Water-Soluble Fullerene C60 Derivatives Are Effective Inhibitors of Influenza Virus Replication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Test Compounds
2.3. Culture Medium
2.4. Synthesis of Water-Soluble Fullerenes
2.5. Cytotoxicity Assay
2.6. CPE Reduction Assay
2.7. Time-of-Addition Experiments
2.8. Virus Yield Reduction Assay
2.9. Statistical Analysis
3. Results
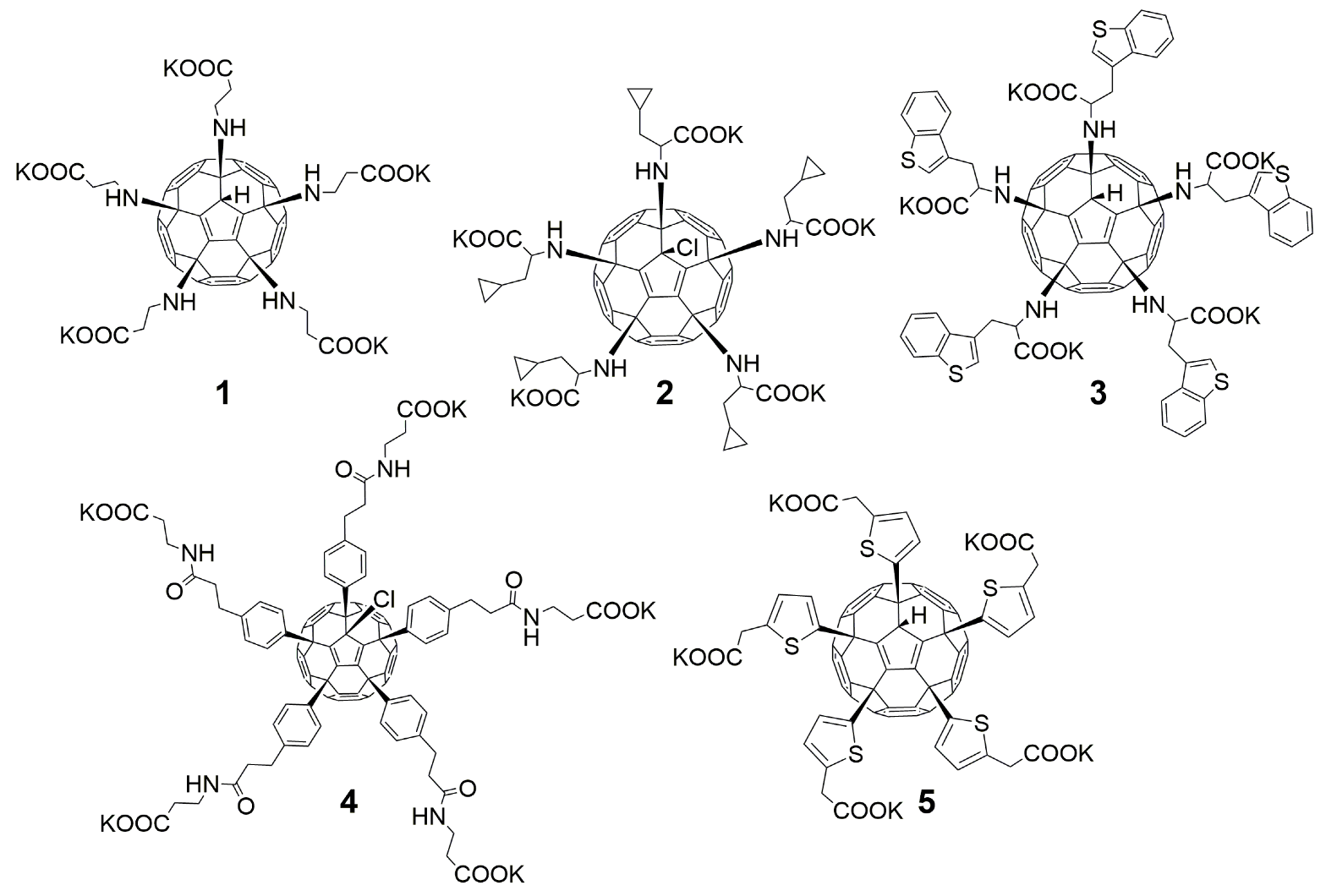
3.1. Anti-Influenza Activity of Fullerene Derivatives
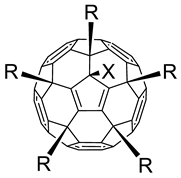 | ||||||
|---|---|---|---|---|---|---|
| # | R | X | CC50 (µg/mL) a | IC50 (µg/mL) b | SI c | Reference for Synthesis and Characterization |
| 1 |  | H | >300 | 6.3 ± 2.5 | 48 | [16,23] |
| 2 | 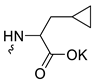 | Cl | >300 | 4.73 ± 2.47 | 64 | [19] |
| 3 | 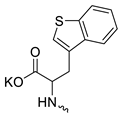 | H | >300 | 5.53 ± 1.72 | 55 | this work |
| 4 | 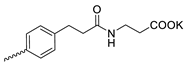 | Cl | >300 | 6.67 ± 2.44 | 45 | [19] |
| 5 | 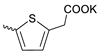 | H | 33.37 ± 9.42 | 0.39 ± 0.07 | 84 | [18] |
| 6 | 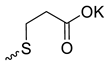 | H | >300 | 106.0 ± 8.54 | 3 | [24] |
| 7 |  | Cl | 35.13 ± 1.06 | >30 | 1 | [15,25,26] |
| 8 |  | H | 14.8 ± 2.10 | >11 | 1 | [26] |
| 9 | 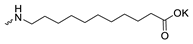 | H | >300 | >300 | 1 | [26] |
| 10 | 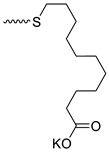 | H | >300 | 31.33 ± 8.66 | 10 | [24] |
| 11 |  | H | >200 | 34.10 ± 8.91 | 6 | [15,16,23] |
| 12 | 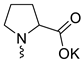 | Cl | <3 | <3 | <1 | [23] |
| 13 | 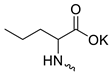 | Cl | >300 | 158.03 ± 13.71 | 2 | [19] |
| 14 |  | Cl | >300 | >300 | 1 | [19] |
| 15 | 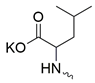 | Cl | >300 | 83.30 ± 7.67 | 4 | [19] |
| 16 | 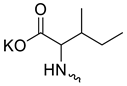 | H | >300 | 40.17 ± 8.26 | 7 | [19] |
| 17 | 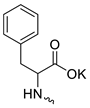 | H | 200.00 ± 5.00 | >100 | 2 | [15,16] |
