.; Zhao, F.; Li, J. Evaluation of rumen bypass effect of different protected methionine. Feed Res. 2008, 6, 9–12. [Google Scholar]
- Fowler, C.M. Evaluation of 2-hydroxy-4-(methylthio)butanoic acid isopropyl ester and methionine supplementation on efficiency of microbial protein synthesis and rumen bacterial populations. J. Dairy Sci. 2011, 94, 3913–3927. [Google Scholar]
- Brosnan, J.T.; Brosnan, M.E.; Bertolo, R.F.P.; Brunton, J.A. Methionine: A metabolically unique amino acid. Livest. Sci. 2007, 112, 2–7. [Google Scholar] [CrossRef]
- Hassan, F.U.; Guo, Y.; Li, M.; Tang, Z.; Peng, L.; Liang, X.; Yang, C. Effect of methionine supplementation on rumen microbiota, fermentation, and amino acid metabolism in in vitro cultures containing nitrate. Microorga. 2021, 9, 1717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, N.; Yu, Z.; Nie, L.; Guan, P.; Zhang, A. Effects of protective methionine and slow-release urea on rumen fermentation parameters and fiber degradation rate in vitro. Heilongjiang Anim. Sci. Vet. Med. 2018, 9, 15–21+27. [Google Scholar]
- Ishigami, K.; Inoue, K. Metabolism of nitrate and methemoglobinemia in ruminants. Research Bulletin of Obihiro Zootechnical University. Res. Bull. Obihiro Univ. 1976, 10, 45–55. [Google Scholar]
- Kuypers, M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 3–12. [Google Scholar] [CrossRef]
- Chen, S.Q. Liver and Kidney Ire1 Pathway and Apoptosis in Grass Carp (Ctenopharyngodon idellus) Induced by Nitrite Acute Exposure. Master’s Thesis, Hebei Medical University, Wuhan, China, 2019. [Google Scholar]
- Zhao, P.; Duan, G.; Du, L.; Xu, D.; Tang, Y.; Han, Y. Systematic evaluation on the efficacy of ademetionine patiens with drug-induced liver injury. Chin. J. Gastroenter. Hepatol. 2011, 20, 341–344. [Google Scholar]
- GB/T 5009.33-2010; Determination of Nitrite and Nitrate in Foods. Ministry of Health of the PRC: Beijing, China, 2010. Available online: http://down.foodmate.net/standard/yulan.php?itemid=21715 (accessed on 11 November 2022).
- Parbkh, H.K. New formula for calculating fat corrected milk (FCM3.5%). Indian J. Anim. Sci. 1986, 56, 608–609. [Google Scholar]
- Weatherburn, M. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Guo, Y.; Li, M.; Tang, Z.; Peng, L.; Peng, K.; Laing, X.; Xie, F.; Yang, C. Effects of disodium fumarate on fermentation parameters, fatty acid composition and the number of key rumen microorganisms of buffalo in vitro. China Anim. Husb. Vet. Med. 2021, 53, 24–31. [Google Scholar]
- Joyce, R.; Kuziene, V.; Zou, X.; Wang, X.; Pullen, F.; Loo, R.L. Development and validation of an ultra-performance liquid chromatography quadrupole time of flight mass spectrometry method for rapid quantification of free amino acids in human urine. Amino Acids 2016, 48, 219–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Rulquin, H.; Graulet, B.; Delaby, L.; Robert, J.C. Effect of different forms of methionine on lactational performance of dairy cows. J. Dairy Sci. 2006, 89, 4387–4394. [Google Scholar] [CrossRef] [PubMed]
