Occurrence and Multidrug Resistance in Strains of Listeria monocytogenes Recovered from the Anaerobic Co-Digestion Sludge Contained in a Single Stage Steel Biodigester: Implications for Antimicrobial Stewardship
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Bacterial Culture for Isolation
2.3. Purification and Preservation of Bacterial Strains/Colonies
2.4. Identification of L. monocytogenes Isolates
2.5. Susceptibility Testing with Conventional Antibiotics
2.6. Calculation of Multiple Antibiotic Resistance (MAR) Index of Bacterial Strains
2.7. Statistical Analysis
3. Results
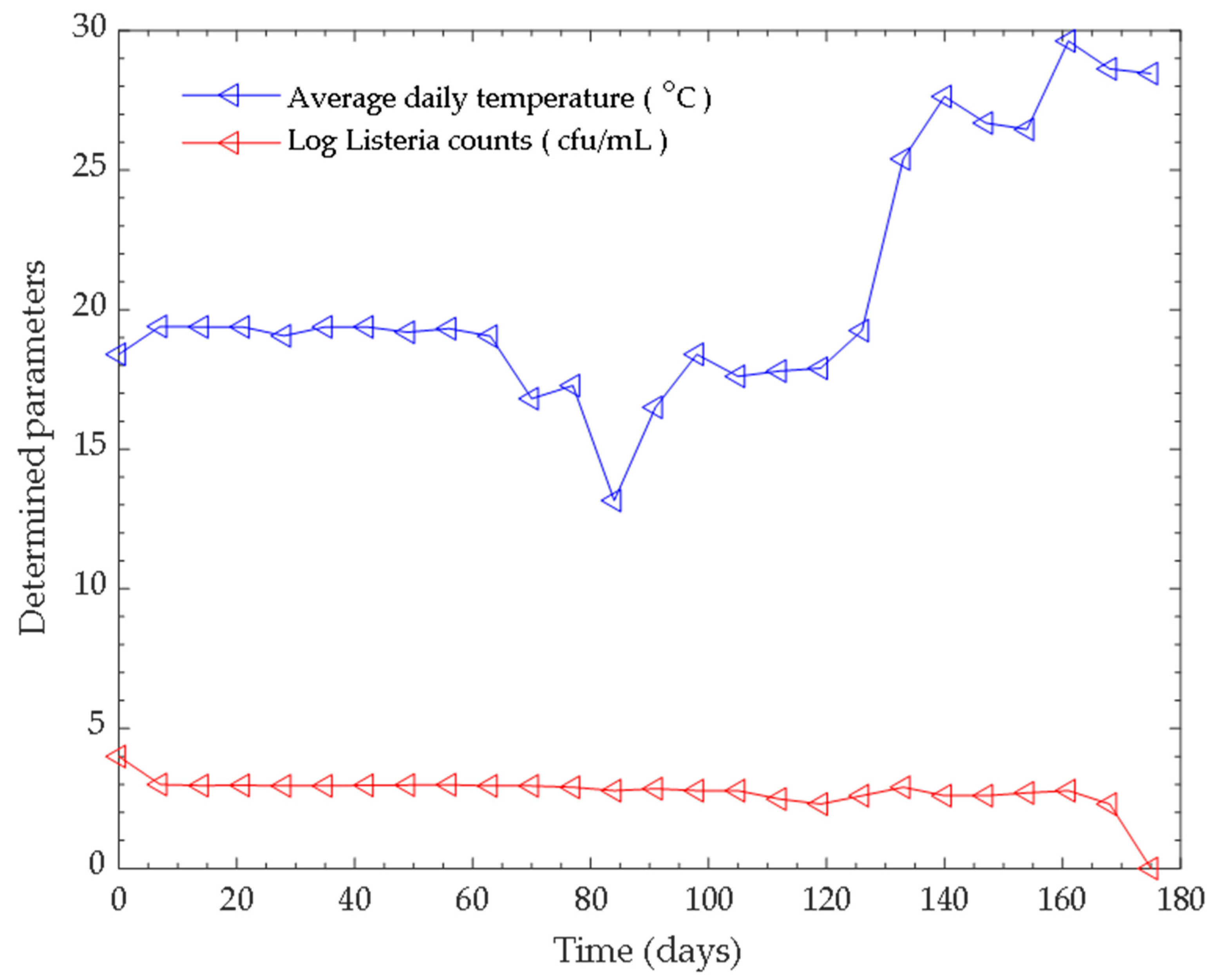
3.1. Monitoring the Parameters (pH and Tempertaure) of the Anaerobic Digestion Process
3.2. Bacterial Counts and Identification
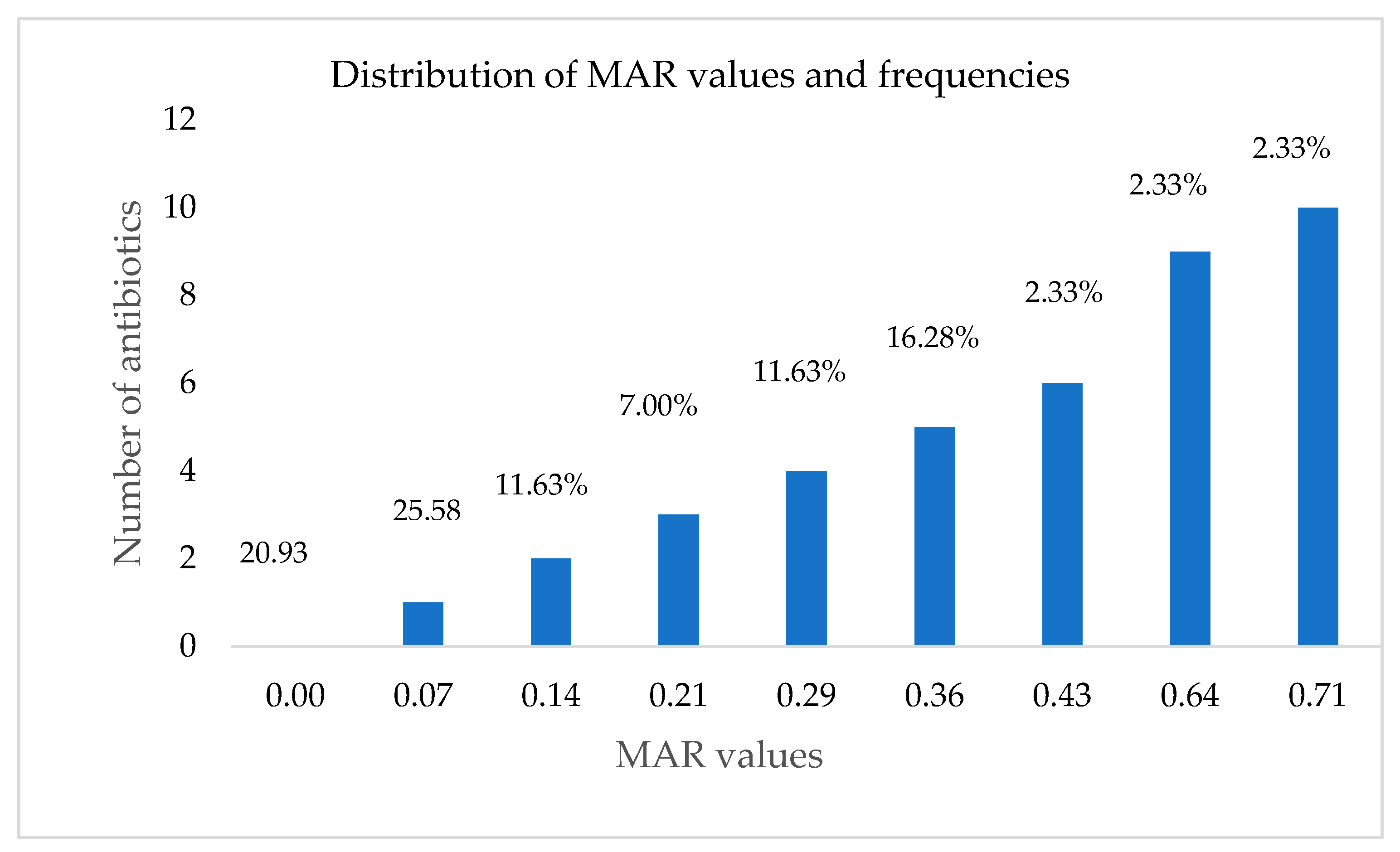
3.3. Antibiotic Susceptibility Testing
4. Discussion
Antimicrobial Stewardship
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Den Bakker, H.C.; Cummings, C.A.; Ferreira, V.; Vatta, P.; Orsi, R.H.; Degoricija, L.; Bakker, M.; Petrauskene, O.; Furtado, M.R.; Wiedmann, M. Comparative genomics of the bacterial genus Listeria: Genome evolution is characterized by limited gene acquisition and limited gene loss. BMC Genom. 2010, 11, 688. [Google Scholar] [CrossRef] [Green Version]
- Raschle, S.; Stephan, R.; Stevens, M.J.A.; Cernela, N.; Zurfuh, K.; Muchaamba, F.; Nüesch-Inderbinen, M. Environmental dissemination of pathogenic Listeria monocytogenes in flowing surface waters in Switzerland. Sci. Rep. 2021, 11, 9066. [Google Scholar] [CrossRef]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to stress conditions encountered in food and food processing environments. Front. Microbiol. 2018, 9, 2700. [Google Scholar] [CrossRef] [Green Version]
- Vivant, A.-L.; Desneux, J.; Pourcher, A.-M.; Pascal Piveteau, P. Transcriptomic Analysis of the Adaptation of Listeria monocytogenes to Lagoon and Soil Matrices Associated with a Piggery Environment: Comparison of Expression Profiles. Front. Microbiol. 2017, 8, 1811. [Google Scholar] [CrossRef]