| 18 | 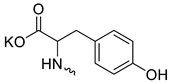 | Cl | >200 | 63.00 ± 10.64 | 3 | [23] |
| 19 |  | Cl | >300 | 33.03 ± 9.87 | 9 | [23,27] |
| 20 |  | Cl | 57.83 ± 6.86 | 45.07 ± 7.19 | 1 | [23] |
| 21 | 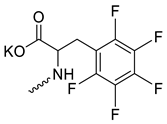 | Cl | 14.70 ± 2.57 | >10 | 1 | [23] |
| 22 |  | Cl | 33.60 ± 6.51 | >30 | 1 | [17,19] |
| 23 | 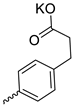 | Cl | 30.50 ± 4.86 | 6.93 ± 2.80 | 4 | [28] |
| 24 | 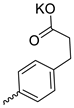 | Me | 43.60 ± 6.55 | >12.5 | 3 | [29] |
| 25 | 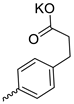 | iPr | 14.70 ± 3.75 | >11 | 1 | [29] |
| 26 |  | Bu | 24.13 ± 4.01 | >12.5 | 2 | [29] |
| 27 | 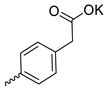 | Cl | 12.23 ± 3.15 | >11 | 1 | [30] |
| 28 |  | Cl | >300 | >300 | 1 | [31] |
| 29 | 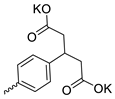 | Cl | 11.87 ± 4.47 | 4.00 ± 1.67 | 3 | [29] |
| 30 |  | H | 9.70 ± 2.40 | 3.70 ± 1.01 | 3 | [29] |
| 31 |  | Et | 3.67 ± 2.10 | >3 | 1 | [29] |
| 32 |  | Cl | 33.00 ± 9.53 | 10.33 ± 2.60 | 3 | [28,32] |
| 33 | 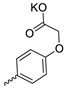 | 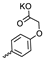 | 36.60 ± 6.62 | 1.10 ± 0.26 | 33 | [32] |
| 34 |  | H | 32.37 ± 7.07 | 11.13 ± 1.95 | 3 | [32] |
| 35 |  | Cl | >300 | 213.07 ± 13.62 | 1 | [28] |
| 36 | 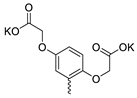 | Cl | 23.23 ± 7.68 | 5.47 ± 2.00 | 4 | [33] |
| 37 | 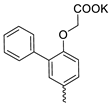 | Cl | 1.47 ± 0.81 | 0.24 ± 0.08 | 6 | this work |
| 38 |  | Cl | 21.03 ± 7.38 | 6.97 ± 1.45 | 3 | [17] |
| 39 | 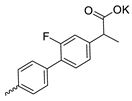 | Et | 126.07 ± 5.25 | 96.87 ± 5.85 | 1 | this work |
| 40 |  | Me | 3.70 ± 1.05 | >3 | 1 | [18] |
| 41 |  | Et | 13.80 ± 4.00 | >11 | 1 | [18] |
| 42 | 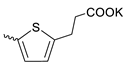 | H | 11.60 ± 5.60 | >11 | 1 | [18] |
| 43 | 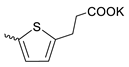 | Cl | 3.63 ± 1.16 | 1.03 ± 0.40 | 4 | [18] |
