New Insights into the Physiology of the Propionate Producers Anaerotignum propionicum and Anaerotignum neopropionicum (Formerly Clostridium propionicum and Clostridium neopropionicum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cultivation
2.2. Analytical Methods
2.3. Gram Staining and Microscopy
3. Results
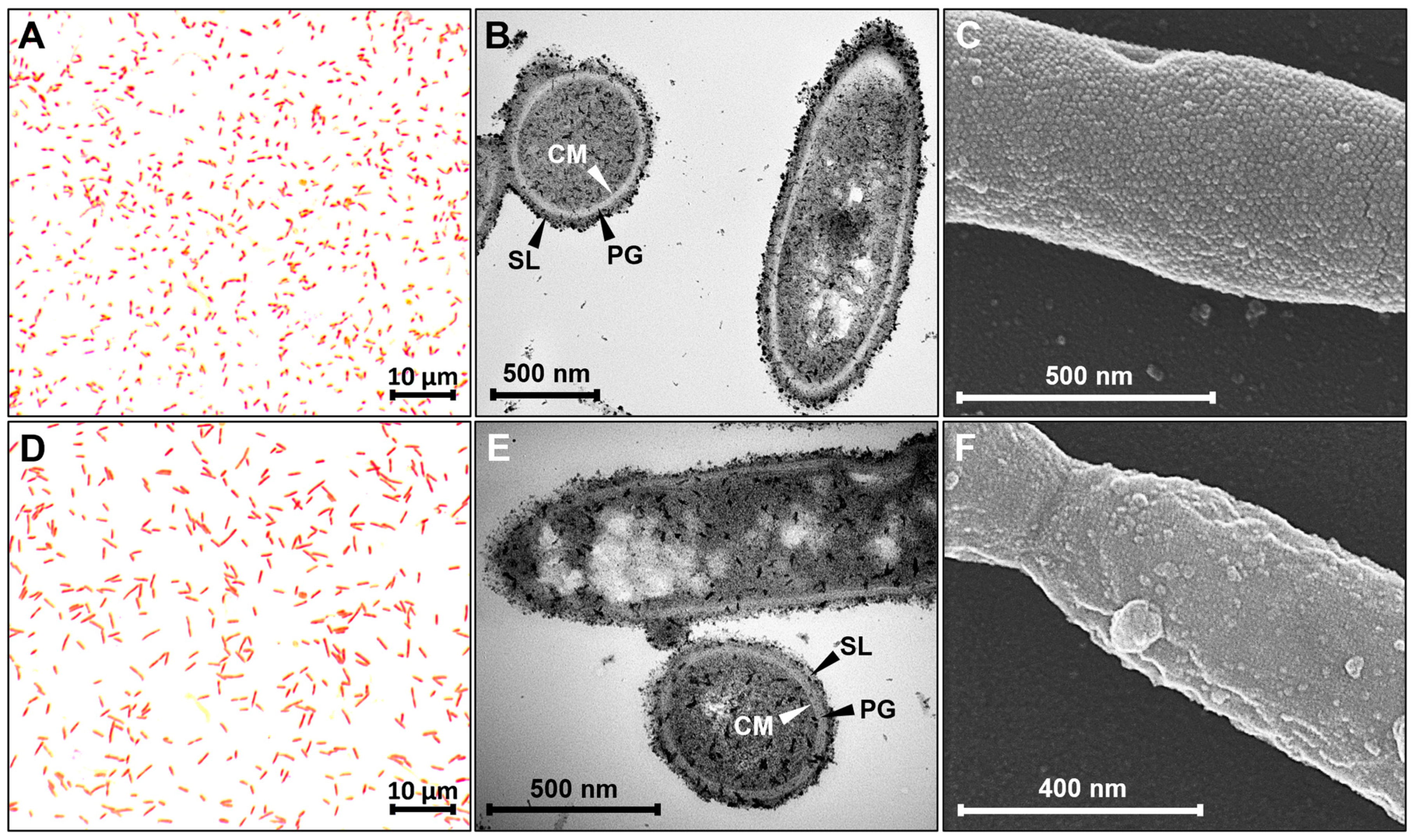
3.1. Investigation of Cell Envelope and Cell Surface
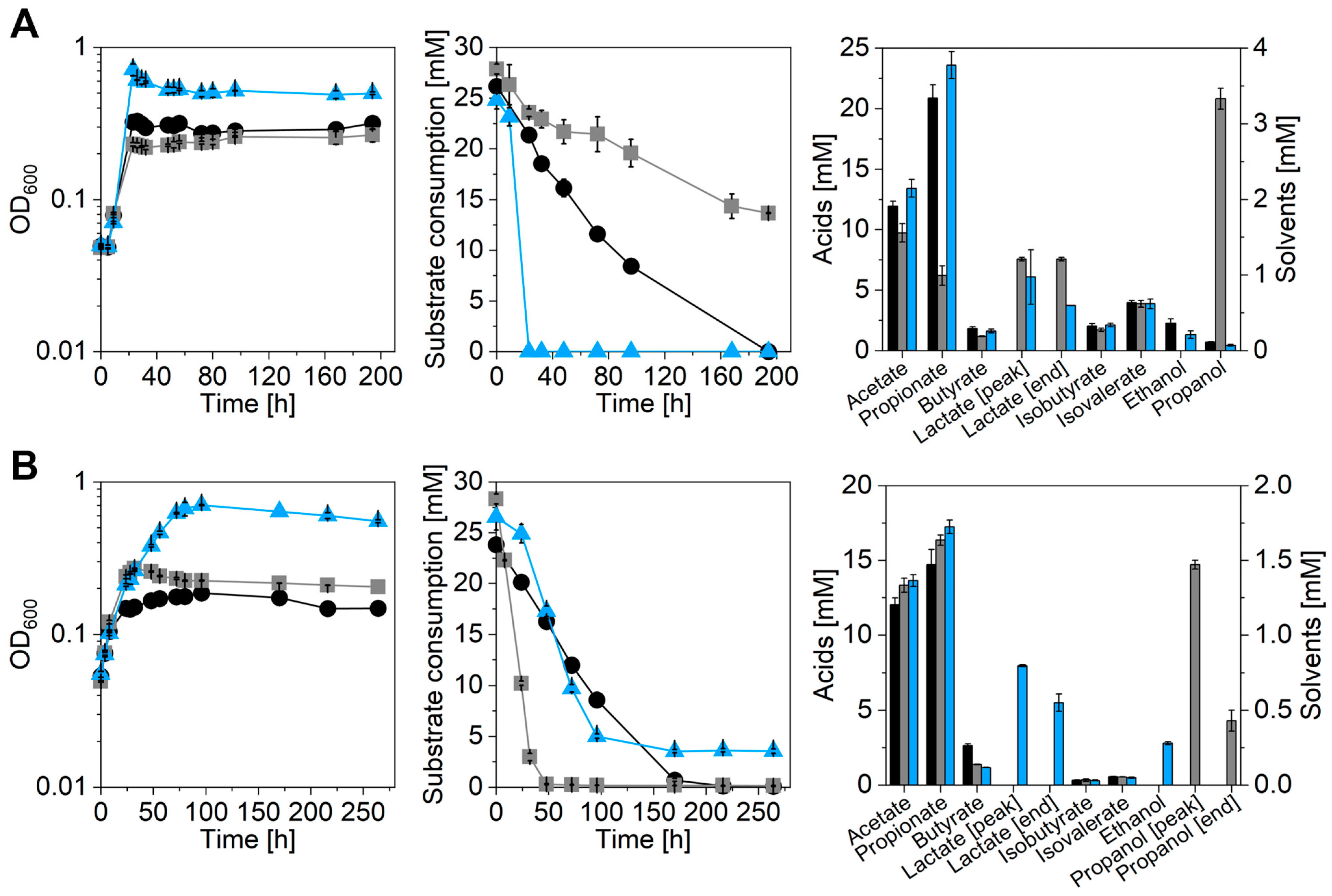
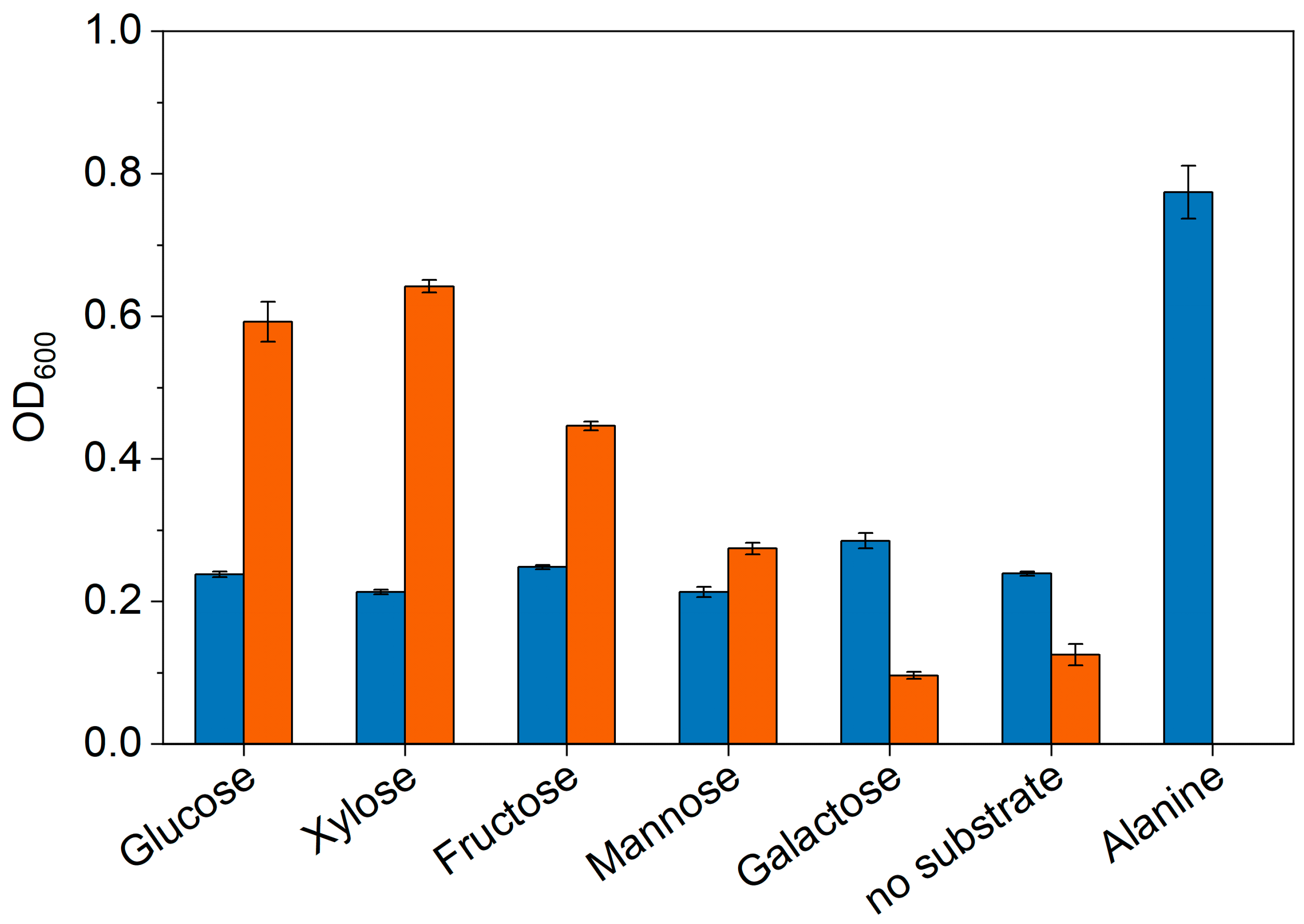
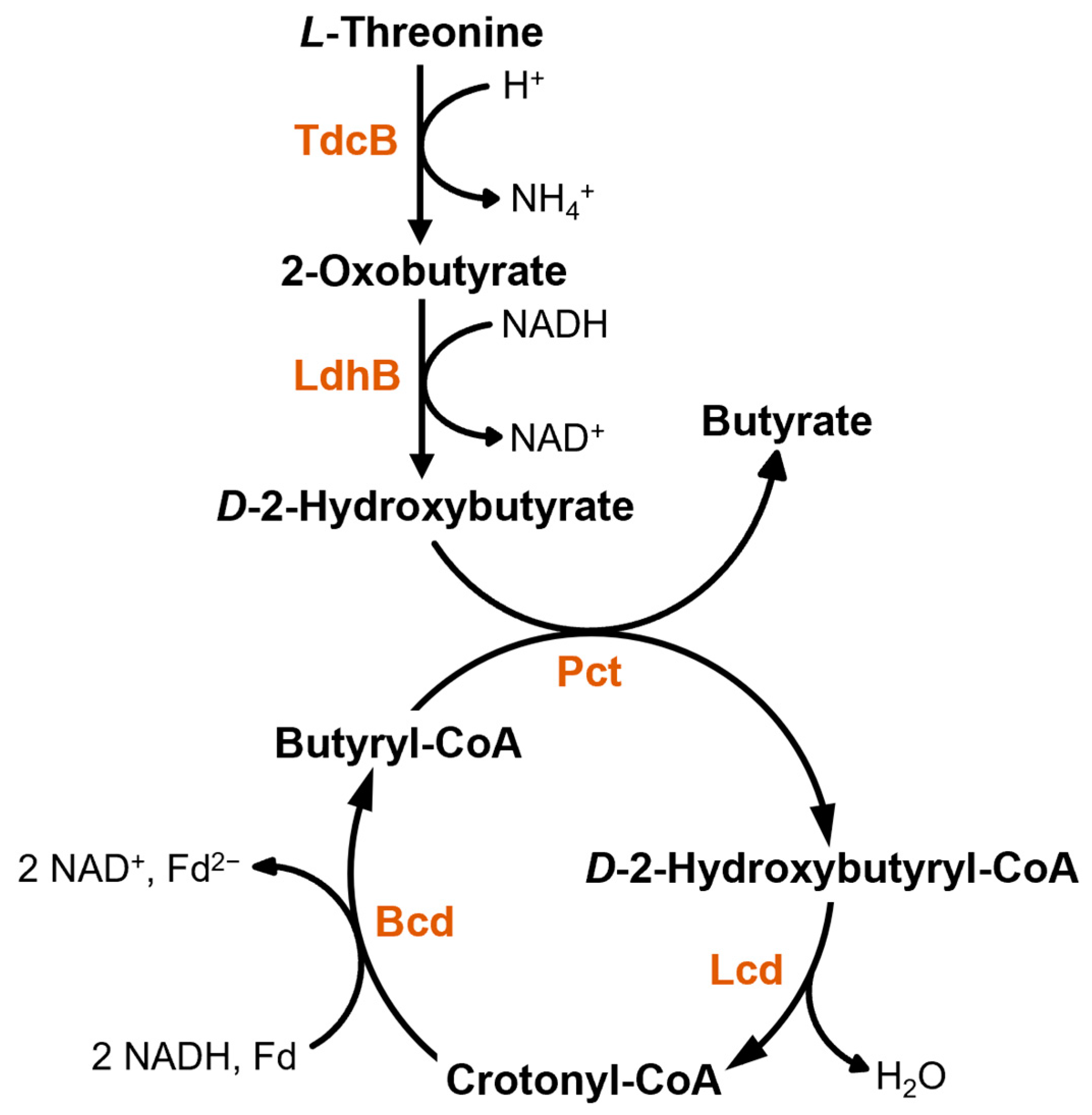
3.2. Investigation of Metabolic Capabilities
| 1% Glucose | 2% ExcelloTM-95 | 2% ExcelloTM-99 | |
|---|---|---|---|
| Growth parameters | |||
| max. OD600 | 0.7 | 0.7 | 0.7 |
| µ [h−1] | 0.05 | 0.04 | 0.05 |
| tD [h] | 13.3 | 15.9 | 15.3 |
| Substrate consumption [mM] | |||
| Glucose | 16.8 | 14.2 | 15.0 |
| Xylose | - | 3.6 | 5.5 |
| Product profile [mM] | |||
| Acetate | 13.5 | 15.4 | 14.1 |
| Propionate | 13.0 | 14.5 | 13.4 |
| Butyrate | 1.5 | 1.7 | 1.5 |
| Lactate | 1.9 | 2.2 | 2.3 |
| Ethanol | 0.5 | 0.3 | 0.3 |
| Propanol | - | - | - |
| Isobutyrate | 0.3 | 0.2 | 0.2 |
| Isovalerate | 0.5 | 0.5 | 0.5 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Garcia, R.A.; McCubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.K.; Marcellin, E. Microbial propionic acid production. Fermentation 2017, 3, 21. [Google Scholar] [CrossRef]
- Heßlinger, C.; Fairhurst, S.A.; Sawers, G. Novel keto acid formate-lyase and propionate kinase enzymes are components of an anaerobic pathway in Escherichia coli that degrades L-threonine to propionate. Mol. Microbiol. 1998, 27, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Schuchmann, K.; Schmidt, S.; Martinez Lopez, A.; Kaberline, C.; Kuhns, M.; Lorenzen, W.; Bode, H.B.; Joos, F.; Müller, V. Nonacetogenic growth of the acetogen Acetobacterium woodii on 1,2-propanediol. J. Bacteriol. 