Fomes fomentarius and F. inzengae—A Comparison of Their Decay Patterns on Beech Wood
Abstract
:1. Introduction
2. Materials and Methods
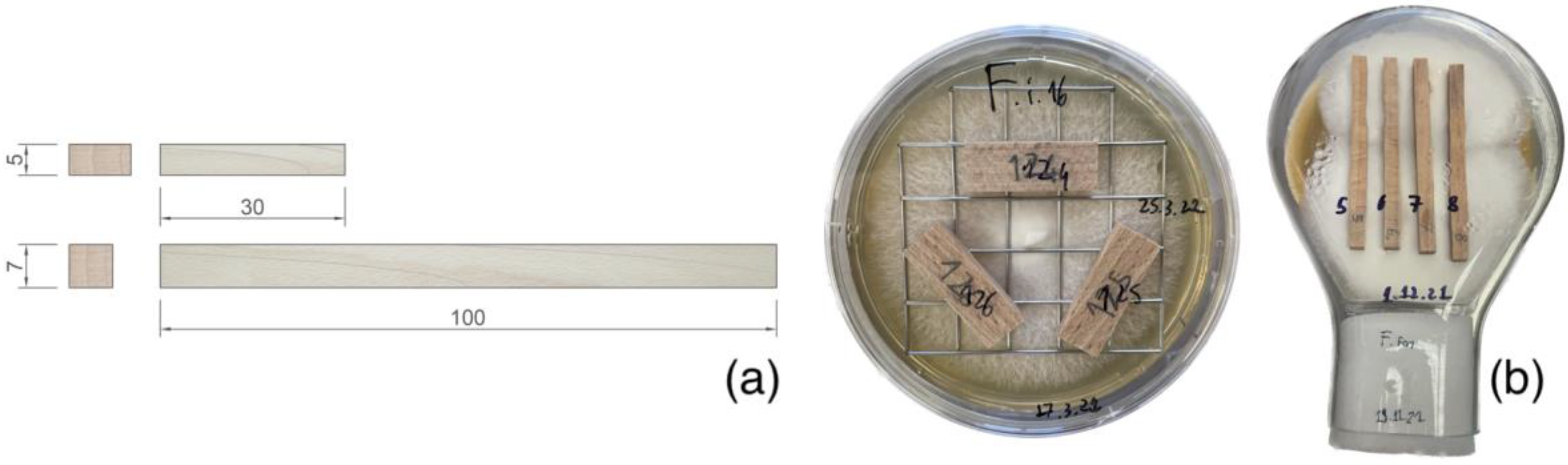
2.1. Sample Preparation
2.2. Fungal Exposure
2.2.1. Selection of Fungal Strains
2.2.2. Sterilisation Process
2.2.3. Mass-Loss Assessment
2.2.4. Fungal Exposure of Samples for Mechanical Testing