| 44 | 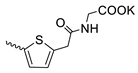 | Cl | 102.27 ± 10.89 | 15.83 ± 1.60 | 6 | [17,19] |
| 45 | 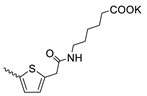 | Cl | >300 | 31.30 ± 5.83 | 10 | [17,19] |
| Oseltamivir carboxylate | >200 d | 0.3 ± 0.02 d | >667 | N/A | ||
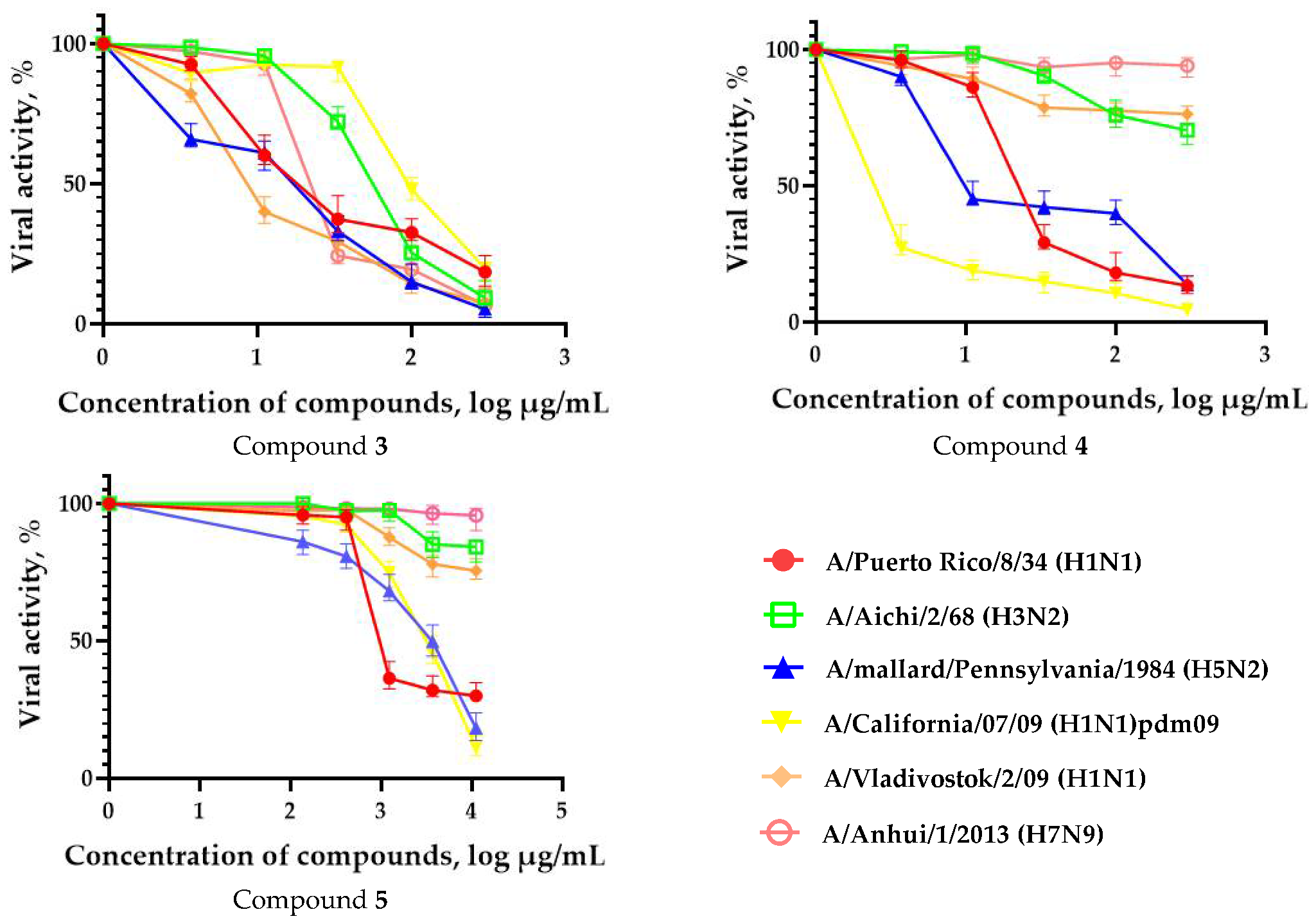
3.2. Time-of-Addition Experiments
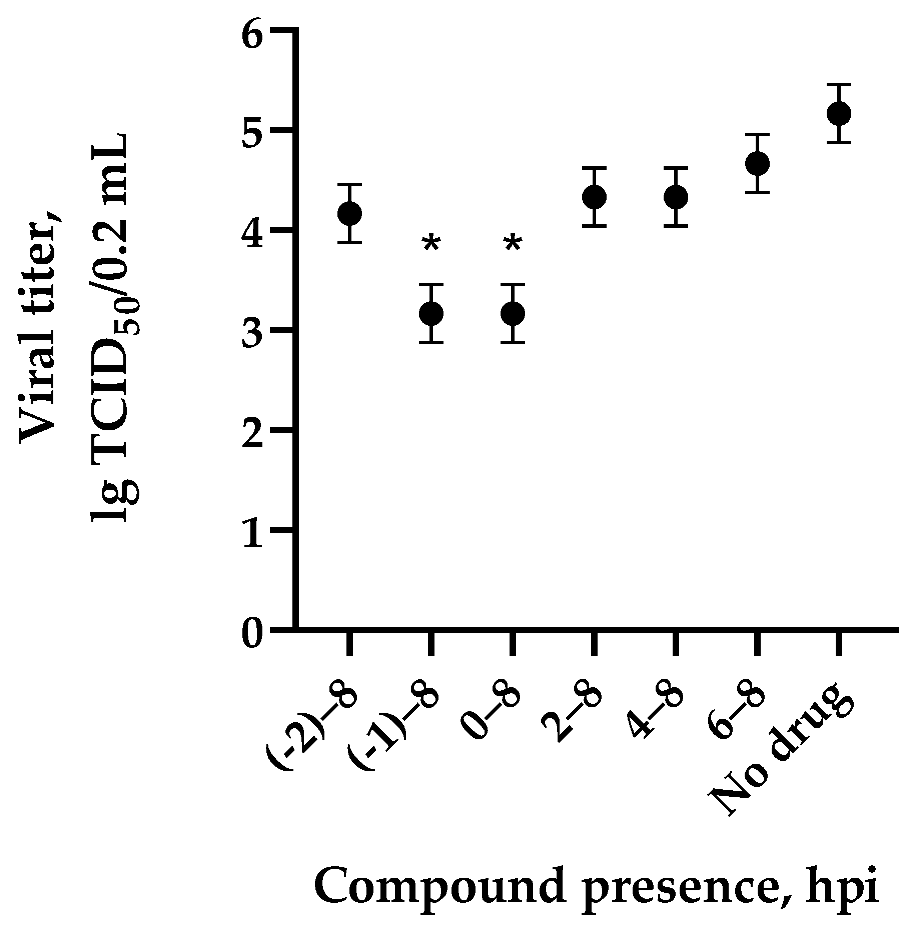
3.3. One-Step Growth Curve in the Presence of the Most Active Compounds 1– 5
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Influenza (Seasonal). Available online: www.who.int/mediacentre/factsheets/fs211/en/ (accessed on 11 October 2022).
- Clark, A.M.; DeDiego, M.L.; Anderson, C.S.; Wang, J.; Yang, H.; Nogales, A.; Martinez-Sobrido, L.; Zand, M.S.; Sangster, M.Y.; Topham, D.J. Antigenicity of the 2015–2016 Seasonal H1N1 Human Influenza Virus HA and NA Proteins. PLoS ONE 2017, 12, e0188267. [Google Scholar] [CrossRef] [Green Version]
- Ison, M.G. Antivirals and Resistance: Influenza Virus. Curr. Opin. Virol. 2011, 1, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Kolosnjaj, J.; Szwarc, H.; Moussa, F. Toxicity Studies of Fullerenes and Derivatives; Springer: New York, NY, USA, 2007; pp. 168–180. [Google Scholar]
- Aschberger, K.; Johnston, H.J.; Stone, V.; Aitken, R.J.; Tran, C.L.; Hankin, S.M.; Peters, S.A.K.; Christensen, F.M. Review of Fullerene Toxicity and Exposure—Appraisal of a Human Health Risk Assessment, Based on Open Literature. Regul. Toxicol. Pharmacol. 2010, 58, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Lu, T.-Y. C60 fullerene derivatized nanoparticles and their application to therapeutics. Recent Pat. Nanotechnol. 2012, 6, 105–113. [Google Scholar]
- Injac, R.; Prijatelj, M.; Strukelj, B. Fullerenol Nanoparticles: Toxicity and Antioxidant Activity; Humana Press: Totowa, NJ, USA, 2013; pp. 75–100. [Google Scholar]
