Diversity of Bacteria with Quorum Sensing and Quenching Activities from Hydrothermal Vents in the Okinawa Trough
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Bacterial Strains from the Okinawa Trough
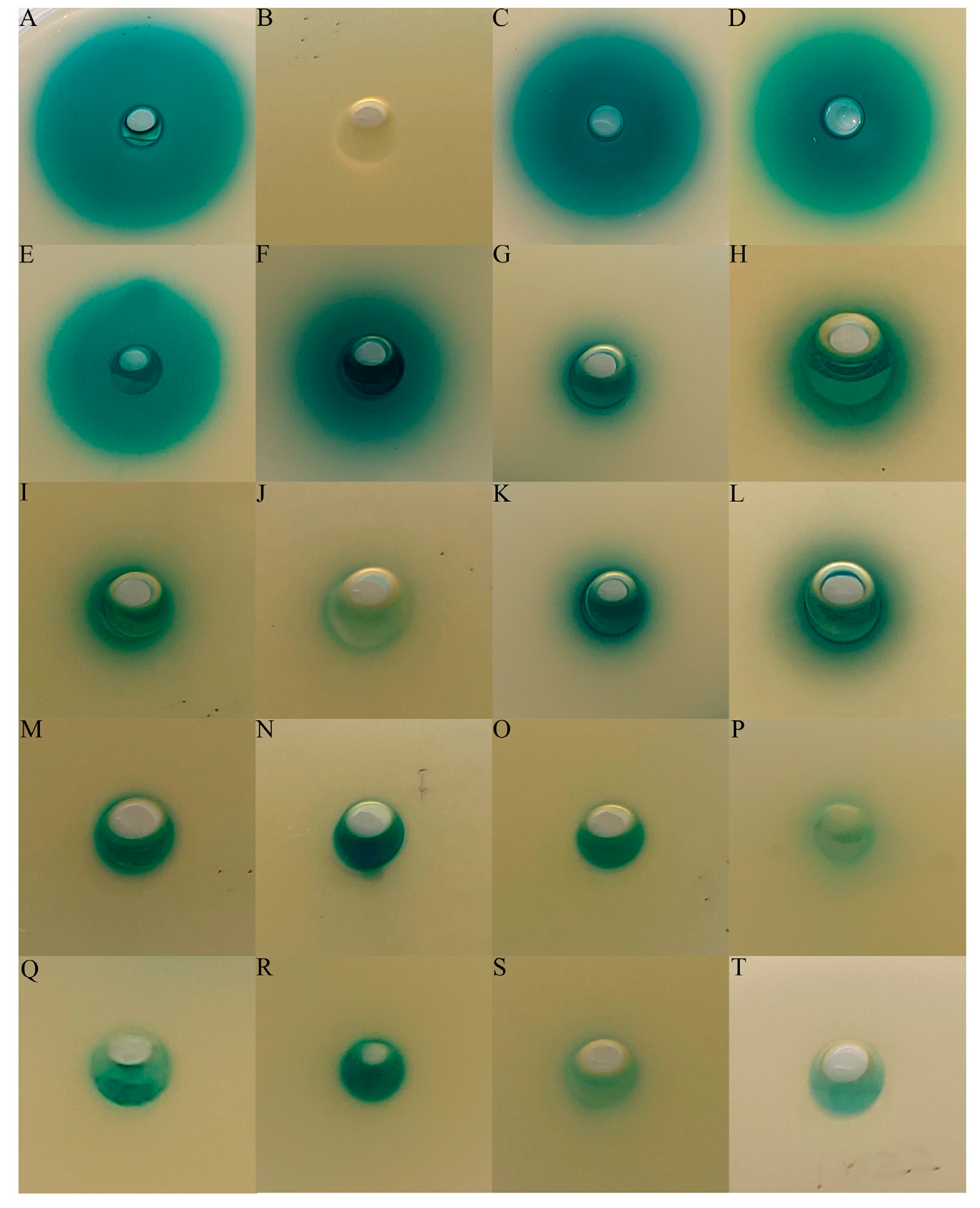
2.2. Screening for AHL-Producing and AHL-Degrading Bacteria
2.3. Identification of QQ Activities in QS Strains
2.4. Biofilm Production in QS and QQ Strains
2.5. Extracellular Enzyme Activities in QS Strains
3. Results
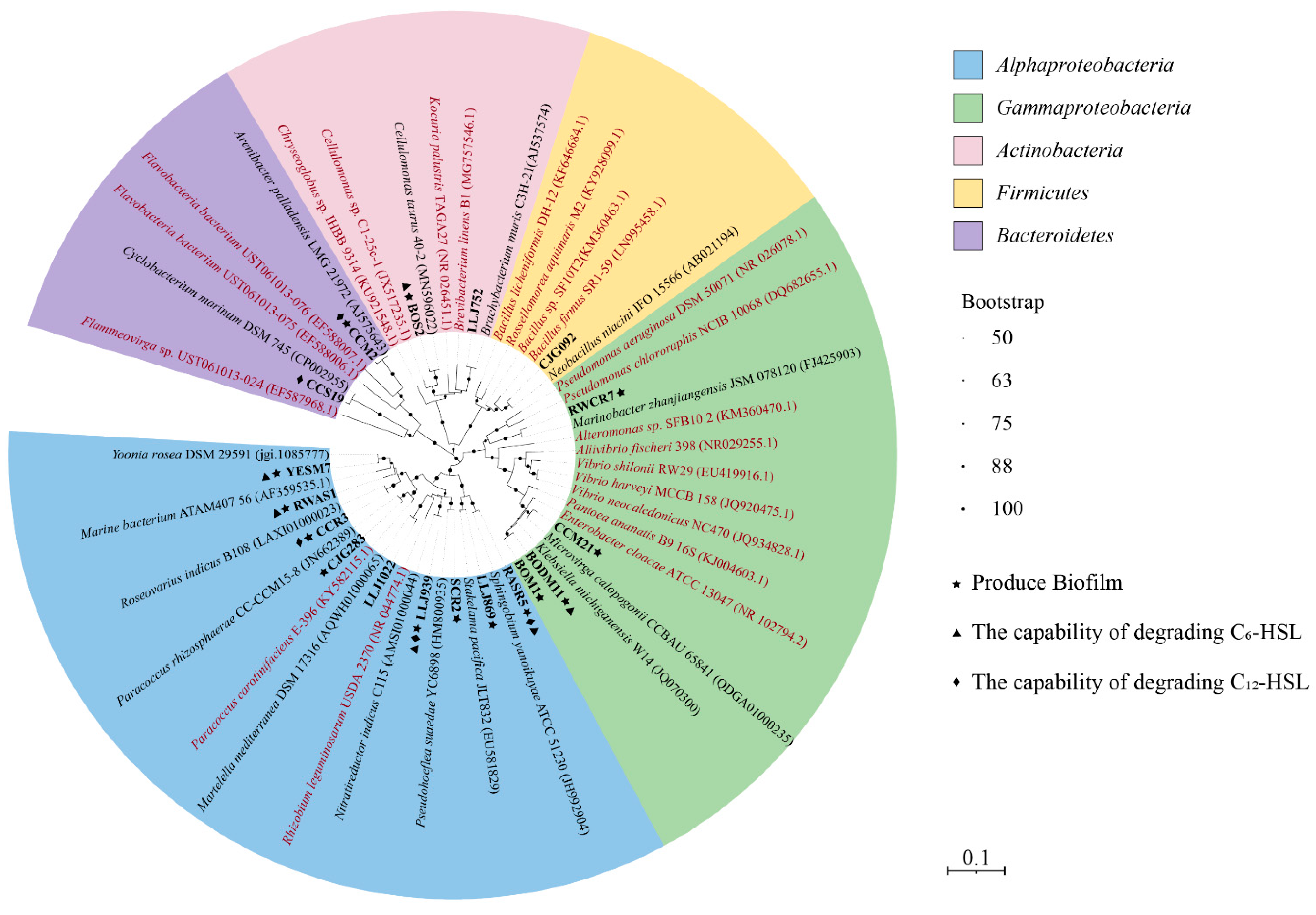
3.1. AHL-Producing Bacteria Isolated from the Hydrothermal Fields of the Okinawa Trough
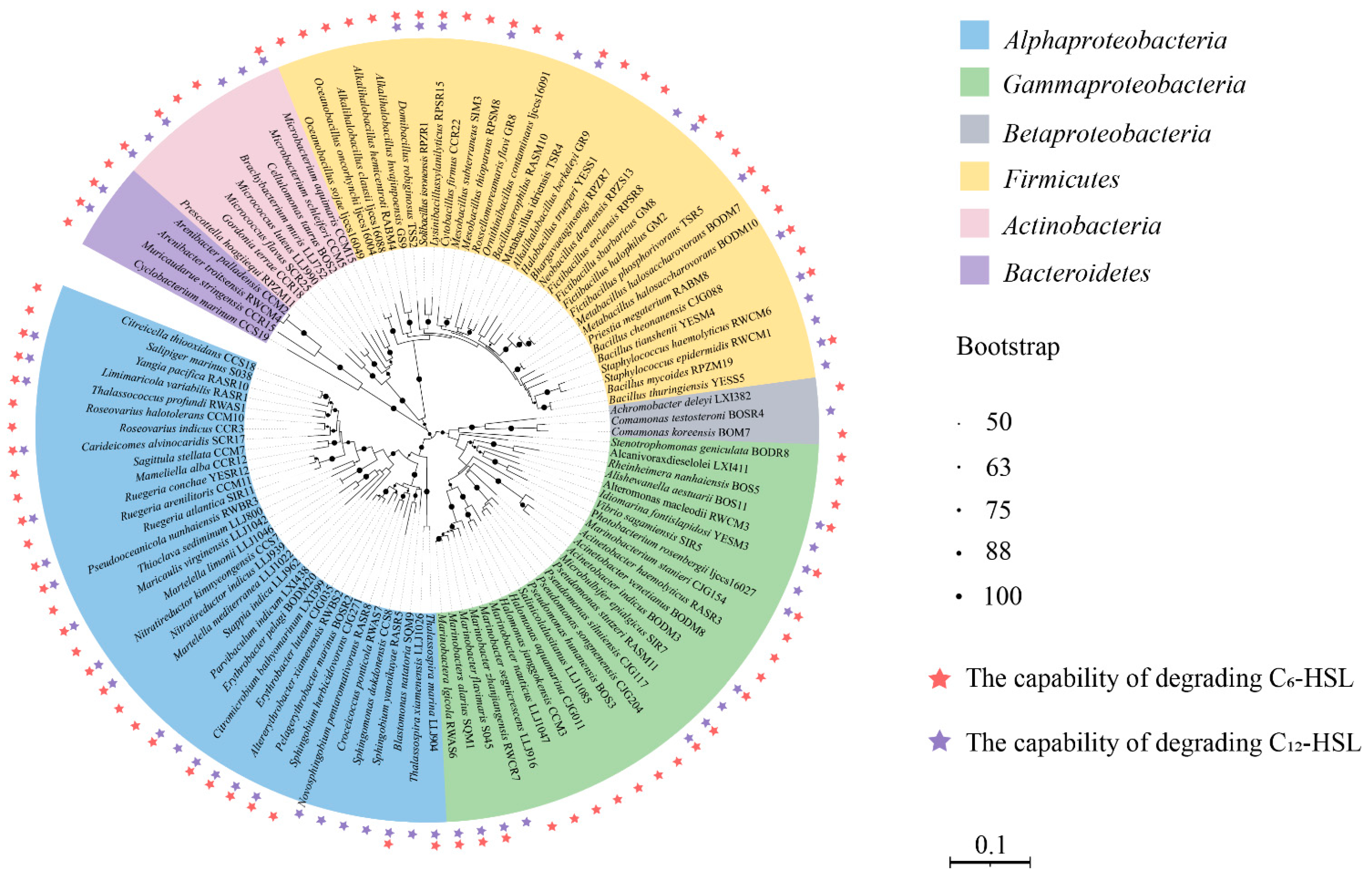
3.2. Identification of Species Capable of AHL Degradation
3.3. Biofilm Production in QS and QQ Cultures
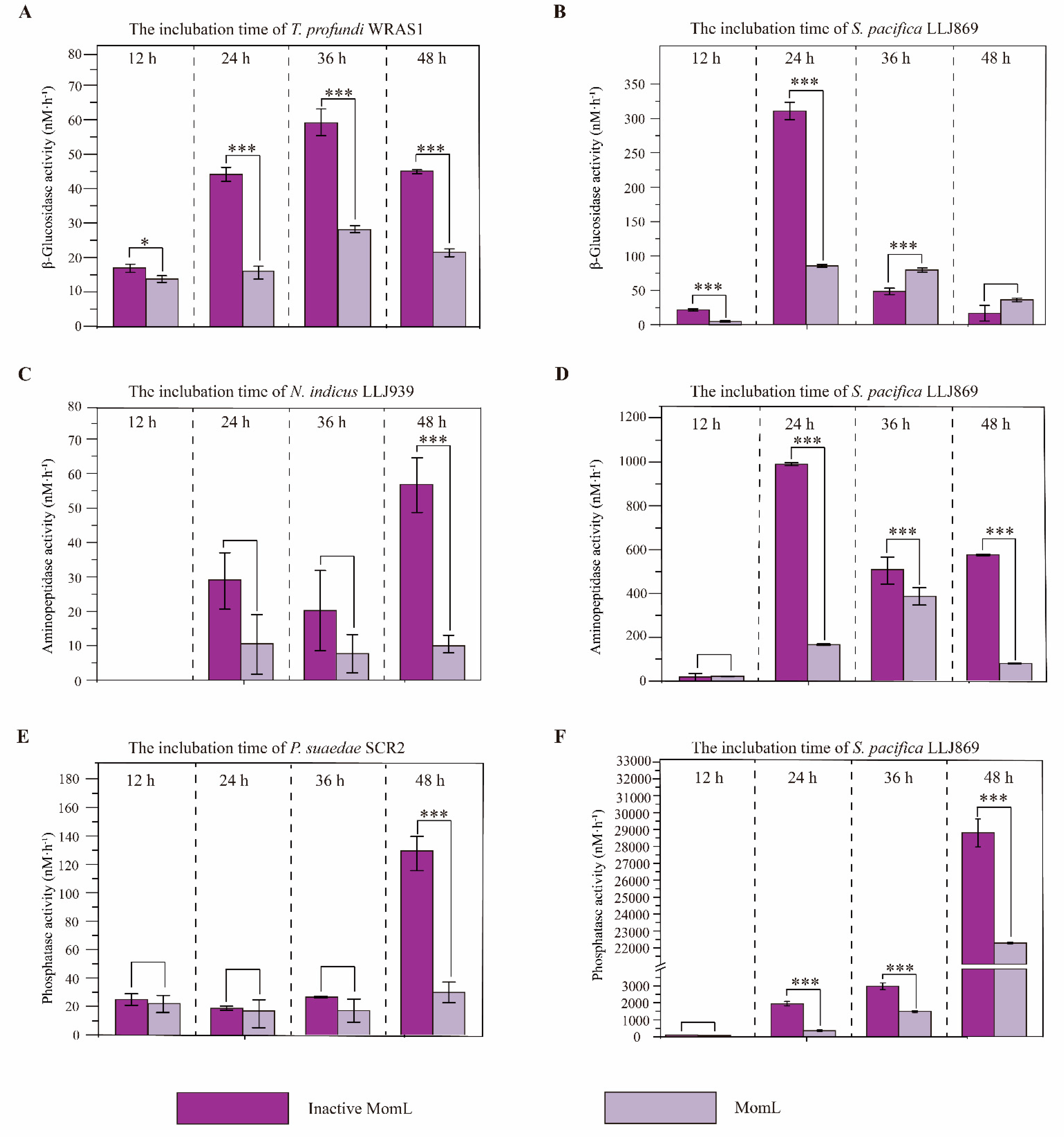
3.4. Extracellular Enzyme Activities in QS Isolates
4. Discussion
4.1. The Diversity of QS Strains in Hydrothermal Fields
4.2. The Diversity of QQ Isolates in Hydrothermal Fields
4.3. Biofilm Formation and the Regulation of EE Activities in QS Isolates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decho, A.W.; Norman, R.S.; Visscher, P.T. Quorum Sensing in Natural Environments: Emerging Views from Microbial Mats. Trends Microbiol. 2010, 18, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hmelo, L.R. Quorum Sensing in Marine Microbial Environments. Annu. Rev. Mar. Sci. 2017, 9, 257–281. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Tang, K.; Liu, J.; Wang, Y.; Zheng, Y.; Zhang, X.-H. Quorum Sensing System of Ruegeria Mobilis Rm01 Controls Lipase and Biofilm Formation. Front. Microbiol. 2019, 9, 3304. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Quadri, L.E.N.; Kuipers, O.P.; De Vos, W.M. Quorum Sensing by Peptide Pheromones and Two-component Signal-transduction Systems in Gram-positive Bacteria. Mol. Microbiol. 1997, 24, 895–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liu, Q.; Dong, D.; Hu, H.; Wu, B.; Ren, H. AHLs-Mediated Quorum Sensing Threshold and Its Response towards Initial Adhesion of Wastewater Biofilms. Water Res. 2021, 194, 116925. [Google Scholar] [CrossRef]