- Giallongo, F.; Harper, M.T.; Oh, J.; Lopes, J.C.; Lapierre, H.; Patton, R.A.; Parys, C.; Shinzato, I.; Hristov, A.N. Effects of rumen-protected methionine, lysine, and histidine on lactation performance of dairy cows. J. Dairy Sci. 2016, 99, 4437–4452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Hristov, A.N.; Cassidy, T.W.; Heyler, K.S.; Lapierre, H.; Varga, G.A.; de Veth, M.J.; Patton, R.A.; Parys, C. Rumen-protected lysine, methionine, and histidine increase milk protein yield in dairy cows fed a metabolizable protein-deficient diet. J. Dairy Sci. 2012, 95, 6042–6056. [Google Scholar] [CrossRef] [Green Version]
- Olika, C.D. Review on Effect of nutrition on milk composition and yield of dairy cows. Eur. J. Sci. Technol. 2021, 1, 24–31. [Google Scholar]
- Patton, R.A. Effect of rumen-protected methionine on feed intake, milk production, true milk protein concentration, and true milk protein yield, and the factors that influence these effects: A meta-analysis. J. Dairy Sci. 2010, 93, 2105–2118. [Google Scholar] [CrossRef]
- Liu, Q.; Fu, C.; Zhou, C.; Chen, L.; Yang, H. Research advances in limiting amino acid metabolism of microorganisms in the rumen. Acta Ecol. Anim. Domestic 2017, 38, 83–87. [Google Scholar]
- Lu, P.; Cao, N.; Wang, F.; Song, X.; Zhang, K. Effect of different doses of nitrate on dry matter digestibility of sheep ruminal in vitro. Feed. Rev. 2015, 23, 1–4. [Google Scholar]
- Chung, Y.H.; Bateman, H.G.; Williams, C.C., II; Stanley, C.C.; Gantt, D.T.; Braud, T.W.; Southern, L.L.; Ward, J.D.; Hoyt, P.G.; Sod, G.A. Effects of methionine and lysine on fermentation in vitro and in vivo, nutrient flow to the intestine, and milk production. J. Dairy Sci. 2006, 89, 1613–1620. [Google Scholar] [CrossRef]
- Sun, Y.K.; Yan, X.G.; Ban, Z.B.; Yang, H.M.; Hegarty, H.M. The effect of cysteamine hydrochloride and nitrate supplementation on in-vitro and in-vivo methane production and productivity of cattle. Anim. Feed Sci. Technol. 2017, 232, 49–56. [Google Scholar] [CrossRef]
- Guyader, J.; Ungerfeld, E.M.; Beauchemin, K.A. Redirection of metabolic hydrogen by inhibiting methanogenesis in the rumen simulation technique (RUSITEC). Front. Microbiol. 2017, 8, 393. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Meng, Q.; Zhou, Z.; Cui, Z.; Ren, L. Effect of nitrate addition level on in vitro ruminal fermentation characteristics and microbial efficiency. Sci. Agric. Sin. 2010, 43, 3418–3424. [Google Scholar]
- Zhao, L.; Meng, Q.; Ren, L.; Liu, W.; Zhang, X.; Huo, Y.; Zhou, Z. Effects of nitrate addition on rumen fermentation, bacterial biodiversity and abundance. Asian-Austr. J. Anim. Sci. 2015, 28, 1433. [Google Scholar] [CrossRef] [Green Version]
- Patra, A.K.; Zhong, T.Y. Effects of adaptation of in vitro rumen culture to garlic oil, nitrate, and saponin and their combinations on methanogenesis, fermentation, and abundances and diversity of microbial populations. Front. Microbiol. 2015, 6, 1434–1444. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, M.; Tang, Z.; Liang, X.; Peng, L.; Peng, K.; Wang, X.; Yang, C. Effects of supplemental feeding of cysteamine and chromium nicotinate on lactation performance, antioxidant performance, rumen fermentation parameters and microbial diversity of buffalo in summer. Chin. J. Anim. Nutr. 2020, 32, 5760–5777. [Google Scholar]