- Zhou, Y.; Li, J.; Ma, H.; Zhen, M.; Guo, J.; Wang, L.; Jiang, L.; Shu, C.; Wang, C. Biocompatible [60]/[70] Fullerenols: Potent Defense against Oxidative Injury Induced by Reduplicative Chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 35539–35547. [Google Scholar] [CrossRef] [PubMed]
- Mashino, T. Development of Bio-Active Fullerene Derivatives Suitable for Drug. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2022, 142, 165–179. [Google Scholar] [CrossRef]
- Hui, M.; Jia, X.; Li, X.; Lazcano-Silveira, R.; Shi, M. Anti-Inflammatory and Antioxidant Effects of Liposoluble C60 at the Cellular, Molecular, and Whole-Animal Levels. J. Inflamm. Res. 2023, 16, 83–93. [Google Scholar] [CrossRef]
- Mizuno, K.; Zhiyentayev, T.; Huangv, L.; Khalil, S.; Nasim, F. Antimicrobial Photodynamic Therapy with Functionalized Fullerenes: Quantitative Structure-Activity Relationships. J. Nanomed. Nanotechnol. 2011, 2, 1–9. [Google Scholar] [CrossRef]
- Misra, C.; Kumar, M.; Sharma, G.; Kumar, R.; Singh, B.; Katare, O.P.; Raza, K. Glycinated Fullerenes for Tamoxifen Intracellular Delivery with Improved Anticancer Activity and Pharmacokinetics. Nanomedicine 2017, 12, 1011–1023. [Google Scholar] [CrossRef]
- Piotrovsky, L.B.; Kiselev, O.I. Fullerenes and Viruses. Fuller. Nanotub. Carbon Nanostruct. 2005, 12, 397–403. [Google Scholar] [CrossRef]
- Kornev, A.B.; Khakina, E.A.; Troyanov, S.I.; Kushch, A.A.; Peregudov, A.; Vasilchenko, A.; Deryabin, D.G.; Martynenko, V.M.; Troshin, P.A. Facile Preparation of Amine and Amino Acid Adducts of [60]Fullerene Using Chlorofullerene C60Cl6 as a Precursor. Chem. Commun. 2012, 48, 5461–5463. [Google Scholar] [CrossRef]
- Hsieh, F.-Y.; Zhilenkov, A.V.; Voronov, I.I.; Khakina, E.A.; Mischenko, D.V.; Troshin, P.A.; Hsu, S. Water-Soluble Fullerene Derivatives as Brain Medicine: Surface Chemistry Determines If They Are Neuroprotective and Antitumor. ACS Appl. Mater. Interfaces 2017, 9, 11482–11492. [Google Scholar] [CrossRef]