- Prazdnova, E.V.; Gorovtsov, A.V.; Vasilchenko, N.G.; Kulikov, M.P.; Statsenko, V.N.; Bogdanova, A.A.; Refeld, A.G.; Brislavskiy, Y.A.; Chistyakov, V.A.; Chikindas, M.L. Quorum-Sensing Inhibition by Gram-Positive Bacteria. Microorganisms 2022, 10, 350. [Google Scholar] [CrossRef]
- Pearson, J.P.; Gray, K.M.; Passador, L.; Tucker, K.D.; Eberhard, A.; Iglewski, B.H.; Greenberg, E.P. Structure of the Autoinducer Required for Expression of Pseudomonas Aeruginosa Virulence Genes. Proc. Natl. Acad. Sci. USA 1994, 91, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Jatt, A.N.; Tang, K.; Liu, J.; Zhang, Z.; Zhang, X.-H. Quorum Sensing in Marine Snow and Its Possible Influence on Production of Extracellular Hydrolytic Enzymes in Marine Snow Bacterium Pantoea Ananatis B9. FEMS Microbiol. Ecol. 2015, 91, 1–13. [Google Scholar] [CrossRef]
- Tait, K.; Hutchison, Z.; Thompson, F.L.; Munn, C.B. Quorum Sensing Signal Production and Inhibition by Coral-Associated Vibrios: Quorum Sensing and Coral-Associated Vibrios. Environ. Microbiol. Rep. 2010, 2, 145–150. [Google Scholar] [CrossRef]
- Golberg, K.; Eltzov, E.; Shnit-Orland, M.; Marks, R.S.; Kushmaro, A. Characterization of Quorum Sensing Signals in Coral-Associated Bacteria. Microb. Ecol. 2011, 61, 783–792. [Google Scholar] [CrossRef]
- Gardères, J.; Taupin, L.; Saïdin, J.B.; Dufour, A.; Le Pennec, G. N-Acyl Homoserine Lactone Production by Bacteria within the Sponge Suberites Domuncula (Olivi, 1792) (Porifera, Demospongiae). Mar. Biol. 2012, 159, 1685–1692. [Google Scholar] [CrossRef]