2015, 197, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Bui, T.P.N.; Stams, A.J.M.; Boeren, S.; Sánchez-Andrea, I.; de Vos, W.M. Comparative genomics and proteomics of Eubacterium maltosivorans: Functional identification of trimethylamine methyltransferases and bacterial microcompartments in a human intestinal bacterium with a versatile lifestyle. Environ. Microbiol. 2022, 24, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Seedorf, H.; Fricke, W.F.; Veith, B.; Brüggemann, H.; Liesegang, H.; Strittmatter, A.; Miethke, M.; Buckel, W.; Hinderberger, J.; Li, F.; et al. The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc. Natl. Acad. Sci. USA 2008, 105, 2128–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitz, A. Über Spaltpilzgährungen. Ber. Dtsch. Chem. Ges. 1878, 11, 1890–1899. [Google Scholar] [CrossRef]
- von Freudenreich, E.; Orla-Jensen, S. Über die in Emmentalerkäse stattfindende Propionsäure-gärung. Zentralbl. Bakteriol. 1906, 17, 529–546. [Google Scholar]
- Sherman, J.M. The cause of eyes and characteristic flavor in emmental or Swiss cheese. J. Bacteriol. 1921, 6, 379–393. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, C.B. The Propionic Acid Bacteria. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 1928. [Google Scholar]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Kieliszek, M.; Ścibisz, I. Propionibacterium spp.—Source of propionic acid, vitamin B12, and other metabolites important for the industry. Appl. Microbiol. Biotechnol. 2018, 102, 515–538. [Google Scholar] [CrossRef] [Green Version]
- Cardon, B.P.; Barker, H.A. Two new amino-acid-fermenting bacteria, Clostridium propionicum and Diplococcus glycinophilus. J. Bacteriol. 1946, 52, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Samain, E.; Albagnac, G.; Dubourguier, H.C.; Touzel, J.P. Characterization of a new propionic acid bacterium that ferments ethanol and displays a growth factor-dependent association with a Gram-negative homoacetogen. FEMS Microbiol. Lett. 1982, 15, 69–74. [Google Scholar] [CrossRef]