- Kraevaya, O.A.; Peregudov, A.S.; Godovikov, I.A.; Shchurik, E.V.; Martynenko, V.M.; Shestakov, A.F.; Balzarini, J.; Schols, D.; Troshin, P.A. Direct Arylation of C60Cl6 and C70Cl8 with Carboxylic Acids: A Synthetic Avenue to Water-Soluble Fullerene Derivatives with Promising Antiviral Activity. Chem. Commun. 2020, 56, 1179–1182. [Google Scholar] [CrossRef]
- Kraevaya, O.A.; Peregudov, A.S.; Fedorova, N.E.; Klimova, R.R.; Godovikov, I.A.; Mishchenko, D.V.; Shestakov, A.F.; Schols, D.; Kushch, A.A.; Troshin, P.A. Thiophene-Based Water-Soluble Fullerene Derivatives as Highly Potent Antiherpetic Pharmaceuticals. Org. Biomol. Chem. 2020, 18, 8702–8708. [Google Scholar] [CrossRef]
- Huang, H.-J.; Chetyrkina, M.; Wong, C.-W.; Kraevaya, O.A.; Zhilenkov, A.V.; Voronov, I.I.; Wang, P.-H.; Troshin, P.A.; Hsu, S. Identification of Potential Descriptors of Water-Soluble Fullerene Derivatives Responsible for Antitumor Effects on Lung Cancer Cells via QSAR Analysis. Comput. Struct. Biotechnol. J. 2021, 19, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Cheng, L.P.; Pang, W.; Ling Guo, L. Design, Synthesis and Biological Evaluation of Novel 1,3,4-Oxadiazole Derivatives as Potent Neuraminidase Inhibitors. Bioorg. Med. Chem. 2022, 57, 116647. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Musharrafieh, R.G.; Ma, C.; Hau, R.; Wang, J. Discovery of Dapivirine, a Nonnucleoside HIV-1 Reverse Transcriptase Inhibitor, as a Broad-Spectrum Antiviral against Both Influenza A and B Viruses. Antivir. Res. 2017, 145, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-W.; Zhilenkov, A.V.; Kraevaya, O.A.; Mischenko, D.V.; Troshin, P.A.; Hsu, S. Toward Understanding the Antitumor Effects of Water-Soluble Fullerene Derivatives on Lung Cancer Cells: Apoptosis or Autophagy Pathways? J. Med. Chem. 2019, 62, 7111–7125. [Google Scholar] [CrossRef] [PubMed]