- Wagner-Döbler, I.; Thiel, V.; Eberl, L.; Allgaier, M.; Bodor, A.; Meyer, S.; Ebner, S.; Hennig, A.; Pukall, R.; Schulz, S. Discovery of Complex Mixtures of Novel Long-Chain Quorum Sensing Signals in Free-Living and Host-Associated Marine Alphaproteobacteria. ChemBioChem 2005, 6, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Van Mooy, B.A.S.; Hmelo, L.R.; Sofen, L.E.; Campagna, S.R.; May, A.L.; Dyhrman, S.T.; Heithoff, A.; Webb, E.A.; Momper, L.; Mincer, T.J. Quorum Sensing Control of Phosphorus Acquisition in Trichodesmium Consortia. ISME J. 2012, 6, 422–429. [Google Scholar] [CrossRef]
- Ransome, E.; Munn, C.B.; Halliday, N.; Cámara, M.; Tait, K. Diverse Profiles of N-Acyl-Homoserine Lactone Molecules Found in Cnidarians. FEMS Microbiol. Ecol. 2014, 87, 315–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Feng, T.; Wang, J.; Wang, Y.; Zhang, X.-H. The Mechanisms and Applications of Quorum Sensing (QS) and Quorum Quenching (QQ). J. Ocean Univ. China 2019, 18, 1427–1442. [Google Scholar] [CrossRef]
- Charlesworth, J.C.; Watters, C.; Wong, H.L.; Visscher, P.T.; Burns, B.P. Isolation of Novel Quorum-Sensing Active Bacteria from Microbial Mats in Shark Bay Australia. FEMS Microbiol. Ecol. 2019, 95, fiz035. [Google Scholar] [CrossRef]
- Freckelton, M.L.; Høj, L.; Bowden, B.F. Quorum Sensing Interference and Structural Variation of Quorum Sensing Mimics in Australian Soft Coral. Front. Mar. Sci. 2018, 5, 198. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, F.; Ding, G.; Li, J.; Guo, X.; Zhu, J.; Zhou, L.; Cai, S.; Liu, X.; Luo, Y.; et al. Acyl Homoserine Lactone-Based Quorum Sensing in a Methanogenic Archaeon. ISME J. 2012, 6, 1336–1344. [Google Scholar] [CrossRef] [Green Version]
- Sharif, D.I.; Gallon, J.; Smith, C.J.; Dudley, E. Quorum Sensing in Cyanobacteria: N-Octanoyl-Homoserine Lactone Release and Response, by the Epilithic Colonial Cyanobacterium Gloeothece PCC6909. ISME J. 2008, 2, 1171–1182. [Google Scholar] [CrossRef] [Green Version]