- Pourcher, A.M.; Ziebal, C.; Kervarrec, M.; Bioteau, T.; Dabert, P. Sanitary status of 44 hog manures in brittany: Comparaison of the effectiveness of manure treatments based on the levels of indicator bacteria and two pathogenic bacteria. J. Agric. Sci. Technol. A 2012, 2, 303–313. [Google Scholar]
- Desneux, J.; Biscuit, A.; Picard, S.; Pourcher, A.-M. Fate of Viable but Non-culturable Listeria monocytogenes in Pig Manure Microcosms. Front. Microbiol. 2016, 7, 245. [Google Scholar] [CrossRef] [Green Version]
- Parussolo, L.; Sfaciotte, R.A.P.; Dalmina, K.A.; Melo, F.D.; da Costa, U.M.; Ferraz, S.M. Detection of virulence genes and antimicrobial susceptibility profile of Listeria monocytogenes isolates recovered from artisanal cheese produced in the Southern region of Brazil. Ann. Braz. Acad. Sci. 2021, 93, e20190200. [Google Scholar] [CrossRef]
- Pagliano, P.; Arslan, F.; Ascione, T. Epidemiology and treatment of the commonest form of listeriosis: Meningitis and bacteraemia. Infez. Med. 2017, 25, 210–216. [Google Scholar]
- Morvan, A.; Moubareck, C.; Leclercq, A.; Herv_e-Bazin, M.; Bremont, S.; Lecuit, M.; Courvalin, P.; Le Monnier, A. Antimicrobial resistance of Listeria monocytogenes strains isolated from humans in France. Antimicrob. Agents Chemother. 2010, 54, 2728–2731. [Google Scholar] [CrossRef] [Green Version]
- Andriyanov, P.A.; Zhurilov, P.A.; Liskova, E.A.; Karpova, T.I.; Sokolova, E.V.; Yushina, Y.K.; Zaiko, E.V.; Bataeva, D.S.; Voronina, O.L.; Psareva, E.K.; et al. Antimicrobial Resistance of Listeria monocytogenes Strains Isolated from Humans, Animals, and Food Products in Russia in 1950–1980, 2000–2005, and 2018–2021. Antibiotics 2021, 10, 1206. [Google Scholar] [CrossRef]
- [WHO] World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. 2014. Available online: http://www.who.int/drugresistance/documents/surveillancereport/en (accessed on 26 October 2016).