2.3. Physical and Mechanical Measurements
2.3.1. Vibroacoustic Measurements
2.3.2. Static Mechanical Testing
2.3.3. Computed Tomography Assessment
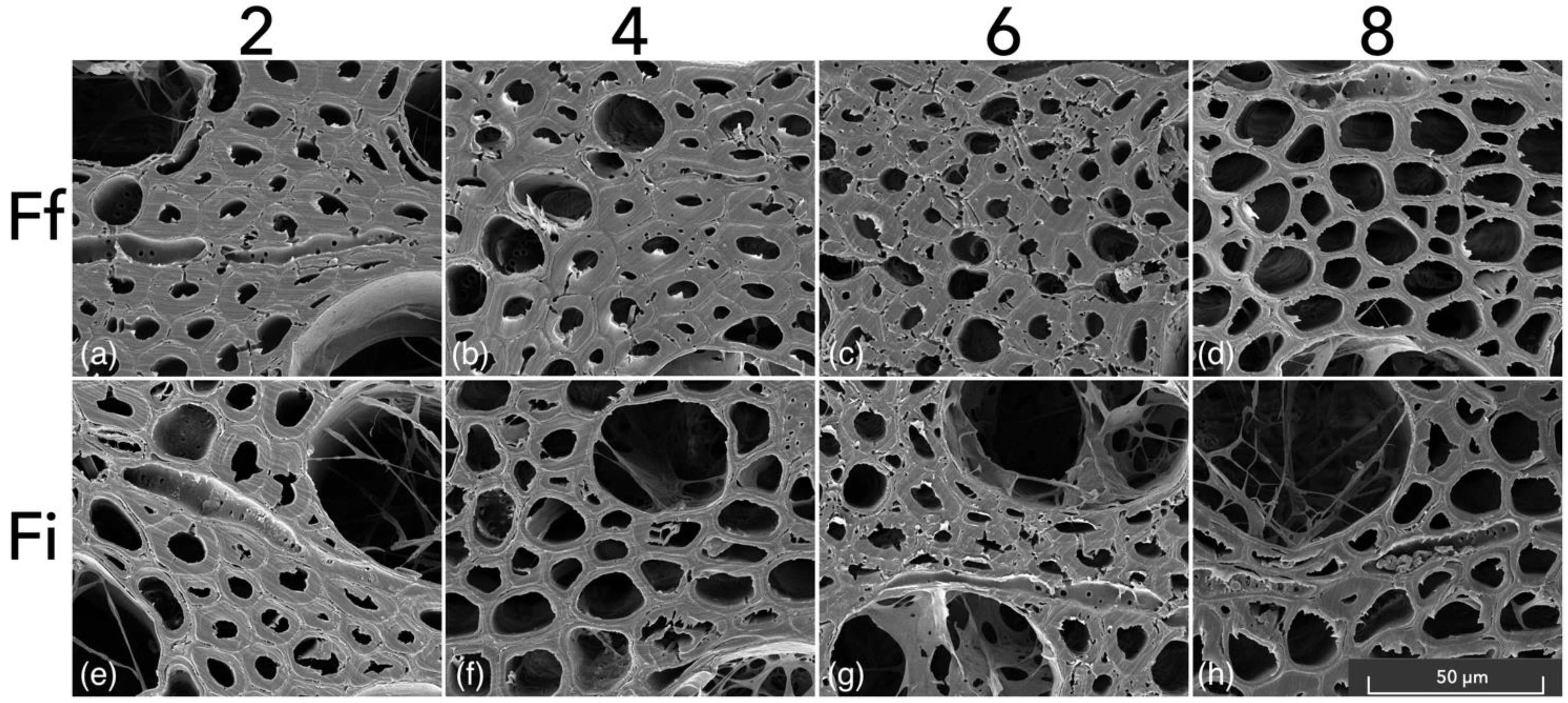
2.4. Scanning Electron Microscopy
2.5. Statistical Analysis
3. Results and Discussion
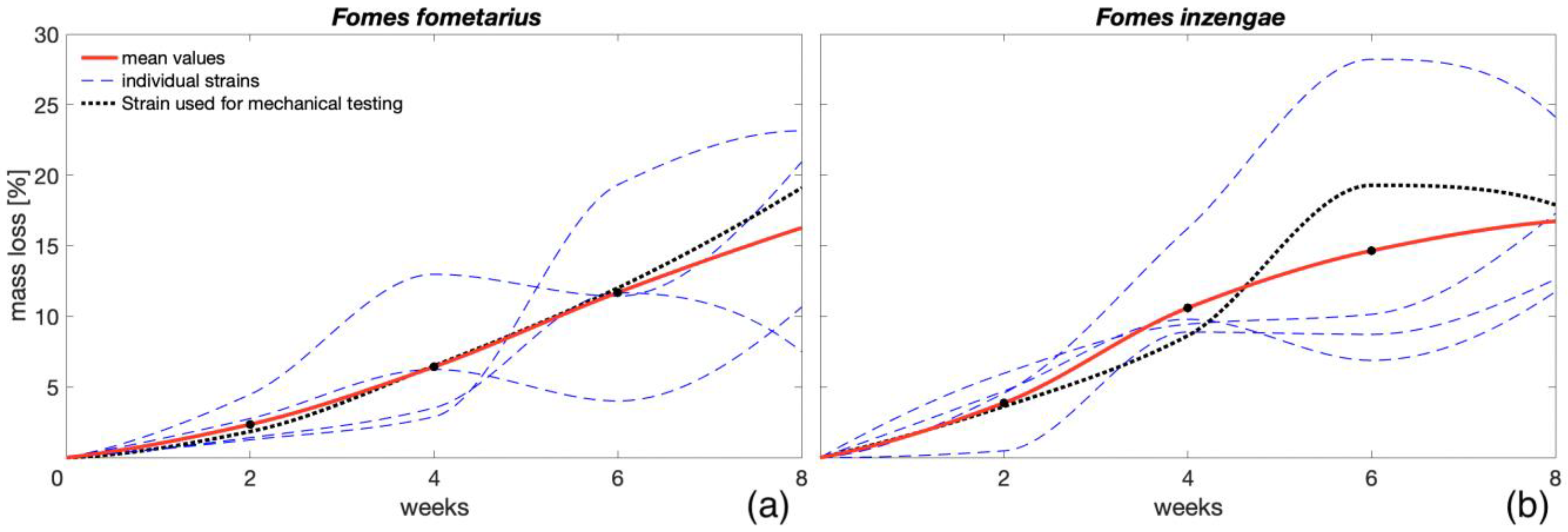
3.1. ML Comparison among Different Fungal Strains
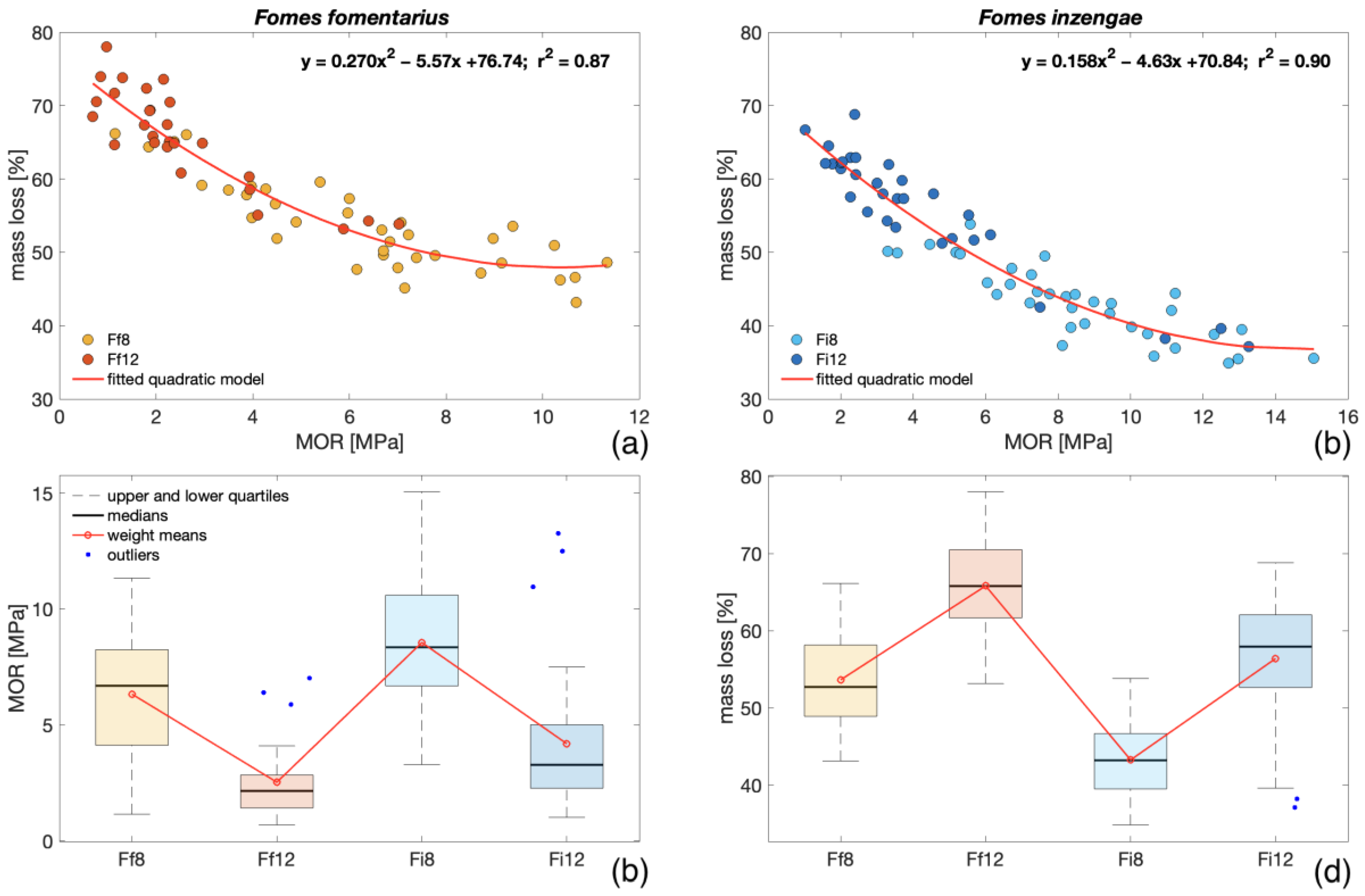
3.2. Physical and Mechanical Properties of Degraded Bending Samples
4. Conclusions
- From the experiment conducted on different strains of F. fomentarius and F. inzengae, no relevant difference in and between the two species was found (> 0.001). Both species showed similar trends and high variability among the different strains. With an increment in the , increased for both species (= 0.74).