- Doberva, M.; Sanchez-Ferandin, S.; Toulza, E.; Lebaron, P.; Lami, R. Diversity of Quorum Sensing Autoinducer Synthases in the Global Ocean Sampling Metagenomic Database. Aquat. Microb. Ecol. 2015, 74, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Nealson, K.H.; Hastings, J.W. Quorum Sensing on a Global Scale: Massive Numbers of Bioluminescent Bacteria Make Milky Seas. Appl. Environ. Microbiol. 2006, 72, 2295–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joint, I.; Tait, K.; Callow, M.E.; Callow, J.A.; Milton, D.; Williams, P.; Cámara, M. Cell-to-Cell Communication Across the Prokaryote-Eukaryote Boundary. Science 2002, 298, 1207. [Google Scholar] [CrossRef] [Green Version]
- Romero, M.; Martin-Cuadrado, A.-B.; Otero, A. Determination of Whether Quorum Quenching Is a Common Activity in Marine Bacteria by Analysis of Cultivable Bacteria and Metagenomic Sequences. Appl. Environ. Microbiol. 2012, 78, 6345–6348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muras, A.; López-Pérez, M.; Mayer, C.; Parga, A.; Amaro-Blanco, J.; Otero, A. High Prevalence of Quorum-Sensing and Quorum-Quenching Activity among Cultivable Bacteria and Metagenomic Sequences in the Mediterranean Sea. Genes 2018, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Blanchet, E.; Prado, S.; Stien, D.; Oliveira da Silva, J.; Ferandin, Y.; Batailler, N.; Intertaglia, L.; Escargueil, A.; Lami, R. Quorum Sensing and Quorum Quenching in the Mediterranean Seagrass Posidonia Oceanica Microbiota. Front. Mar. Sci. 2017, 4, 218. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Ki, J.; Case, R.; Qian, P. Diversity and Acyl-Homoserine Lactone Production among Subtidal Biofilm-Forming Bacteria. Aquat. Microb. Ecol. 2008, 52, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Rehman, Z.U.; Leiknes, T. Quorum-Quenching Bacteria Isolated From Red Sea Sediments Reduce Biofilm Formation by Pseudomonas Aeruginosa. Front. Microbiol. 2018, 9, 1354. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-H.; Xu, J.-L.; Li, X.-Z.; Zhang, L.-H. AiiA, an Enzyme That Inactivates the Acylhomoserine Lactone Quorum-Sensing Signal and Attenuates the Virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar] [CrossRef]