- Moreira, J.P.C.; Diender, M.; Arantes, A.L.; Boeren, S.; Stams, A.J.M.; Alves, M.M.; Alves, J.I.; Sousa, D.Z. Propionate production from carbon monoxide by synthetic cocultures of Acetobacterium wieringae and propionigenic bacteria. Appl. Environ. Microbiol. 2021, 87, e02839-20. [Google Scholar] [CrossRef] [PubMed]
- Benito-Vaquerizo, S.; Parera Olm, I.; de Vroet, T.; Schaap, P.J.; Sousa, D.Z.; Martins dos Santos, V.A.P.; Suarez-Diez, M. Genome-scale metabolic modelling enables deciphering ethanol metabolism via the acrylate pathway in the propionate-producer Anaero-tignum neopropionicum. Microb. Cell Factories 2022, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Tholozan, J.L.; Touzel, J.P.; Samain, E.; Grivet, J.P.; Prensier, G.; Albagnac, G. Clostridium neopropionicum sp. nov., a strict anaero-bic bacterium fermenting ethanol to propionate through acrylate pathway. Arch. Microbiol. 1992, 157, 249–257. [Google Scholar] [CrossRef]
- Selmer, T.; Willanzheimer, A.; Hetzel, M. Propionate CoA-transferase from Clostridium propionicum. Cloning of the gene and identification of glutamate 324 at the active site. Eur. J. Biochem. 2002, 269, 372–380. [Google Scholar] [CrossRef]
- Schweiger, G.; Buckel, W. On the dehydration of (R)-lactate in the fermentation of alanine to propionate by Clostridium propioni-cum. FEBS Lett. 1984, 171, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Brunelle, S.L.; Abeles, R.H. The stereochemistry of hydration of acrylyl-CoA catalyzed by lactyl-CoA dehydratase. Bioorg. Chem. 1993, 21, 118–126. [Google Scholar] [CrossRef]
- Hofmeister, A.E.M.; Buckel, W. (R)-Lactyl-CoA dehydratase from Clostridium propionicum. Stereochemistry of the dehydration of (R)-2-hydroxybutyryl-CoA to crotonyl-CoA. Eur. J. Biochem. 1992, 206, 547–552. [Google Scholar] [CrossRef]
- Kuchta, R.D.; Abeles, R.H. Lactate reduction in Clostridium propionicum. J. Biol. Chem. 1985, 260, 13181–13189. [Google Scholar] [CrossRef]
- Hetzel, M.; Brock, M.; Selmer, T.; Pierik, A.J.; Golding, B.T.; Buckel, W. Acryloyl-CoA reductase from Clostridium propionicum. An enzyme complex of propionyl-CoA dehydrogenase and electron-transferring flavoprotein. Eur. J. Biochem. 2003, 270, 902–910. [Google Scholar] [CrossRef]