- The degraded bending samples showed higher heterogeneity of density distribution than the intact ones (< 0.001). After 8 weeks of fungal exposure, a difference in densities was observed between the two species. Nevertheless, after 12 weeks, no relevant difference was found.
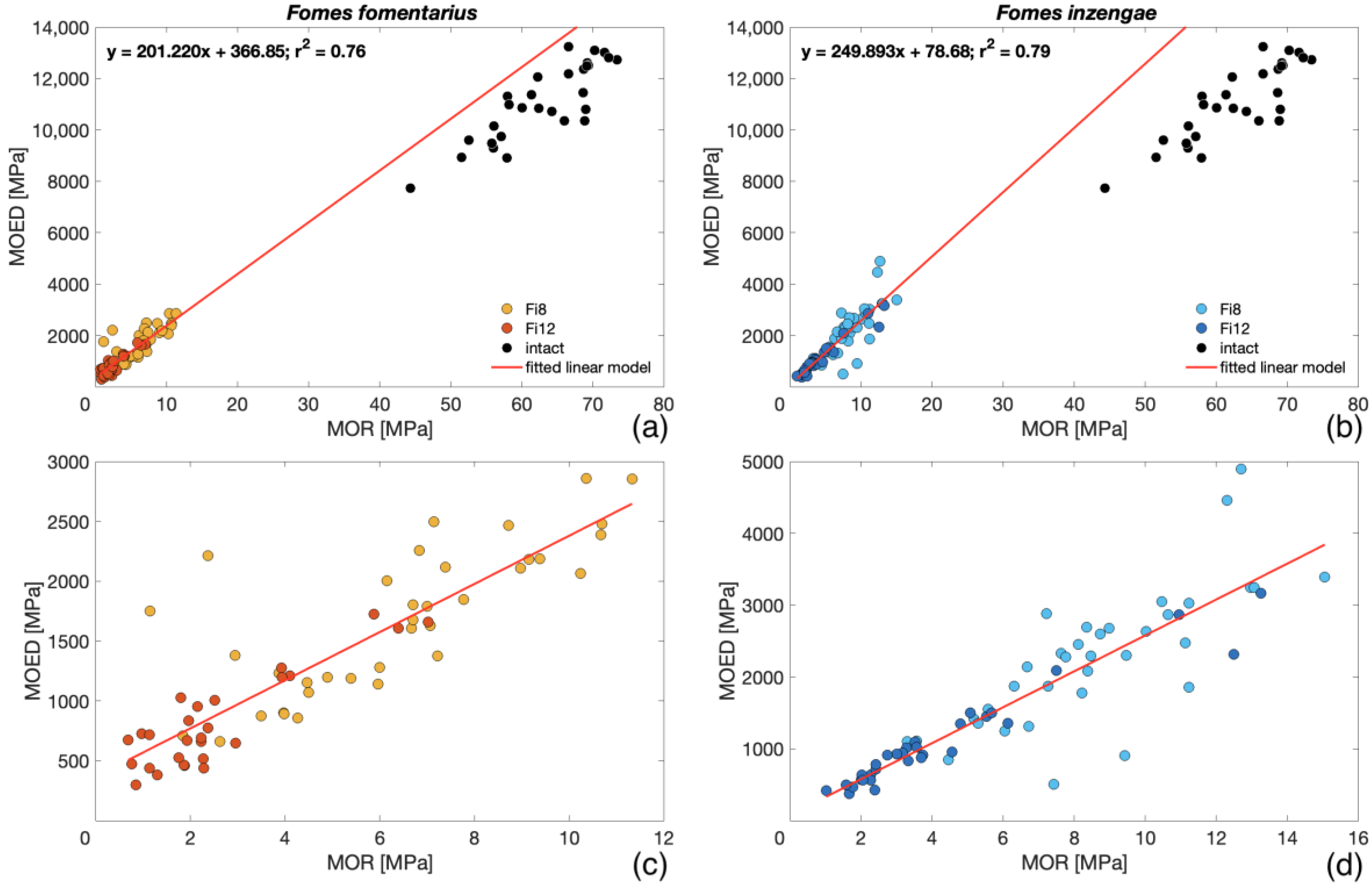
- A strong relationship between and was found for both species (Figure 5). After both exposure periods, no relevant difference in the between the two species was evident (> 0.001).
- The higher of bending samples compared with the specimens exposed in Petri dishes was probably caused by the samples’ geometry and environmental conditions. The negative correlation between the and for both species ( between −0.54 and −0.74) was caused by the increasing trend of with .