- Khakina, E.A.; Yurkova, A.A.; Peregudov, A.S.; Troyanov, S.I.; Trush, V.V.; Vovk, A.I.; Mumyatov, A.V.; Martynenko, V.M.; Balzarini, J.; Troshin, P.A. Highly Selective Reactions of C60Cl6 with Thiols for the Synthesis of Functionalized [60]Fullerene Derivatives. Chem. Commun. 2012, 48, 7158–7160. [Google Scholar] [CrossRef] [Green Version]
- Zhilenkov, A.V.; Khakina, E.A.; Troshin, P.A.; Inchagova, K.S.; Deryabin, D.G. Synthesis and Antibacterial Activity of Hybrid Supramolecular Complexes Based on Tetracycline/Doxycycline and Water-Soluble C60-Fullerene Derivatives. Pharm. Chem. J. 2017, 50, 637–641. [Google Scholar] [CrossRef]
- Deryabin, D.G.; Efremova, L.V.; Vasilchenko, A.S.; Saidakova, E.V.; Sizova, E.A.; Troshin, P.A.; Zhilenkov, A.V.; Khakina, E.A. A Zeta Potential Value Determines the Aggregate’s Size of Penta-Substituted [60]Fullerene Derivatives in Aqueous Suspension Whereas Positive Charge Is Required for Toxicity against Bacterial Cells. J. Nanobiotechnol. 2015, 13, 50. [Google Scholar] [CrossRef] [Green Version]
- Soldatova, Y.V.; Kotelnikova, R.A.; Zhilenkov, A.V.; Faingold, I.I.; Troshin, P.A.; Kozlova, M.A.; Areshidze, D.A.; Aldoshin, S.M. Potassium Salt of Fullerenylpenta-N-Dihydroxytyrosine Effects on Type 2 Diabetes Mellitus Therapeutic Targets. Dokl. Biochem. Biophys. 2019, 488, 320–323. [Google Scholar] [CrossRef]
- Fedorova, N.E.; Klimova, R.R.; Tulenev, Y.A.; Chichev, E.V.; Kornev, A.B.; Troshin, P.A.; Kushch, A.A. Carboxylic Fullerene C60 Derivatives: Efficient Microbicides Against Herpes Simplex Virus And Cytomegalovirus Infections In Vitro. Mendeleev Commun. 2012, 22, 254–256. [Google Scholar] [CrossRef]
- Kraevaya, O.A.; Peregudov, A.S.; Troyanov, S.I.; Godovikov, I.; Fedorova, N.E.; Klimova, R.R.; Sergeeva, V.A.; Kameneva, L.V.; Ershova, E.S.; Martynenko, V.M.; et al. Diversion of the Arbuzov Reaction: Alkylation of C–Cl Instead of Phosphonic Ester Formation on the Fullerene Cage. Org. Biomol. Chem. 2019, 17, 7155–7160. [Google Scholar] [CrossRef] [PubMed]