- Van der Wielen, P.W.J.J.; Rovers, G.M.L.L.; Scheepens, J.M.A.; Biesterveld, S. Clostridium lactatifermentans sp. nov., a lactate-fermenting anaerobe isolated from the caeca of a chicken. Int. J. Syst. Evol. Microbiol. 2002, 52, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Goto, K.; Ohtaki, Y.; Kaku, N.; Ueki, K. Description of Anaerotignum aminivorans gen. nov., sp. nov., a strictly anaerobic, amino-acid-decomposing bacterium isolated from a methanogenic reactor, and reclassification of Clostridium propionicum, Clostridium neopropionicum and Clostridium lactatifermentans as species of the genus Anaerotignum. Int. J. Syst. Evol. Microbiol. 2017, 67, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Kim, J.-S.; Park, J.-E.; Lee, K.C.; Eom, M.K.; Oh, B.S.; Yu, S.Y.; Kang, S.W.; Han, K.-I.; Suh, M.K.; et al. Anaerotignum faecicola sp. nov., isolated from human faeces. J. Microbiol. 2019, 57, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Dávila, C.A.; Zuidema, N.; Buisman, C.J.N.; Strik, D.P.B.T.B. Reactor microbiome enriches vegetable oil with n-caproate and n-caprylate for potential functionalized feed additive production via extractive lactate-based chain-elongation. Biotechnol. Biofuels 2021, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Zhang, M. Constraints in anaerobic microbial dichlorination, fermentation, and sulfate-reduction induced by high concentrations of tetrachloroethylene. Water Air Soil Pollut. 2020, 231, 390. [Google Scholar] [CrossRef]
- Roblin, C.; Chiumento, S.; Jacqueline, C.; Pinloche, E.; Nicoletti, C.; Olleik, H.; Courvoisier-Dezord, E.; Amouric, A.; Basset, C.; Dru, L.; et al. The multifunctional sactipeptide Rumminococcin C1 displays potent antibacterial activity in vivo as well as other beneficial properties for human health. Int. J. Mol. Sci. 2021, 22, 3253. [Google Scholar] [CrossRef]
- Touzel, J.P.; Albagnac, G. Isolation and characterization of Methanococcus mazei strain MC3. FEMS Microbiol. Lett. 1983, 16, 241–245. [Google Scholar] [CrossRef]