- For both species, the and showed a strong linear relationship (Figure 6). As for the , no relevant difference between the two species after both exposure times was observed.
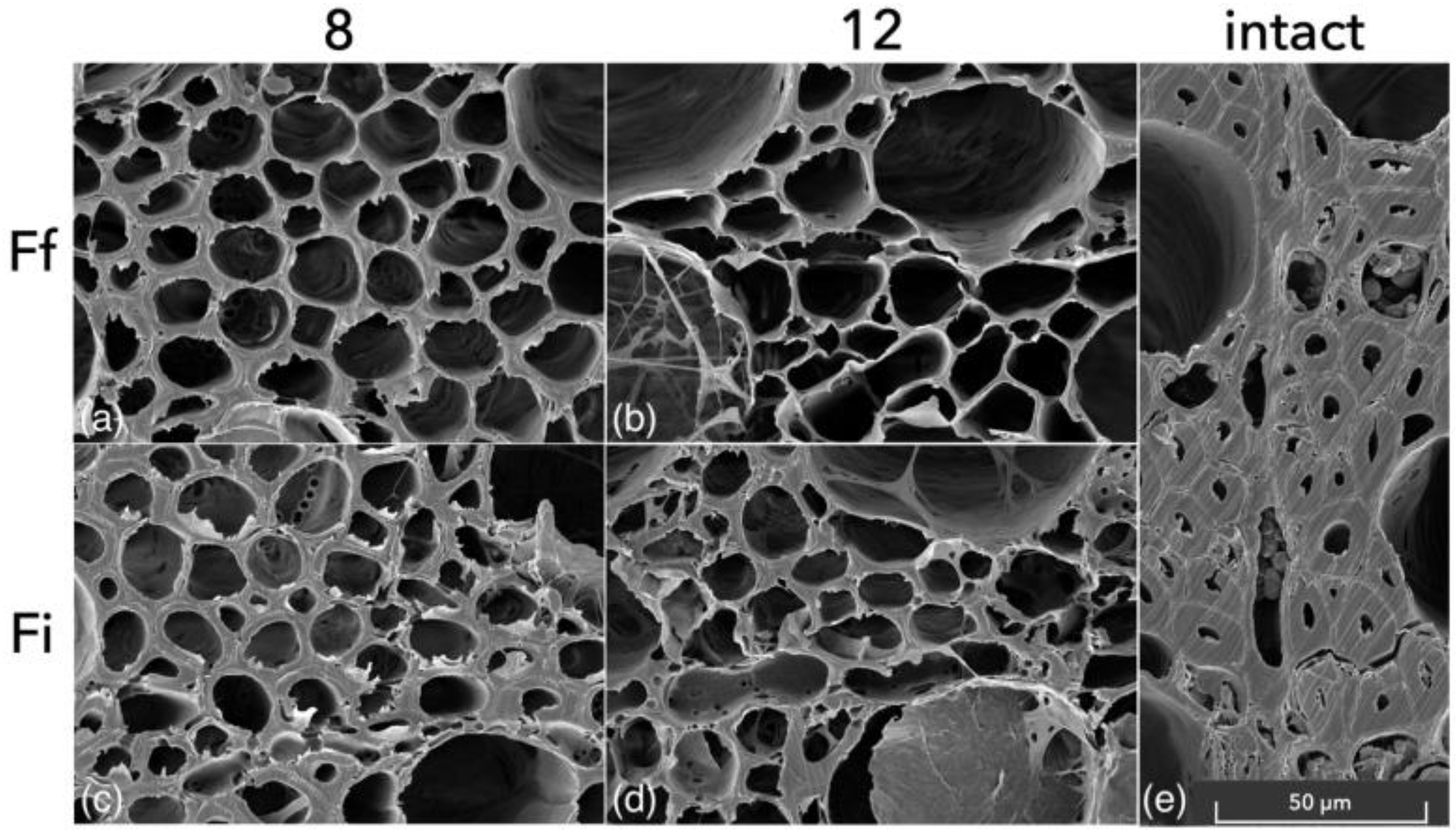
- According to our SEM assessment, both species showed the morphological patterns of simultaneous white rot and soft-rot type I.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukasawa, Y. Ecological Impacts of Fungal Wood Decay Types: A Review of Current Knowledge and Future Research Directions. Ecol. Res. 2021, 36, 910–931. [Google Scholar] [CrossRef]
- Wainhouse, M.; Boddy, L. Making Hollow Trees: Inoculating Living Trees with Wood-Decay Fungi for the Conservation of Threatened Taxa—A Guide for Conservationists. Glob. Ecol. Conserv. 2022, 33, e01967. [Google Scholar] [CrossRef]
- Czaja, M.; Kołton, A.; Muras, P. The Complex Issue of Urban Trees-Stress Factor Accumulation and Ecological Service Possibilities. Forests 2020, 11, 932. [Google Scholar] [CrossRef]
- le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Manning, A.D.; Gibbons, P. The Future of Large Old Trees in Urban Landscapes. PLoS ONE 2014, 9, 99403. [Google Scholar] [CrossRef]
- Ordóñez-Barona, C.; Sabetski, V.; Millward, A.A.; Urban, J.; Steenberg, J.; Grant, A. The Influence of Abiotic Factors on Street Tree Condition and Mortality in a Commercial-Retail Streetscape. Arboric. Urban For. 2018, 44, 133–144. [Google Scholar]
- Schwarze, F.W.M.R.; Engels, J.; Mattheck, C. Fungal Strategies of Wood Decay in Trees; Springer: Berlin/Heidelberg, Germany, 2004; ISBN 978-3-642-63133-7. [Google Scholar]
- Soge, A.O.; Popoola, O.I.; Adetoyinbo, A.A. Detection of Wood Decay and Cavities in Living Trees: A Review. Can. J. For. Res. 2021, 51, 937–947. [Google Scholar] [CrossRef]
- Cristini, V.; Tippner, J.; Vojáčková, B.; Paulić, V. Comparison of Variability in Results of Acoustic Tomographs in Pedunculate Oak (Quercus robur L.). Bioresources 2021, 16, 3046–3058. [Google Scholar] [CrossRef]
- Schmidt, O. Wood and Tree Fungi—Biology, Damage, Protection and Use; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 978-3-642-06875-1. [Google Scholar]
- McCormick, M.A.; Cubeta, M.A.; Grand, L.F. Geography and Hosts of the Wood Decay Fungi Fomes fasciatus and Fomes fomentarius in the United States. N. Am. Fungi 2013, 8, 1–53. [Google Scholar] [CrossRef]