- Troshina, O.A.; Troshin, P.A.; Peregudov, A.S.; Kozlovskiy, V.I.; Balzarini, J.; Lyubovskaya, R.N. Chlorofullerene C60Cl6: A Precursor for Straightforward Preparation of Highly Water-Soluble Polycarboxylic Fullerene Derivatives Active against HIV. Org. Biomol. Chem. 2007, 5, 2783. [Google Scholar] [CrossRef] [PubMed]
- Kotelnikova, R.A.; Smolina, A.V.; Grigoryev, V.V.; Faingold, I.I.; Mischenko, D.V.; Rybkin, A.Y.; Poletayeva, D.A.; Vankin, G.I.; Zamoyskiy, V.L.; Voronov, I.I.; et al. Influence of Water-Soluble Derivatives of [60]Fullerene on Therapeutically Important Targets Related to Neurodegenerative Diseases. Medchemcomm 2014, 5, 1664–1668. [Google Scholar] [CrossRef]
- Huang, H.-J.; Kraevaya, O.A.; Voronov, I.I.; Troshin, P.A.; Hsu, S. Fullerene Derivatives as Lung Cancer Cell Inhibitors: Investigation of Potential Descriptors Using QSAR Approaches. Int. J. Nanomed. 2020, 15, 2485–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voronov, I.I.; Martynenko, V.M.; Chernyak, A.V.; Godovikov, I.; Peregudov, A.S.; Balzarini, J.; Shestakov, A.F.; Schols, D.; Troshin, P.A. Synthesis, Characterization and Anti-HIV Activity of Polycarboxylic [60]Fullerene Derivatives Obtained in the Reaction of C60Cl6 with a Hydroquinone Ether. Tetrahedron Lett. 2020, 61, 151598. [Google Scholar] [CrossRef]
- Pochkaeva, E.I.; Podolsky, N.E.; Zakusilo, D.N.; Petrov, A.V.; Charykov, N.A.; Vlasov, T.D.; Penkova, A.V.; Vasina, L.V.; Murin, I.V.; Sharoyko, V.V.; et al. Fullerene Derivatives with Amino Acids, Peptides and Proteins: From Synthesis to Biomedical Application. Prog. Solid State Chem. 2020, 57, 100255. [Google Scholar] [CrossRef]
- Du, C.-X.; Xiong, H.-R.; Ji, H.; Liu, Q.; Xiao, H.; Yang, Z.-Q. The Antiviral Effect of Fullerene-Liposome Complex against Influenza Virus (H1N1) in Vivo. Sci. Res. Essays 2012, 7, 7111–7125. [Google Scholar]