- Gomez, D.; Iguacel, L.P.; Rota, M.C.; Carramiñana, J.J.; Ariño, A.; Yangüela, J. Occurrence of Listeria monocytogenes in ready-to-eat meat products and meat processing plants in Spain. Foods 2015, 4, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Moreno, L.Z.; Paixao, R.; Gobbi, D.S.; Raimundo, D.C.; Ferreira, T.P.; Moreno, A.M.; Hofer, E.; Reis, C.M.F.; Matte´, G.R.; Matte´, M.H. Characterization of antibiotic resistance in Listeria spp. isolated from slaughterhouse environments, pork and human infections. J. Infect. Dev. Ctries 2014, 8, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Şanlibaba, P.; Uymaz tezel, B.; Çakmak, G.A.; Keskin, R.; Akçelik, M. Occurrence of listeria spp. and antibiotic resistance profiles of listeria monocytogenes from raw meat at retail in turkey. Italy J. Food Sci. 2020, 32, 234–250. [Google Scholar]
- Escolar, C.; Diego Gomez, D.; Garcıa, M.D.C.R.; Conchello, P.; Herrera, A. Antimicrobial resistance profiles of Listeria monocytogenes and Listeria innocua Isolated from Ready-to-Eat Products of Animal Origin in Spain. Foodborne Pathog. Dis. 2017, 14, 357–363. [Google Scholar] [CrossRef]
- Lakicevic, B.Z.; Den Besten, H.M.W.; De Biase, D. Landscape of stress response and virulence genes among Listeria monocytogenes strains. Front. Microbiol. 2022, 12, 738470. [Google Scholar] [CrossRef]
- Wi´sniewski, P.; Zakrzewski, A.J.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Antimicrobial Resistance and Virulence Characterization of Listeria monocytogenes Strains Isolated from Food and Food Processing Environments. Pathogens 2022, 11, 1099. [Google Scholar] [CrossRef]
- Muchaamba, F.; Eshwar, A.K.; Stevens, M.J.A.; Stephan, R.; Tasara, T. Different Shades of Listeria monocytogenes: Strain, Serotype, and Lineage-Based Variability in Virulence and Stress Tolerance Profiles. Front. Microbiol. 2022, 12, 792162. [Google Scholar] [CrossRef]
- Bilung, L.M.; Chai, L.S.; Tahar, A.S.; Ted, C.K.; Apun, K. Prevalence, Genetic Heterogeneity, and Antibiotic Resistance Profile of Listeria spp. And Listeria monocytogenes at Farm Level: A Highlight of ERIC- and BOX-PCR to Reveal Genetic Diversity. BioMed Res. Int. 2018, 2018, 3067494. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority). Analysis of the baseline survey on the prevalence of Listeria monocytogenesin certain ready-to-eat foods in the EU, 2010–2011 Part A: Listeria monocytogenes prevalence estimates. EFSA J. 2013, 11, 3241. [Google Scholar] [CrossRef]
- Samtiya, M.; Matthews, K.R.; Dhewa, T.; Puniya, A.K. Antimicrobial resistance in the food Chain: Trends, mecha-nisms, pathways, and possible regulation strategies. Foods 2022, 11, 2966. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Okoh, A.I.; Lues, R. Prevalence of multidrug-resistant bacteria (Enteropathogens) recovered from a blend of pig manure and pinewood saw dust during anaerobic co-digestion in a steel biodigester. Int. J. Environ. Res. Public Health 2023, 20, 984. [Google Scholar] [CrossRef]
- Baloyi, T.; Duvenage, S.; Du Plessis, E.; Villamizar- Rodríguez, G.; Korsten, L. Multidrug resistant Escherichia coli from fresh produce sold by street vendors in South African informal settlements. Int. J. Environ. Health Res. 2021, 32, 1513–1528. [Google Scholar] [CrossRef]
- Mthembu, T.P.; Zishiri, O.T.; El Zowalaty, M.E. Molecular detection of multidrug-resistant Salmonella Iso-lated from livestock production. Infect. Drug Resist. 2019, 12, 3537–3548. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, S.E.; Abia, A.L.K.; Amoako, D.G.; Perrett, K.; Bester, L.A.; Essack, S.Y. Food animals as reservoirs and potential sources of multidrug-resistant diarrheagenic E. coli pathotypes: Focus on intensive pig farming in South Africa. Onderstepoort J. Vet Res. 2022, 89, a1963. [Google Scholar] [CrossRef]