- Toki, T.; Itoh, M.; Iwata, D.; Ohshima, S.; Shinjo, R.; Ishibashi, J.; Tsunogai, U.; Takahata, N.; Sano, Y.; Yamanaka, T.; et al. Geochemical Characteristics of Hydrothermal Fluids at Hatoma Knoll in the Southern Okinawa Trough. Geochem. J. 2016, 50, 493–525. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Yu, M.; Zhang, W.; He, K.; Pan, H.; Cui, K.; Zhao, Y.; Zhang, X.-H.; Xiao, T.; Zhang, W.; et al. Metagenomic and Microscopic Analysis of Magnetotactic Bacteria in Tangyin Hydrothermal Field of Okinawa Trough. Front. Microbiol. 2022, 13, 887136. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, F. Sulfurimonas autotrophica Gen. Nov., Sp. Nov., a Novel Sulfur-Oxidizing -Proteobacterium Isolated from Hydrothermal Sediments in the Mid-Okinawa Trough. Int. J. Syst. Evol. Microbiol. 2003, 53, 1801–1805. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, F.; Takai, K.; Nealson, K.H.; Horikoshi, K. Sulfurovum lithotrophicum Gen. Nov., Sp. Nov., a Novel Sulfur-Oxidizing Chemolithoautotroph within the ε-Proteobacteria Isolated from Okinawa Trough Hydrothermal Sediments. Int. J. Syst. Evol. Microbiol. 2004, 54, 1477–1482. [Google Scholar] [CrossRef] [Green Version]
- Nunoura, T.; Oida, H.; Miyazaki, M.; Suzuki, Y. Thermosulfidibacter takaii Gen. Nov., Sp. Nov., a Thermophilic, Hydrogen-Oxidizing, Sulfur-Reducing Chemolithoautotroph Isolated from a Deep-Sea Hydrothermal Field in the Southern Okinawa Trough. Int. J. Syst. Evol. Microbiol. 2008, 58, 659–665. [Google Scholar] [CrossRef]