- Evans, N.J.; Brown, J.M.; Murray, R.D.; Getty, B.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Appl. Environ. Microbiol. 2011, 77, 138–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehority, B.A. Numbers, factors affecting the population and distribution of rumen bacteria. In Rumen Microbiology; Dehority, B.A., Ed.; Nottingham University Press: Nottingham, UK, 2003; pp. 265–294. [Google Scholar]
- Pitta, D.W.; Pinchak, W.E.; Indugu, N.; Vecchiarelli, B.; Sinha, R.; Fulford, J.D. Metagenomic analysis of the rumen microbiome of steers with wheat-induced frothy bloat. Front. Microbiol. 2016, 7, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, M.; Reddy, C. Adaptations of Castrointestinal Bacteria in Response to Changes in Dietary Oxralate and Nitrate; American Society for Microbiology: Washington, DC, USA, 1984. [Google Scholar]
- Fan, P.; Bian, B.; Teng, L.; Nelson, C.D.; Driver, J.; Elzo, M.A.; Jeong, K.C. Host genetic effects upon the early gut microbiota in a bovine model with graduated spectrum of genetic variation. ISME J. 2020, 14, 302–317. [Google Scholar] [CrossRef] [Green Version]
- Derakhshani, H.; Tun, H.M.; Cardoso, F.C.; Plaizier, J.C.; Khafipour., E.; Loor, J.J. Linking peripartal dynamics of ruminal microbiota to dietary changes and production parameters. Front. Microbiol. 2016, 7, 2143. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, C.; Chen, Y.; Liu, J.; Zhang, C.; Irving, B.; Fitzsimmons, C.; Plastow, G.; Guan, L.L. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle. Microbiome 2019, 7, 92. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Li, S.; Jiao, Y.; Wu, Z.; Sun, W.; Sun, J.; Lu, J.; Hao, X. Isolation, identification and cellulase-producing characteristics of a cellulose-degrading strain Acinetobacter sp. XG. BMP 2022, 1–20. Available online: http://kns.cnki.net/kcms/detail/46.1068.S.20221123.0937.010.html (accessed on 2 December 2022).
- Mukhopadhyay, B.C.; Mitra, S.; Kazi, T.A.; Mandal, S.; Biswas, S.R. Draft genome sequence of cold tolerant Kurthia gibsonii B83, isolated from spinach leaf. Microbiol. Resour. Announc. 2019, 8, e01480-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Zhou, S.; Wang, Y.W.; Song, J.; Wang, H.; Kong, D.; Zhu, J.; Dong, W.; He, M.; Hu, G.; et al. Characterization of a highly thermostable and organic solvent-tolerant copper containing polyphenol oxidase with dyedecolorizingability from Kurthia huakuii LAM0618T. PLoS ONE 2016, 11, e0164810. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Zhu, J.; Guo, X.; Kong, D.; Zhang, Q.; Zhou, Y.; Liu, X.; Zhao, S.; Ruan, Z. Characterization of AiiK, an AHL lactonase, from Kurthia huakui LAM0618 T and its application in quorum quenching on Pseudomonas aeruginosa PAO1. Sci. Rep. 2018, 8, 6013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, P.L. Domestication and Screening of High-Temperature Lignin Degradtion Bacteria and Its Application in Sheep Manure-Mushroom Residue Composting. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2021. [Google Scholar]