- Gáper, J.; Pristaš, P.; Gáperová, S.; Maliničová, L. Molecular Identification of Fomes fomentarius in Hosts from Urban and Suburban Areas in Slovakia. Folia Oecologica 2013, 40, 22–27. [Google Scholar]
- Větrovský, T.; Voříšková, J.; Šnajdr, J.; Gabriel, J.; Baldrian, P. Ecology of Coarse Wood Decomposition by the Saprotrophic Fungus Fomes fomentarius. Biodegradation 2011, 22, 709–718. [Google Scholar] [CrossRef]
- Bari, E.; Pizzi, A.; Schmidt, O.; Amirou, S.; Tajick-Ghanbary, M.A.; Humar, M. Differentiation of Fungal Destructive Behaviour of Wood by the White-Rot Fungus Fomes fomentarius by Maldi-Tof Mass Spectrometry. J. Renew. Mater. 2021, 9, 381–397. [Google Scholar] [CrossRef]
- Suvorov, P.A. Biological Characteristics of Fomes Fomentarius Found on Spruce and Birch. Can. J. Bot. 1967, 45, 1853–1857. [Google Scholar] [CrossRef]
- Boddy, L. Fungi and Trees—Their Complex Relationships; Arboricultural Association: Stonehouse, UK, 2021; ISBN 978-0-900978-70-8. [Google Scholar]
- Baum, S.; Sieber, T.N.; Schwarze, F.W.M.R.; Fink, S. Latent Infections of Fomes fomentarius in the Xylem of European Beech (Fagus Sylvatica). Mycol. Prog. 2003, 2, 141–148. [Google Scholar] [CrossRef]
- Niemelä, T.; Renvall, P.; Penttilä, R. Interactions of Fungi at Late Stages of Wood Decomposition. Ann. Bot. Fenn. 1995, 32, 141–152. [Google Scholar]
- Peintner, U.; Kuhnert-Finkernagel, R.; Wille, V.; Biasioli, F.; Shiryaev, A.; Perini, C. How to Resolve Cryptic Species of Polypores: An Example in Fomes. IMA Fungus 2019, 10, 17. [Google Scholar] [CrossRef] [Green Version]
- Badalyan, S.M.; Zhuykova, E.; Mukhin, V. The Phylogenetic Analysis of Armenian Collections of Medicinal Tinder Polypore Fomes fomentarius (Agaricomycetes, Polyporaceae). Ital. J. Mycol. 2022, 51, 23–33. [Google Scholar] [CrossRef]
- Fink, S. Hazard Tree Identification by Visual Tree Assessment (VTA): Scientifically Solid and Practically Approved. Arboric. J. 2009, 32, 139–155. [Google Scholar] [CrossRef]
- Gunduz, G.; Aydemir, D. The Influence of Mass Loss on the Mechanical Properties of Heat-Treated Black Pine Wood. Wood Res. 2009, 54, 33–42. [Google Scholar]