- Wolin, E.A.; Wolin, M.J.; Wolfe, R.S. Formation of methane by bacterial extracts. J. Biol. Chem. 1963, 28, 2882–2886. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor: New York, NY, USA, 2012; p. 1100. [Google Scholar]
- Baur, T.; Wentzel, A.; Dürre, P. Production of propionate using metabolically engineered strains of Clostridium saccharoperbutylacetonicum. Appl. Microbiol. Biotechnol. 2022, 106, 7547–7562. [Google Scholar] [CrossRef]
- Blombach, B.; Schreiner, M.E.; Holátko, J.; Bartek, T.; Oldiges, M.; Eikmanns, B.J. L-Valine production with pyruvate dehydrogenase complex-deficient Corynebacterium glutamicum. Appl. Environ. Microbiol. 2007, 73, 2079–2084. [Google Scholar] [CrossRef] [Green Version]
- Baur, T. Expanding the Knowledge on Bacterial Propionate Production by Metabolic Engineering of Solventogenic Clostridia and Characterization of Natural Clostridial Propionate Producers. Ph.D. Thesis, University of Ulm, Ulm, Germany, 2022. [Google Scholar] [CrossRef]
- Jag, V. Characterization of Oerskovia enterophila and Investigation of Its Isophorone Metabolism. Ph.D. Thesis, University of Ulm, Ulm, Germany, 2018. [Google Scholar]
- Cheng, K.-J.; Costerton, J.W. Ultrastructure of Butyrivibrio fibrisolvens: A Gram-positive bacterium? J. Bacteriol. 1977, 129, 1506–1512. [Google Scholar] [CrossRef] [Green Version]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Rohde, M. The Gram-positive bacterial cell wall. Microbiol. Spectrum 2019, 7, GPP3–GPP0044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glauert, A.M.; Thornley, M.J. The topography of the bacterial cell wall. Annu. Rev. Microbiol. 1969, 23, 159–198. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.J. Use of the Gram stain in microbiology. Biotech. Histochem. 2001, 76, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.M.; Messner, P. Improved description of the cell wall architecture of the xylanolytic eubacterium Clostridium xylanolyticum. Int. J. Syst. Bacteriol. 1992, 42, 492–493. [Google Scholar] [CrossRef] [Green Version]