| Items | Ingredient (%) | |||
|---|---|---|---|---|
| CON | SN | MET | SN+MET | |
| Brewer’s grain | 50.00 | 50.00 | 50.00 | 50.00 |
| Corn leaf | 16.00 | 16.00 | 16.00 | 16.00 |
| Silage elephant grass | 20.00 | 20.00 | 20.00 | 20.00 |
| Corn | 3.60 | 3.60 | 3.60 | 3.60 |
| Soybean meal | 7.10 | 7.10 | 7.10 | 7.10 |
| Wheat bran | 1.00 | 1.00 | 1.00 | 1.00 |
| Cottonseed meal | 1.25 | 1.25 | 1.25 | 1.25 |
| CaHPO4 | 0.10 | 0.10 | 0.10 | 0.10 |
| Shell meal | 0.10 | 0.10 | 0.10 | 0.10 |
| NaCl | 0.15 | 0.15 | 0.15 | 0.15 |
| NaHCO3 | 0.20 | 0.20 | 0.20 | 0.20 |
| Premix 1 | 0.50 | 0.50 | 0.50 | 0.50 |
| Urea (g) | 26.41 | 1.51 | 24.89 | 50.00 |
| Sodium nitrate (g) | 70.00 | 70.00 | ||
| L-methionine (g) | 15.00 | 15.00 | ||
| Chemical composition | ||||
| NEL 2 (MJ/kg) | 5.45 | 5.45 | 5.45 | 5.45 |
| Crude protein (%) | 20.75 | 20.75 | 20.75 | 20.75 |
| Neutral detergent fiber (%) | 50.61 | 50.61 | 50.61 | 50.61 |
| Acid detergent Fiber (%) | 21.56 | 21.56 | 21.56 | 21.56 |
| Ash (%) | 5.87 | 5.87 | 5.87 | 5.87 |
| Ca (%) | 0.97 | 0.97 | 0.97 | 0.97 |
| P (%) | 0.61 | 0.61 | 0.61 | 0.61 |
| Items | CON | SN | MET | SN+MET | SEM | p Value |
|---|---|---|---|---|---|---|
| Dry matter intake (DMI, kg/d) | 8.44 a | 8.35 a | 7.73 b | 8.17 a | 0.12 | 0.001 |
| Milk yield (kg/d) | 6.62 | 5.63 | 6.72 | 6.61 | 0.62 | 0.570 |
| 3.5% fat-corrected milk yield (3.5% FCM, kg/d) | 3.17 | 2.72 | 3.22 | 3.18 | 0.29 | 0.585 |
| Lactation efficiency (%) | 78.86 | 67.50 | 87.54 | 80.43 | 7.82 | 0.342 |
| Milk protein (%) | 4.99 | 4.94 | 4.76 | 4.80 | 0.12 | 0.527 |
| Milk fat (%) | 8.43 | 8.55 | 8.37 | 8.86 | 0.31 | 0.695 |
| Lactose (%) | 5.18 | 5.11 | 5.39 | 5.34 | 0.08 | 0.060 |
| Total solid content (%) | 19.66 | 19.67 | 19.49 | 20.02 | 0.41 | 0.831 |
| Sodium nitrate residue in milk (mg/kg) | 3.72 | 2.13 | 2.96 | 2.96 | 0.45 | 0.113 |
| Items | CON | SN | MET | SN+MET | SEM | p Value |
|---|---|---|---|---|---|---|
| pH | 6.48 | 6.63 | 6.64 | 6.73 | 0.08 | 0.208 |
| Ammonia nitrogen (NH3-N, mg/mL) | 2.03 | 1.55 | 2.36 | 1.75 | 0.59 | 0.790 |
| Microbial protein (MCP, mg/mL) | 0.65 | 0.63 | 0.77 | 0.77 | 0.06 | 0.169 |
| Acetate (mmol/L) | 35.29 a | 35.54 a | 29.69 b | 31.39 ab | 1.54 | 0.031 |
| Propionate (mmol/L) | 19.24 a | 18.68 a | 14.75 b | 14.95 b | 1.18 | 0.020 |
| Isobutyrate (mmol/L) | 0.67 | 0.72 | 0.59 | 0.61 | 0.06 | 0.485 |
| Butyrate (mmol/L) | 11.75 | 12.07 | 10.12 | 10.38 | 0.84 | 0.296 |
| Isovalerate (mmol/L) | 1.48 | 1.40 | 1.13 | 1.07 | 0.20 | 0.142 |
| Valerate (mmol/L) | 1.46 a | 1.62 a | 1.00 b | 0.85 b | 0.19 | 0.003 |
| Total volatile fatty acids (TVFA, mmol/L) | 69.90 a | 70.03 a | 57.29 b | 59.24 ab | 2.08 | 0.031 |
| Acetate-to-propionate ratio (A/P) | 1.86 | 1.92 | 2.02 | 2.14 | 0.09 | 0.154 |