- Sineke, N.; Asante, J.; Amoako, D.G.; Abia, A.L.K.; Perrett, K.; Bester, L.A.; Essack, S.Y. Staphylococcus aureus in intensive pig production in South Africa: Antibiotic resistance, virulence determinants, and clonality. Pathogens 2021, 10, 317. [Google Scholar] [CrossRef]
- Ayandel, A.A.; Oladipo, E.K.; Oyebisi, O.; Kaka, M.O. Prevalence of Multi-Antibiotic Resistant Escherichia coli and Klebsiella species obtained from a Tertiary Medical Institution in Oyo State, Nigeria. Qatar Med. J. 2020, 2020, 9. [Google Scholar] [CrossRef]
- Keet, R.; Rip, D. Listeria monocytogenes isolates from Western Cape, South Africa exhibit resistance to multiple antibiotics and contradicts certain global resistance patterns. AIMS Microbiol. 2021, 7, 40–58. [Google Scholar] [CrossRef]
- Riaz, S.; Faisal, M.; Hasnain, S. Antibiotic susceptibility pattern and multiple antibiotic resistances (MAR) calculation of extended spectrum βlactamase (ESBL) producing Escherichia coli and Klebsiella species in Pakistan. Afr. J. Biotechnol. 2011, 10, 6325–6331. [Google Scholar]
- Rajput, A.A.; Zeshan Sheikh, Z. Effect of inoculum type and organic loading on biogas production of sunflower meal and wheat straw. Sustain. Environ. Res. 2019, 29, 4. [Google Scholar] [CrossRef] [Green Version]
- Manyi-Loh, C.; Lues, R. Reduction in bacterial pathogens in a single-stage steel biodigester co-digesting saw dust and pig manure at psychrophilic temperature. Appl. Sci. 2022, 12, 10071. [Google Scholar] [CrossRef]
- Singh, B.; Szamosi, Z.; Siménfalvi, Z. Impact of mixing intensity and duration on biogas production in an anaerobic digester: A review. Crit. Rev. Biotechnol. 2020, 40, 508–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaker, H.C.; Brahmbhatt, M.N.; Nayak, J.B. Study on occurrence and antibiogram pattern of Escherichia coli from raw milk samples in Anand, Gujarat, India. Vet. World 2012, 5, 556–559. [Google Scholar] [CrossRef]
- Rowbotham, R.F.; Ruegg, P.L. Bacterial counts on teat skin and in new sand, recycled sand, and recycled manure solids used as bedding in freestalls. J. Dairy Sci. 2016, 99, 6594–6608. [Google Scholar] [CrossRef]
- Dunka, H.I.; Bello, M.; Lawan, M.K. Prevalence and Antibiogram of Listeria Monocytogenes Contamination of Liver, Spleen, Ruminal Content and Effluent in Jos, Nigeria. J. Vet.Med. Animal Sci. 2021, 4, 1072. [Google Scholar]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Characterisation and antibiotic resistance of selected bacterial pathogens recovered from dairy cattle manure during anaerobic mono-digestion in a balloon-type digester. Appl. Sci. 2018, 8, 2088. [Google Scholar] [CrossRef] [Green Version]
- Cheesbrough, M. District Laboratory Practice in Tropical Country, Part 2: Microbiology; Cambridge University Press: Cambridge, UK, 2000; pp. 234–280. ISBN 9780521676311. [Google Scholar]
- Sanlıbaba, P.; Tezel, B.U.; Çakmak, G.A. Prevalence and Antibiotic Resistance of Listeria monocytogenes isolated from ready-to-eat foods in Turkey. Hindawi J. Food Qual. 2018, 2018, 7693782. [Google Scholar] [CrossRef] [Green Version]
- Clinical Laboratory Standard Institute. M100. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Du, X.J.; Zhang, X.; Wang, X.Y.; Su, Y.L.; Li, P.; Wang, S. Isolation and characterization of Listeria monocytogenes in Chinese food obtained from the central area of China. Food Control 2017, 74, 9–16. [Google Scholar] [CrossRef]
- Joseph, A.A.; Odimayo, M.S.; Olokoba, L.; Olokoba, A.B.; Popoola, G.O. Multiple antibiotic resistance index of Escherichia coli isolates in a tertiary hospital in South-West Nigeria. Med. J. Zamb. 2017, 44, 225–232. [Google Scholar]
- Mitchell, E. Gram Positive and Gram Negative Bacteria and Fight against HAIs. 2020, Health.Care/Educational Block. Available online: https://blog.eoscu.com/blog/gram-positive-vs-gram-positive (accessed on 28 February 2023).