- Sushko, E.S.; Vnukova, N.G.; Churilov, G.N.; Kudryasheva, N.S. Endohedral Gd-Containing Fullerenol: Toxicity, Antioxidant Activity, and Regulation of Reactive Oxygen Species in Cellular and Enzymatic Systems. Int. J. Mol. Sci. 2022, 23, 5152. [Google Scholar] [CrossRef] [PubMed]
- Mikheev, I.V.; Sozarukova, M.M.; Izmailov, D.Y.; Kareev, I.E.; Proskurnina, E.V.; Proskurnin, M.A. Antioxidant Potential of Aqueous Dispersions of Fullerenes C60, C70, and Gd@C82. Int. J. Mol. Sci. 2021, 22, 5838. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Sozarukova, M.M.; Proskurnina, E.V.; Kareev, I.E.; Proskurnin, M.A. Non-Functionalized Fullerenes and Endofullerenes in Aqueous Dispersions as Superoxide Scavengers. Molecules 2020, 25, 2506. [Google Scholar] [CrossRef]
- Kovel, E.S.; Kicheeva, A.G.; Vnukova, N.G.; Churilov, G.N.; Stepin, E.A.; Kudryasheva, N.S. Toxicity and Antioxidant Activity of Fullerenol C60,70 with Low Number of Oxygen Substituents. Int. J. Mol. Sci. 2021, 22, 6382. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinegubova, E.O.; Kraevaya, O.A.; Volobueva, A.S.; Zhilenkov, A.V.; Shestakov, A.F.; Baykov, S.V.; Troshin, P.A.; Zarubaev, V.V. Water-Soluble Fullerene C60 Derivatives Are Effective Inhibitors of Influenza Virus Replication. Microorganisms 2023, 11, 681. https://doi.org/10.3390/microorganisms11030681
Sinegubova EO, Kraevaya OA, Volobueva AS, Zhilenkov AV, Shestakov AF, Baykov SV, Troshin PA, Zarubaev VV. Water-Soluble Fullerene C60 Derivatives Are Effective Inhibitors of Influenza Virus Replication. Microorganisms. 2023; 11(3):681. https://doi.org/10.3390/microorganisms11030681
Chicago/Turabian StyleSinegubova, Ekaterina O., Olga A. Kraevaya, Aleksandrina S. Volobueva, Alexander V. Zhilenkov, Alexander F. Shestakov, Sergey V. Baykov, Pavel A. Troshin, and Vladimir V. Zarubaev. 2023. "Water-Soluble Fullerene C60 Derivatives Are Effective Inhibitors of Influenza Virus Replication" Microorganisms 11, no. 3: 681. https://doi.org/10.3390/microorganisms11030681