- Nunoura, T.; Oida, H.; Miyazaki, M.; Suzuki, Y.; Takai, K.; Horikoshi, K. Desulfothermus okinawensis sp. Nov., a Thermophilic and Heterotrophic Sulfate-Reducing Bacterium Isolated from a Deep-Sea Hydrothermal Field. Int. J. Syst. Evol. Microbiol. 2007, 57, 2360–2364. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Kawagucci, S.; Makabe, A.; Takahashi, A.; Kitada, K.; Torimoto, J.; Matsui, Y.; Tasumi, E.; Shibuya, T.; Nakamura, K.; et al. Deepest and Hottest Hydrothermal Activity in the Okinawa Trough: The Yokosuka Site at Yaeyama Knoll. R. Soc. Open Sci. 2017, 4, 171570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGonigle, J.M.; Lang, S.Q.; Brazelton, W.J. Genomic Evidence for Formate Metabolism by Chloroflexi as the Key to Unlocking Deep Carbon in Lost City Microbial Ecosystems. Appl. Environ. Microbiol. 2020, 86, e02583-19. [Google Scholar] [CrossRef] [Green Version]
- Patrick, F.M. The Use of Membrane Filtration and Marine Agar 2216E to Enumerate Marine Heterotrophic Bacteria. Aquaculture 1978, 13, 369–372. [Google Scholar] [CrossRef]
- Massa, S.; Caruso, M.; Trovatelli, F.; Tosques, M. Comparison of Plate Count Agar and R2A Medium for Enumeration of Heterotrophic Bacteria in Natural Mineral Water. World J. Microbiol. Biotechnol. 1998, 14, 727–730. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Fuqua, C.; Winans, S.C. Conserved Cis-Acting Promoter Elements Are Required for Density-Dependent Transcription of Agrobacterium Tumefaciens Conjugal Transfer Genes. J. Bacteriol. 1996, 178, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Bernier, S.P.; Beeston, A.L.; Sokol, P.A. Detection of N-Acyl Homoserine Lactones Using a TraI-LuxCDABE-Based Biosensor as a High-Throughput Screening Tool. BMC Biotechnol. 2008, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Beaber, J.W.; Moré, M.I.; Fuqua, C.; Eberhard, A.; Winans, S.C. Analogs of the Autoinducer 3-Oxooctanoyl-Homoserine Lactone Strongly Inhibit Activity of the TraR Protein of Agrobacterium Tumefaciens. J. Bacteriol. 