- Jiang, Y.; Xie, S.H.; Dennehy, C.; Lawlor, P.G.; Hu, Z.H.; Wu, G.X.; Zhan, X.M.; Gardiner, G.E. Inactivation of pathogens in anaerobic digestion systems for converting biowastes to bioenergy: A review. Renew. Sustain. Energy Rev. 2020, 120, 109654. [Google Scholar] [CrossRef]
- Lin, M.; Wang, A.; Ren, L.; Qiao, W.; Wandera, S.M.; Dong, R. Challenges of pathogen inactivation in animal manure through anaerobic digestion: A short review. Bioengineered 2022, 13, 1149–1161. [Google Scholar] [CrossRef]
- Bhajani, S.S.; Pal, S.L. Review: Factors affecting biogas production. Int. J. Res. Appl. Sci. Eng. Technol. 2022, 10, 79–85. [Google Scholar] [CrossRef]
- Jin, Q.; Kirk, M.F. pH as a primary control in environmental microbiology: 1. Thermodynamic Perspective. Front. Environ. Sci. 2018, 6, 21. [Google Scholar] [CrossRef]
- Saraswat, M.; Garg, M.; Bhardwaj, M.; Mehrotra, M.; Singhal, R. Impact of variables affecting biogas production from biomass. IOP Conf. Ser. Mater. Sci. Eng. 2019, 691, 012043. [Google Scholar] [CrossRef] [Green Version]
- Tessema, G.T.; Møretrø, T.; Snipen, L.; Heir, E.; Holck, A.; Naterstad, K.; Axelsson, L. Microarray-based transcriptome of Listeria monocytogenes adapted to sublethal concentrations of acetic acid, lactic acid, and hydrochloric acid. Can. J. Microbiol. 2012, 58, 1112–1123. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, e177–e208. [Google Scholar] [CrossRef] [PubMed]
- Lund, P.A.; De Biase, D.; Liran, O.; Scheler, O.; Mira, N.P.; Cetecioglu, Z.; Noriega Fernández, E.; Bover-Cid, S.; Hall, R.; Sauer, M.; et al. Understanding how microorganisms respond to acid pH Is central to their control and successful exploitation. Front. Microbiol. 2020, 11, 556140. [Google Scholar] [CrossRef]
- Ratzke, C.; Gore, J. Modifying and reacting to the environmental pH can drive bacterial interactions. PLoS Biol. 2018, 16, e2004248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosso, L.; Lobry, J.R.; Bajard, S.; Flandrois, J.P. Convenient model to describe the combined effects of temperature and pH on microbial growth. Appl. Environ. Microbiol. 1995, 61, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.L.; Liu, Y.; Paoli, G.C. How does Listeria monocytogenes combat acid conditions? Can. J. Microbiol. 2013, 59, 141–152. [Google Scholar] [CrossRef]
- Shamloo, E.; Abdimoghadam, Z.; Nazari, K.; Hosseini, S.M.; Hosseini, H.; Alebouyeh, M. Long term survival of Listeria monocytogenes in stress conditions: High pH and salt concentrations. J. Res. Med. Dental Sci. 2018, 6, 96–100. [Google Scholar]
- Liu, X.; Lendormi, T.; Dong, P.; Lanoisellé, J.-L. Conventional and innovative hygienisation of feedstock for biogas production: Resistance of indicator bacteria to thermal pasteurisation, pulsed and electric field treatment and anaerobic digestion. Energies 2021, 14, 1938. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Ma, R.; Kumar, V.; Tong, Y.W.; He, Y.; Mao, F. Mesophilic and thermophilic anaerobic digestion of animal manure: Integrated insights from biogas productivity. Microbial viability and enzymatic activity. Fuel 2022, 320, 123990. [Google Scholar] [CrossRef]
- Akindolire, M.A.; Rama, H.; Roopnarain, A. Psychrophilic anaerobic digestion: A critical evaluation of microorganisms and enzymes to drive the process. Renew. Sustain. Energy Rev. 2022, 161, 112394. [Google Scholar] [CrossRef]
- Gaby, J.C.; Zamanzadeh, M.; Horn, S.J. The effect of temperature and retention time on methane production and microbial community composition in staged anaerobic digesters fed with food waste. Biotechnol. Biofuels. 2017, 10, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Jain, V.K.; Singh, A. Effect of Temperature and other factors on anaerobic digestion process, responsible for Biogas production. Int. J. Theor. Appl. Mech. 2017, 12, 637–657. [Google Scholar]