- Warnick, T.A.; Methé, B.A.; Leschine, S.B. Clostridium phytofermentans sp. nov., a cellulolytic mesophile from forest soil. Int. J. Syst. Evol. Microbiol. 2002, 52, 1155–1160. [Google Scholar] [CrossRef]
- Van Gylswyk, N.O.; Morris, E.J.; Els, H.J. Sporulation and cell wall structure of Clostridium polysaccharolyticum comb. nov. (formerly Fusobacterium polysaccharolyticum). J. Gen. Microbiol. 1980, 121, 491–493. [Google Scholar] [CrossRef] [Green Version]
- Freier, D.; Mothershed, C.P.; Wiegel, J. Characterization of Clostridium thermocellum JW20. Appl. Environ. Microbiol. 1988, 54, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Soh, A.L.A.; Ralambotiana, H.; Ollivier, B.; Prensier, G.; Tine, E.; Garcia, J.-L. Clostridium thermopalmarium sp. nov., a moderately thermophilic butyrate-producing bacterium isolated from palm wine in Senegal. Syst. Appl. Microbiol. 1991, 14, 135–139. [Google Scholar] [CrossRef]
- Lemaire, M.; Miras, I.; Gounon, P.; Béguin, P. Identification of a region responsible for binding to the cell wall within the S-layer protein of Clostridium thermocellum. Microbiology 1998, 144, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.-J.; Hironaka, R.; Roberts, D.W.A.; Costerton, J.W. Cytoplasmic glycogen inclusions in cells of anaerobic gram-negative rumen bacteria. Can. J. Microbiol. 1973, 19, 1501–1506. [Google Scholar] [CrossRef]
- McCready, R.G.L.; Costerton, J.W.; Laishley, E.J. Morphological modifications of cells of Clostridium pasteurianum caused by growth on sulfite. Can. J. Microbiol. 1976, 22, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Rehm, B.H.A. Bacterial polymers: Biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010, 8, 578–592. [Google Scholar] [CrossRef]