1998, 180, 5398–5405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, W.; Vattem, D.A.; Maitin, V.; Barnes, M.B.; McLean, R.J.C. Bioassays of Quorum Sensing Compounds Using Agrobacterium Tumefaciens and Chromobacterium Violaceum. In Quorum Sensing; Rumbaugh, K.P., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 692, pp. 3–19. ISBN 978-1-60761-970-3. [Google Scholar]
- Tempé, J.; Petit, A.; Holsters, M.; van Montagu, M.; Schell, J. Thermosensitive Step Associated with Transfer of the Ti Plasmid during Conjugation: Possible Relation to Transformation in Crown Gall. Proc. Natl. Acad. Sci. USA 1977, 74, 2848–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, K.; Zhang, Y.; Yu, M.; Shi, X.; Coenye, T.; Bossier, P.; Zhang, X.-H. Evaluation of a New High-Throughput Method for Identifying Quorum Quenching Bacteria. Sci. Rep. 2013, 3, 2935. [Google Scholar] [CrossRef] [Green Version]
- Hmelo, L.R.; Mincer, T.J.; Van Mooy, B.A.S. Possible Influence of Bacterial Quorum Sensing on the Hydrolysis of Sinking Particulate Organic Carbon in Marine Environments: Quorum Sensing on Sinking Particles. Environ. Microbiol. Rep. 2011, 3, 682–688. [Google Scholar] [CrossRef]
- Tang, K.; Su, Y.; Brackman, G.; Cui, F.; Zhang, Y.; Shi, X.; Coenye, T.; Zhang, X.-H. MomL, a Novel Marine-Derived N-Acyl Homoserine Lactonase from Muricauda Olearia. Appl. Environ. Microbiol. 2015, 81, 774–782. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Yang, Y.; Zhu, X.-Y.; Zhang, X.-H.; Yu, M. Metagenomic Insights Into the Microbial Assemblage Capable of Quorum Sensing and Quorum Quenching in Particulate Organic Matter in the Yellow Sea. Front. Microbiol. 2021, 11, 602010. [Google Scholar] [CrossRef] [PubMed]
- Zan, J.; Liu, Y.; Fuqua, C.; Hill, R. Acyl-Homoserine Lactone Quorum Sensing in the Roseobacter Clade. Int. J. Mol. Sci. 2014, 15, 654–669. [Google Scholar] [CrossRef]