- Noll, P.; Lilge, L.; Hausmann, R.; Marius Henkel, M. Modeling and exploiting microbial temperature response. Processes 2020, 8, 121. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; He, S.; Kang, X.; Sun, Y.; Yuan, Z.; Xing, T.; Guo, Y.; Li, L. Effect of organic loading rate and temperature on the anaerobic digestion of municipal solid wastes: Process performance and energy recovery. Front. Energy Res. 2020, 8, 89. [Google Scholar] [CrossRef]
- Mateos-Rivera, A.; Yde, J.C.; Wilson, B.; Finster, K.W. The effect of temperature change on the icrobial diversity and communitystructure along the chronosequence of the sub-artic glacier forefield of Styggedalsbreen (Norway). FEMS Microbiol. Ecol. 2019, 92, fiw038. [Google Scholar] [CrossRef] [Green Version]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes—How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef]
- Shourav, A.H.; Hasan, M.; Ahmed, S. Antibiotic susceptibility pattern of Listeria spp. isolated from cattle farm environment in Bangladesh. J. Agric Food Res. 2020, 2, 100082. [Google Scholar] [CrossRef]
- Londero, A.; Costa, M.; Galli, L.; Brusa, V.; Linares, L.; Prieto, M.; Leotta, G. Characterization and subtyping of Listeria monocytogenes strains from butcher shops. LWT Food Sci. Technol. 2019, 113, 108363. [Google Scholar] [CrossRef]
- Govender, L.; Pillay, K.; Siwela, M.; Modi, A.T.; Mabhaudhi, T. Assessment of the Nutritional Status of Four Selected Rural Communities in KwaZulu-Natal, South Africa. Nutrients 2021, 13, 2920. [Google Scholar] [CrossRef]
- Milford, A.B.; Le Mouël, C.; Bodirsky, B.L.; Rolinski, S. Drivers of meat consumption. Appetite 2019, 141, 104313. [Google Scholar] [CrossRef] [PubMed]
- Achoki, T.; Sartorius, B.; Watkins, D.; Glenn, S.D.; Kengne, A.P.; Oni, T.; Wiysonge, C.S.; Walker, A.; Adetokunboh, O.O.; Babalola, T.K.; et al. Health trends, inequalities and opportunities in South Africa’s provinces, 1990–2019: Findings from the Global Burden of Disease 2019 Study. J. Epidemiol. Community Health 2022, 76, 471–481. [Google Scholar] [CrossRef]
- Narsai, K.; Leufkens, H.G.M.; Mantel-Teeuwisse, A.K. Linking market authorizations of medicines with disease burden in South Africa. J. Pharm Policy Pract. 2021, 14, 33. [Google Scholar] [CrossRef]
- Fish, D.N.; Ohlinger, M.J. Antimicrobial Resistance: Factors and Outcomes. Crit. Care Clin. 2006, 22, 291–311. [Google Scholar] [CrossRef] [PubMed]
- Geta, K.; Kibret, M. Knowledge, attitudes and practices of animal farm owners/workers on antibiotic use and resistance in Amhara region, northwestern Ethiopia. Sci. Rep. 2021, 11, 21211. [Google Scholar] [CrossRef] [PubMed]
- Noll, M.; Kleta, S.; Al Dahouk, S. Antibiotic susceptibility of 259 Listeria monocytogenes strains isolated from food, food-processing plants and human samples in Germany. J. Infect. Public Health 2018, 11, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Acciari, V.A.; Ruolo, A.; Torresi, M.; Ricci, L.; Pompei, A.; Marfoglia, C.; Valente, F.M.; Centorotol, G.; Conte, A.; Salini, R.; et al. Genetic diversity of Listeria monocytogenes strains contaminating food and food producing environment as single based sample in Italy (retrospective study). Int. J. Food Microbiol. 2022, 366, 10956210.53. [Google Scholar] [CrossRef]
- Troxler, R.; Von Graevenitz, A.; Funke, G.; Wiedemann, B.; Stock, I. Natural antibiotic susceptibility of Listeria species: L. grayi, L. innocua, L. ivanovii, L. monocytogenes, L. seeligeri and L. welshimeri strains. Clin. Microbiol. Infect. 2000, 6, 525–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Nabulsi, A.A.; Osaili, T.M.; Shaker, R.R.; Olaimat, A.N.; Jaradat, Z.W.; Elabedeen, N.A.Z.; Holley, R.A. Effects of osmotic pressure, acid, or cold stresses on antibiotic susceptibility of Listeria monocytogenes. Food Microbiol. 2015, 46, 154–160. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram- negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Azim, A.; Gurjar, M.; Baronia, A.K. Current concepts in combination antibiotic therapy for critically ill patients. Indian J. Crit. Care Med. 2014, 18, 310–314. [Google Scholar]