- Xu, H.; Di, Y.; Cappellazzi, J.; Morrell, J.J. Effect of Brown Rot Degradation on Mass Loss and Compressive Strength of Chinese Poplar (Populus simonii). Maderas Cienc. Tecnol. 2019, 21, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Bari, E.; Daniel, G.; Yilgor, N.; Kim, J.S.; Tajick-Ghanbary, M.A.; Singh, A.P.; Ribera, J. Comparison of the Decay Behavior of Two White-Rot Fungi in Relation to Wood Type and Exposure Conditions. Microorganisms 2020, 8, 1931. [Google Scholar] [CrossRef]
- Chauhan, S.; Sethy, A. Differences in Dynamic Modulus of Elasticity Determined by Three Vibration Methods and Their Relationship with Static Modulus of Elasticity. Maderas Cienc. Tecnol. 2016, 18, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Machek, L.; Militz, H.; Sierra-Alvarez, R. The Use of an Acoustic Technique to Assess Wood Decay in Laboratory Soil-Bed Tests. Wood Sci. Technol. 2001, 34, 467–472. [Google Scholar] [CrossRef]
- Schwarze, F.W.M.R.; Lonsdale, D.; Fink, S. Soft Rot and Multiple T-Branching by the Basidiomycete Inonotus hispidus in Ash and London Plane. Mycol. Res. 1995, 99, 813–820. [Google Scholar] [CrossRef]
- Deflorio, G.; Fink, S.; Schwarze, F.W.M.R. Detection of Incipient Decay in Tree Stems with Sonic Tomography after Wounding and Fungal Inoculation. Wood Sci. Technol. 2008, 42, 117–132. [Google Scholar] [CrossRef]
- Cristini, V.; Tippner, J.; Nop, P.; Zlámal, J.; Hassan Vand, M.; Šeda, V. Degradation of Beech Wood by Kretzschmaria deusta: Its Heterogeneity and Influence on Dynamic and Static Bending Properties. Holzforschung 2022, 76, 813–824. [Google Scholar] [CrossRef]
- Rypáček, V. Biologie Drevokaznych Hub, 1st ed.; ČSAV: Praha, Czech Republic, 1957. [Google Scholar]
- Fukasawa, Y.; Osono, T.; Takeda, H. Wood Decomposing Abilities of Diverse Lignicolous Fungi on Nondecayed and Decayed Beech Wood. Mycologia 2011, 103, 474–482. [Google Scholar] [CrossRef]
- Bravery, A.F. A Miniaturised Wood-Block Test for the Rapid Evaluation of Wood Preservative Fungicides; International Research Group on Wood Protection: Stockholm, Sweden, 1978. [Google Scholar]
- Tomšovský, M. Delimitation of an Almost Forgotten Species Spongipellis litschaueri (Polyporales, Basidiomycota) and Its Taxonomic Position within the Genus. Mycol. Prog. 2012, 11, 415–424. [Google Scholar] [CrossRef]
- CEN/TS 15083-1:2006; Durability of Wood and Wood-Based Products—Determination of the Natural Durability of Solid Wood against Wood-Destroying Fungi, Test Methods—Part 1: Basidiomycetes. Slovenski inštitut za standardizacijo: Ljubljana, Slovenia, 2006.