- Bertsch, J.; Siemund, A.L.; Kremp, F.; Müller, V. A novel route for ethanol oxidation in the acetogenic bacterium Acetobacterium woodii: The acetaldehyde/ethanol dehydrogenase pathway. Environ. Microbiol. 2016, 18, 2913–2922. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.H.; Poehlein, A.; Bengelsdorf, F.R.; Schiel-Bengelsdorf, B.; Daniel, R.; Dürre, P. Draft genome sequence of the strict anaerobe Clostridium neopropionicum X4 (DSM 3847T). Genome Announc. 2016, 4, e00209-16. [Google Scholar] [CrossRef] [Green Version]
- Poehlein, A.; Schlien, K.; Chowdhury, N.P.; Gottschalk, G.; Buckel, W.; Daniel, R. Complete genome sequence of the amino acid-fermenting Clostridium propionicum X2 (DSM 1682). Genome Announc. 2016, 4, e00294-16. [Google Scholar] [CrossRef] [Green Version]
- Buckel, W.; Thauer, R.K. Flavin-based electron bifurcation, a new mechanism of biological energy coupling. Chem. Rev. 2018, 118, 3862–3886. [Google Scholar] [CrossRef] [Green Version]
- Weghoff, M.C.; Bertsch, J.; Müller, V. A novel mode of lactate metabolism in strictly anaerobic bacteria. Environ. Microbiol. 2015, 17, 670–677. [Google Scholar] [CrossRef]
- Poehlein, A.; Krabben, P.; Dürre, P.; Daniel, R. Complete genome sequence of the solvent producer Clostridium saccharoperbutylacetonicum strain DSM 14923. Genome Announc. 2014, 2, e01056-14. [Google Scholar] [CrossRef] [Green Version]
- Poehlein, A.; Montoya Solano, J.D.; Flitsch, S.K.; Krabben, P.; Winzer, K.; Reid, S.J.; Jones, D.T.; Green, E.; Minton, N.P.; Daniel, R.; et al. Microbial solvent formation revisited by comparative genome analysis. Biotechnol. Biofuels 2017, 10, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köpke, M.; Simpson, S.D. Pollution to products: Recycling of ‘above ground’ carbon by gas fermentation. Curr. Opin. Biotechnol. 2020, 65, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.E.; Nogle, R.; Abdalla, T.; Rasor, B.J.; Canter, C.; Jensen, R.O.; Wang, L.; Strutz, J.; Chirania, P.; de Tissera, S.; et al. Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale. Nat. Biotechnol. 2022, 40, 335–344. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Strain | Relevant Characteristics | Origin |
|---|---|---|
| Anaerotignum neopropionicum DSM 3847 | Type strain | DSMZ * GmbH, Brunswick, Germany |