- Angst, D.C.; Tepekulea, B.; Suna, L.; Bogosa, B.; Bonhoeffera, S. Comparing treatment strategies to reduce antibiotic resistance in an in vitro epidemiological setting. Proc. Natl. Acad. Sci. USA 2021, 13, e2023467118. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Luo, J.; Deng, F.; Huang, Y.; Zhou, H. Antibiotic Combination Therapy: A Strategy to Overcome Bacterial Resistance to Aminoglycoside Antibiotics. Front. Pharmacol. 2022, 13, 839808. [Google Scholar] [CrossRef]
- Hombach, B.; Böttger, E.C.; Roos, M. The critical influence of the intermediate category on interpretation errors in revised EUCAST and CLSI antimicrobial susceptibility testing guidelines. Clin. Microbiol. Infect. 2013, 19, E59–E71. [Google Scholar] [CrossRef] [Green Version]
- Kahlmeter, G. On behalf of the EUCAST Steering Committee (EUCAST proposes to change the definition and usefulness of the susceptibility category ‘Intermediate’. Clin. Microbiol. Infect. 2017, 23, 894–895. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, J.F.; Ferraro, M.J. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef]
- Iwu, C.D.; Okoh, A.I. Characterisation of antibiogram fingerprints in Listeria moncytogenes recovered from irrigation water and agricultural soil. PLoS ONE 2020, 15, e0228956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayode, A.J.; Semerjian, L.; Osaili, T.; Olapade, O.; Okoh, A.I. Occurrence of Multidrug-Resistant Listeria monocytogenes in Environmental Waters: A Menace of Environmental and Public Health Concern. Front. Environ. Sci. 2020, 12, 737435. [Google Scholar] [CrossRef]
- Mpondo, L.; Ebomah, K.E.; Okoh, A.I. Multidrug-resistant listeria Species Shows Abundance in Environmental Waters of a Key District Municipality in South Africa. Ijerph 2021, 18, 481. [Google Scholar] [CrossRef]
- Klibi, N.; Said, L.B.; Jouini, A.; Slama, K.B.; Lopez, M.; Sallem, R.B.; Boudabous, A.; Torres, C. Species distribution, antibiotic resistance and virulence traits in enterococci from meat in Tunisia. Meat Sci. 2013, 93, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F. Sustainable farming: Get pigs off antibiotics. Nature 2012, 486, 465–466. [Google Scholar] [CrossRef]
- Kimera, Z.I.; Shana, S.E.; Rweyemamu, M.M.; Mboera, L.E.G.; Mecky, I.N.; Matee, M.I.N. Antimicrobial use and resistance in food producing animals and the environment: An African perspective. Antimicrob. Resist. Infect. Control 2020, 9, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selaledi, L.A.; Hassan, Z.M.; Manyelo, T.G.; Mabelebele, M. The Current Status of the Alternative Use to Antibiotics in Poultry Production: An African Perspective. Antibiotics 2020, 9, 594. [Google Scholar] [CrossRef]
- Van, T.T.H.; Yidana, Z.; Smooker, P.M.; Coloe, P.J. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J. Global Antimicrob. Resist. 2020, 20, 170–177. [Google Scholar] [CrossRef]
- Bertsch, D.; Muelli, M.; Weller, M.; Uruty, A.; Lacroix, C.; Meile, L. Antimicrobial susceptibility and antibiotic resistance gene transfer analysis of foodborne, clinical, and environmental Listeria spp. isolates including Listeria monocytogenes. MicrobiologyOpen 2014, 3, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Quaik, S.; Embrandiri, A.; Ravindran, B.; Hossain, K.; Al-Dhabi, N.A.; Arasu, M.V.; Ignacimuthu, S.; Ismail, N. Veterinary antibiotics in animal manure and manure laden soil: Scenario and challenges in Asian countries. J. King Saud Univer. Sci. 2020, 32, 1300–1305. [Google Scholar] [CrossRef]
- Agga, G.E.; Couch, M.; Parekh, R.R.; Mahmoudi, F.; Appala, K.; Kasumba, J.; Loughrin, J.H.; Conte, E.D. Lagoon, anaerobic digestion, and composting of animal manure treatments impact on tetracycline resistance genes. Antibiotics 2022, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, A.; Al Mutair, A.; Alhumaid, S.; Salih, S.; Alanazi, A.; Albarsan, H.; Abourayan, M.; Al Subaie, M. The impact of antimicrobial stewardship program implementation at four tertiary private hospitals: Results of a five-years prepost analysis. Antimicrob. Resist. Infect. Control 2020, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-J.; Chen, S.