| Items | CON | SN | MET | SN+MET | SEM | p Value |
|---|---|---|---|---|---|---|
| Alanine | 21.56 | 29.63 | 28.42 | 13.75 | 5.30 | 0.161 |
| Valine | 7.54 ab | 13.74 a | 8.22 ab | 4.90 b | 2.05 | 0.041 |
| Histidine | 3.21 b | 5.39 a | 3.67 b | 2.86 b | 0.63 | 0.045 |
| Arginine | 1.04 | 1.24 | 1.06 | 0.83 | 0.12 | 0.152 |
| Glycine | 8.90 | 17.09 | 11.15 | 6.23 | 2.70 | 0.060 |
| Glutamine | 15.60 b | 35.372 a | 16.79 b | 12.90 b | 5.08 | 0.020 |
| Glutamate | 24.37 b | 47.18 a | 25.64 b | 17.19 b | 7.07 | 0.040 |
| Proline | 8.48 ab | 14.84 a | 6.17 ab | 4.54 b | 2.25 | 0.022 |
| Leucine | 7.59 b | 15.64 a | 7.77 b | 4.99 b | 2.33 | 0.024 |
| Lysine | 16.14 b | 35.37 a | 17.30 b | 12.80 b | 5.37 | 0.032 |
| Methionine | 3.84 b | 7.94 a | 4.36 b | 2.89 b | 1.15 | 0.029 |
| Tryptophan | 0.67 | 1.21 | 0.62 | 0.83 | 0.21 | 0.223 |
| Phenylalanine | 6.71 b | 12.68 a | 5.12 b | 3.53 b | 1.84 | 0.012 |
| Threonine | 8.35 b | 16.84 a | 8.70 b | 5.61 b | 2.38 | 0.019 |
| Isoleucine | 7.46 b | 14.93 a | 7.54 b | 4.80 b | 2.23 | 0.026 |
| Tyrosine | 4.29 b | 9.04 a | 4.26 b | 3.02 b | 1.34 | 0.023 |
| Serine | 8.55 b | 14.74 a | 8.56 b | 5.33 b | 2.17 | 0.042 |
| Aspartic acid | 7.05 b | 13.79 a | 7.27 b | 4.9 b | 2.09 | 0.038 |
| Asparagine | 0.90 | 1.09 | 0.91 | 0.98 | 0.06 | 0.096 |
| Cysteine | 0.74 | 0.73 | 1.50 | 1.34 | 0.24 | 0.065 |
| Essential amino acids 1 | 58.30 b | 118.35 a | 53.97 b | 47.14 b | 10.13 | 0.032 |
| Non-essential amino acids 2 | 104.67 ab | 190.13 a | 103.87 ab | 88.03 b | 15.67 | 0.071 |
| Total amino acids | 162.99 b | 308.48 a | 175.03 b | 114.2 b | 44.81 | 0.036 |
| Items | CON | SN | MET | SN+MET | SEM | p Value |
|---|---|---|---|---|---|---|
| Shannon | 5.18 | 5.38 | 5.24 | 4.86 | 0.16 | 0.161 |
| Simpson | 0.02 b | 0.02 b | 0.02 b | 0.04 a | 0.01 | 0.025 |
| Ace | 1611.18 b | 1768.28 b | 1658.22 b | 2121.93 a | 74.94 | 0.001 |
| Chao | 1591.28 b | 1777.19 b | 1668.88 b | 2126.42 a | 80.47 | 0.001 |
| Coverage (%) | 98.89 | 98.97 | 99.09 | 99.04 | 0.06 | 0.674 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Fan, Z.; Li, M.; Xie, H.; Peng, L.; Yang, C. Effects of Sodium Nitrate and Coated Methionine on Lactation Performance, Rumen Fermentation Characteristics, Amino Acid Metabolism, and Microbial Communities in Lactating Buffaloes. Microorganisms 2023, 11, 675. https://doi.org/10.3390/microorganisms11030675
Guo Y, Fan Z, Li M, Xie H, Peng L, Yang C. Effects of Sodium Nitrate and Coated Methionine on Lactation Performance, Rumen Fermentation Characteristics, Amino Acid Metabolism, and Microbial Communities in Lactating Buffaloes. Microorganisms. 2023; 11(3):675. https://doi.org/10.3390/microorganisms11030675
Chicago/Turabian StyleGuo, Yanxia, Zexiang Fan, Mengwei Li, Huade Xie, Lijuan Peng, and Chengjian Yang. 2023. "Effects of Sodium Nitrate and Coated Methionine on Lactation Performance, Rumen Fermentation Characteristics, Amino Acid Metabolism, and Microbial Communities in Lactating Buffaloes" Microorganisms 11, no. 3: 675. https://doi.org/10.3390/microorganisms11030675