- van Duong, D.; Matsumura, J. Transverse Shrinkage Variations within Tree Stems of Melia azedarach Planted in Northern Vietnam. J. Wood Sci. 2018, 64, 720–729. [Google Scholar] [CrossRef] [Green Version]
- Zelinka, S.L.; Kirker, G.T.; Bishell, A.B.; Glass, S.V. Effects of Wood Moisture Content and the Level of Acetylation on Brown Rot Decay. Forests 2020, 11, 299. [Google Scholar] [CrossRef] [Green Version]
- Schwarze, F.W.M.R. Wood Decay under the Microscope. Fungal. Biol. Rev. 2007, 21, 133–170. [Google Scholar] [CrossRef]
- Schwarze, F.W.M.R.; Fink, S. Host and Cell Type Affect the Mode of Degradation by Meripilus giganteus. New Phytol. 1998, 139, 721–731. [Google Scholar] [CrossRef]
- Skyba, O.; Niemz, P.; Schwarze, F.W.M.R. Resistance of Thermo-Hygro-Mechanically (THM) Densified Wood to Degradation by White Rot Fungi. Holzforschung 2009, 63, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wang, C.; Fei, B.; Ma, X.; Zhang, B.; Zhang, S.; Huang, A. Biological Degradation of Chinese Fir with Trametes versicolor (l.) Lloyd. Materials 2017, 10, 834. [Google Scholar] [CrossRef] [Green Version]
- Leviu, L.; Castro, M.A. Anatomical Study of the Decay Caused by the White-Rot Fungus Tramentes trogii (Aphyllophorales) in Wood of Salix and Populus. IAWA J. 1998, 19, 169–180. [Google Scholar] [CrossRef]
- Fuhr, M.J.; Schubert, M.; Stührk, C.; Schwarze, F.W.; Herrmann, H.J. Penetration Capacity of the Wood-Decay Fungus Physisporinus vitreus. Complex Adapt. Syst. Model. 2013, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Freyburger, C.; Longuetaud, F.; Mothe, F.; Constant, T.; Leban, J.M. Measuring Wood Density by Means of X-Ray Computer Tomography. Ann. For. Sci. 2009, 66, 804. [Google Scholar] [CrossRef] [Green Version]
- Brischke, C.; Grünwald, L.K.; Bollmus, S. Effect of Size and Shape of Specimens on the Mass Loss Caused by Coniophora puteana in Wood Durability Tests. Eur. J. Wood Wood Prod. 2020, 78, 811–819. [Google Scholar] [CrossRef]
- Kazemi, S.M.; Dickinson, D.J.; Murphy, R.J. Effects of Initial Moisture Content on Wood Decay at Different Levels of Gaseous Oxygen Concentrations. J. Agric. Sci. Technol. 2001, 3, 293–304. [Google Scholar]

| Species | Fomes fomentarius | Fomes inzengae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Ff1 | Ff2 | Ff3 | Ff4 | Ff5 | Fi1 | Fi2 | Fi3 | Fi4 | Fi5 |
| Host | Fagus sylvatica | Betula sp. | Betula sp. | Fagus sylvatica | Fagus sylvatica | Fagus sylvatica | Quercus petraea | Fagus sylvatica | Alnus glutinosa | Tilia cordata |
| Accession N.* | OQ474927 | OQ474923 | OQ474926 | OQ474928 | OQ474930 | OQ474914 | OQ474913 | OQ474917 | OQ474918 | OQ474921 |
| MOR (MPa) | MOED (MPa) | (kg/m3) | (kg/m3) | MC (%) | ML (%) | |
|---|---|---|---|---|---|---|
| Ff 8 | 6.3 (42.9%) | 1 685 (36.8%) | 681.7 (15.9%) | 265.8 (12.6%) | 161.3 (36.4%) | 53.6 (11.2%) |
| Ff 12 | 2.5 (66.2%) | 817 (49.1%) | 561.2 (27.3%) | 196.4 (19.5%) | 203.4 (59.2%) | 65.8 (10.2%) |
| Fi 8 | 8.5 (33%) | 2 250 (43.1%) | 941.7 (19%) | 326.5 (8.9%) | 190.5 (31.3%) | 43.3 (11.6%) |
| Fi 12 | 4.2 (73.5%) | 1 088 (63.7%) | 1017 (8.73%) | 252.7 (17.9%) | 312.2 (21.8%) | 56.4 (14.2%) |
| Ref | 63 (11.5%) | 11 102 (13.3%) | 925.9 (3.1%) | 558.9 (3.5%) | 65.8 (9.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristini, V.; Nop, P.; Zlámal, J.; Vand, M.H.; Šeda, V.; Tippner, J. Fomes fomentarius and F. inzengae—A Comparison of Their Decay Patterns on Beech Wood. Microorganisms 2023, 11, 679. https://doi.org/10.3390/microorganisms11030679
Cristini V, Nop P, Zlámal J, Vand MH, Šeda V, Tippner J. Fomes fomentarius and F. inzengae—A Comparison of Their Decay Patterns on Beech Wood. Microorganisms. 2023; 11(3):679. https://doi.org/10.3390/microorganisms11030679
Chicago/Turabian StyleCristini, Valentino, Patrik Nop, Jan Zlámal, Mojtaba Hassan Vand, Vít Šeda, and Jan Tippner. 2023. "Fomes fomentarius and F. inzengae—A Comparison of Their Decay Patterns on Beech Wood" Microorganisms 11, no. 3: 679. https://doi.org/10.3390/microorganisms11030679