-J.; Hu, Y.-W.; Liu, C.-Y.; Wu, P.-F.; Sun, S.-M.; Lee, S.-Y.; Chen, Y.-Y.; Lee, C.-Y.; Chan, Y.-J.; et al. The impact of antimicrobial stewardship program designed to shorten antibiotics use on the incidence of resistant bacterial infections and mortality. Sci. Rep. 2022, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Lekagul, A.; Tangcharoensathien, V.; Mills, A.; Rushton, J.; Yeung, S. How antibiotics are used in pig farming: A mixed-methods study of pig farmers, feed mills and veterinarians in Thailand. BMJ Glob. Health 2020, 5, e001918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Alzahrani, A.J.; Tobaiqy, M.; Alresasi, A.M.; Bu-Shehab, I.; Al-Hadary, I.; Alhmeed, N.; Alismail, M.; et al. Antimicrobial susceptibility of gram-positive and gram-negative bacteria: A 5-year retrospective analysis at a multi-hospital healthcare system in Saudi Arabia. Clin. Microbiol. Antimicrob. 2021, 20, 43. [Google Scholar] [CrossRef] [PubMed]

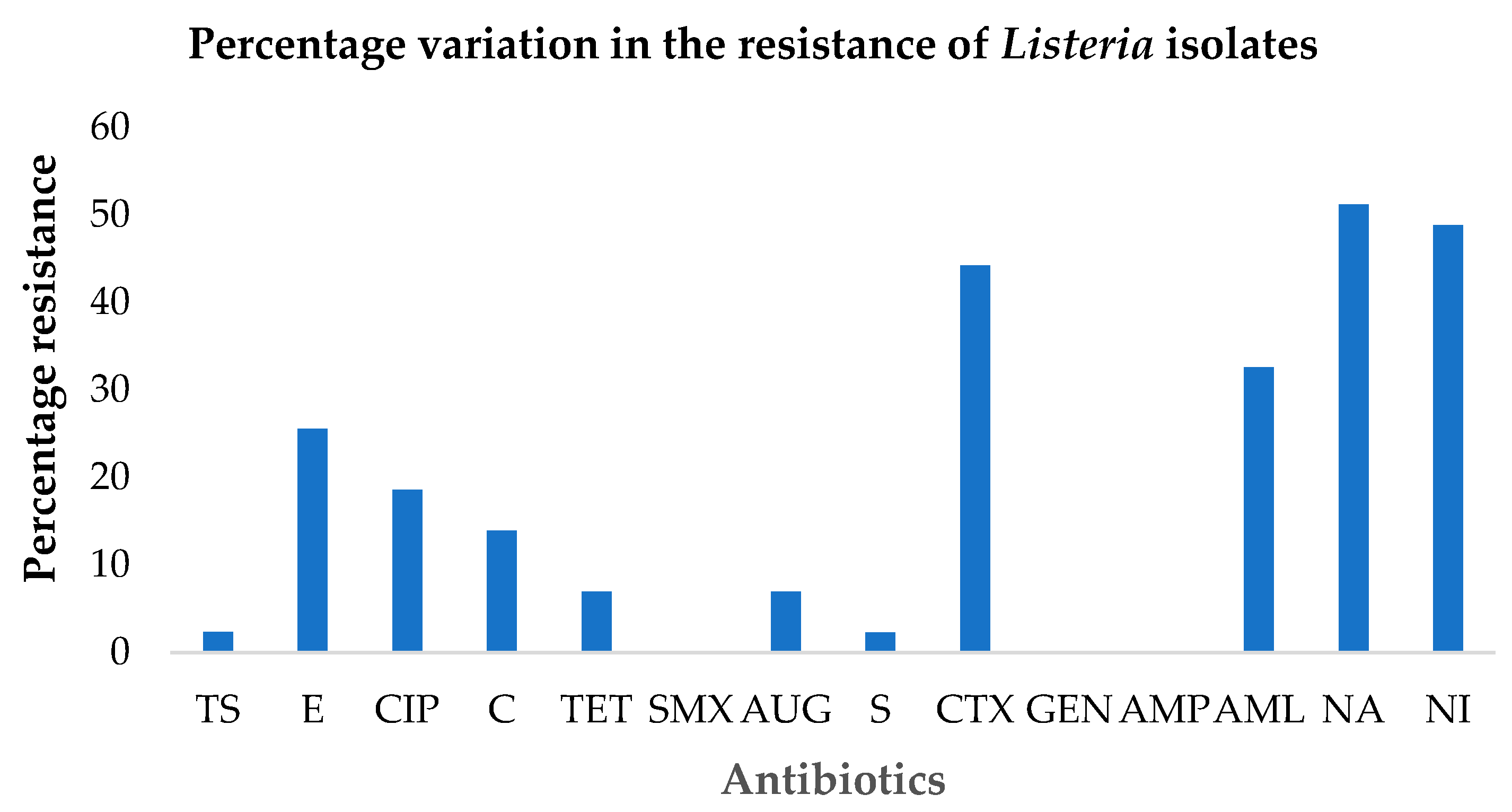
| Antibiotic Classes | Antibiotic Agents a | Percentage Susceptibility of L. monocytogenes (%) b | ||
|---|---|---|---|---|
| Susceptible (S) | Intermediate (I) | Resistance (R) | ||
| Aminoglycosides | Gentamicin (GEN) | 100 | 0 | 0 |
| Streptomycin (S) c | 90.70 | 6.98 | 2.33 | |
| Amphenicols | Chloramphenicol (C) | 74.42 | 11.63 | 13.95 |
| Penicillins | Ampicillin (AMP) | 100 | - d | 0 |
| Amoxicillin (AML) | 67.44 | 0 | 32.56 | |
| Cephalosporins | Cefotaxime (CTX) | 30.23 | 25.58 | 44.19 |
| Macrolides | Erythromycin (E) | 67.44 | 4.65 | 25.58 |
| Polypeptides Quinolones | Nalidixic acid (NA) c | 41.86 | 6.98 | 51.16 |
| Ciprofloxacin (CIP) | 58.14 | 23.26 | 18.60 | |
| Sulfonamides | Sulfamethoxazole (SMX) | 100 | 0 | 0 |
| Tetracyclines | Tetracycline (TET) | 79.07 | 13.95 | 6.98 |
| Nitrofurans | Nitrofurantoin (NI) | 51.16 | 0 | 48.84 |
| Combinations | Augmentin (AUG) e | 93.02 | 0 | 6.98 |
| Co-trimoxazole (COT) e | 97.67 | 0 | 2.33 | |
| Number of Antibiotics | Number of Resistant Isolates | MAR Phenotypes |
|---|---|---|
| 2 | 3 | NA, NI |
| 1 | E, C | |
| 1 | NA, E | |
| 3 | 2 | NA, NI. AML |
| 1 | NA, NI, CTX | |
| 4 | 1 | NA, NI, AML, E |
| 1 | NA, NI, E, CIP | |
| 1 | NA, NI, AML, CIP | |
| 1 | NA, NI, E, C | |
| 1 | NA, NI, AML, CTX | |
| 5 | 2 | NA, NI, AML, CTX, TET |
| 2 | NA, NI, AML, CTX, CIP | |
| 1 | NA, NI, CTX, CIP, E | |
| 1 | NA, NI AML, CTX, E | |
| 1 | NA, NI, CIP, E, C | |
| 6 | 1 | NA, NI, CTX, E, C, AUG |
| 9 | 1 | NA, NI, AML, CTX, CIP, COT, E, C |
| 10 | 1 | NA, NI, AML, CTX, CIP, TET, AUG, E, C, S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manyi-Loh, C.E.; Okoh, A.I.; Lues, R. Occurrence and Multidrug Resistance in Strains of Listeria monocytogenes Recovered from the Anaerobic Co-Digestion Sludge Contained in a Single Stage Steel Biodigester: Implications for Antimicrobial Stewardship. Microorganisms 2023, 11, 725. https://doi.org/10.3390/microorganisms11030725
Manyi-Loh CE, Okoh AI, Lues R. Occurrence and Multidrug Resistance in Strains of Listeria monocytogenes Recovered from the Anaerobic Co-Digestion Sludge Contained in a Single Stage Steel Biodigester: Implications for Antimicrobial Stewardship. Microorganisms. 2023; 11(3):725. https://doi.org/10.3390/microorganisms11030725
Chicago/Turabian StyleManyi-Loh, Christy Echakachi, Anthony Ifeanyin Okoh, and Ryk Lues. 2023. "Occurrence and Multidrug Resistance in Strains of Listeria monocytogenes Recovered from the Anaerobic Co-Digestion Sludge Contained in a Single Stage Steel Biodigester: Implications for Antimicrobial Stewardship" Microorganisms 11, no. 3: 725. https://doi.org/10.3390/microorganisms11030725
APA StyleManyi-Loh, C. E., Okoh, A. I., & Lues, R. (2023). Occurrence and Multidrug Resistance in Strains of Listeria monocytogenes Recovered from the Anaerobic Co-Digestion Sludge Contained in a Single Stage Steel Biodigester: Implications for Antimicrobial Stewardship. Microorganisms, 11(3), 725. https://doi.org/10.3390/microorganisms11030725