| Anaerotignum propionicum DSM 1682 | Type strain | DSMZ * GmbH, Brunswick, Germany |
| Bacillus subtilis DSM 402 | trp− or ind− | DSMZ * GmbH, Brunswick, Germany |
| Escherichia coli XL1-Blue MRF’ | Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac (F’proAB lacIq ZΔM15 Tn10 (TetR)) | Agilent Technologies, Santa Clara, CA, USA |
| A. propionicum | A. neopropionicum | |||||
|---|---|---|---|---|---|---|
| Ethanol | D-Lactate | L-Alanine | Ethanol | D-Lactate | Xylose | |
| Growth parameters | ||||||
| max. OD600 | 0.3 | 0.3 | 0.7 | 0.3 | 0.2 | 0.7 |
| µ [h−1] | 0.09 | 0.11 | 0.15 | 0.05 | 0.04 | 0.05 |
| tD [h] | 8.0 | 6.6 | 4.7 | 13.1 | 16.3 | 14.2 |
| Product profile [mM] | ||||||
| Acetate | 9.7 | 11.9 | 13.4 | 13.3 | 12.0 | 13.6 |
| Propionate | 6.2 | 20.9 | 23.6 | 16.4 | 14.7 | 17.2 |
| Butyrate | 1.2 | 1.8 | 1.6 | 1.4 | 2.6 | 1.2 |
| Lactate | 7.6 | - | 3.7 | - | - | 5.5 |
| Ethanol | - | 0.4 | 0.2 | - | - | 0.3 |
| Propanol | 3.3 | 0.1 | 0.1 | 0.4 | - | - |
| Isobutyrate | 1.7 | 2.0 | 2.1 | 0.3 | 0.3 | 0.3 |
| Isovalerate | 3.9 | 4.0 | 3.9 | 0.5 | 0.6 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baur, T.; Dürre, P. New Insights into the Physiology of the Propionate Producers Anaerotignum propionicum and Anaerotignum neopropionicum (Formerly Clostridium propionicum and Clostridium neopropionicum). Microorganisms 2023, 11, 685. https://doi.org/10.3390/microorganisms11030685
Baur T, Dürre P. New Insights into the Physiology of the Propionate Producers Anaerotignum propionicum and Anaerotignum neopropionicum (Formerly Clostridium propionicum and Clostridium neopropionicum). Microorganisms. 2023; 11(3):685. https://doi.org/10.3390/microorganisms11030685
Chicago/Turabian StyleBaur, Tina, and Peter Dürre. 2023. "New Insights into the Physiology of the Propionate Producers Anaerotignum propionicum and Anaerotignum neopropionicum (Formerly Clostridium propionicum and Clostridium neopropionicum)" Microorganisms 11, no. 3: 685. https://doi.org/10.3390/microorganisms11030685