- Berger, M.; Brock, N.L.; Liesegang, H.; Dogs, M.; Preuth, I.; Simon, M.; Dickschat, J.S.; Brinkhoff, T. Genetic Analysis of the Upper Phenylacetate Catabolic Pathway in the Production of Tropodithietic Acid by Phaeobacter Gallaeciensis. Appl. Environ. Microbiol. 2012, 78, 3539–3551. [Google Scholar] [CrossRef] [Green Version]
- Prol García, M.J.; D’Alvise, P.W.; Gram, L. Disruption of Cell-to-Cell Signaling Does Not Abolish the Antagonism of Phaeobacter Gallaeciensis toward the Fish Pathogen Vibrio Anguillarum in Algal Systems. Appl. Environ. Microbiol. 2013, 79, 5414–5417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urvoy, M.; Lami, R.; Dreanno, C.; Daudé, D.; Rodrigues, A.M.S.; Gourmelon, M.; L’Helguen, S.; Labry, C. Quorum Sensing Disruption Regulates Hydrolytic Enzyme and Biofilm Production in Estuarine Bacteria. Environ. Microbiol. 2021, 23, 7183–7200. [Google Scholar] [CrossRef]
- Deng, Z.; Luo, X.M.; Liu, J.; Wang, H. Quorum Sensing, Biofilm, and Intestinal Mucosal Barrier: Involvement the Role of Probiotic. Front. Cell. Infect. Microbiol. 2020, 10, 538077. [Google Scholar] [CrossRef] [PubMed]
- Urvoy, M.; Labry, C.; L’Helguen, S.; Lami, R. Quorum Sensing Regulates Bacterial Processes That Play a Major Role in Marine Biogeochemical Cycles. Front. Mar. Sci. 2022, 9, 834337. [Google Scholar] [CrossRef]
- Bose, U.; Ortori, C.A.; Sarmad, S.; Barrett, D.A.; Hewavitharana, A.K.; Hodson, M.P.; Fuerst, J.A.; Shaw, P.N. Production of N -Acyl Homoserine Lactones by the Sponge-Associated Marine Actinobacteria Salinispora Arenicola and Salinispora Pacifica. FEMS Microbiol. Lett. 2017, 364, fnx002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, M.; Martin-Cuadrado, A.-B.; Roca-Rivada, A.; Cabello, A.M.; Otero, A. Quorum Quenching in Cultivable Bacteria from Dense Marine Coastal Microbial Communities: Quorum Quenching in Marine Bacteria. FEMS Microbiol. Ecol. 2011, 75, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, X.; Hou, X.; Quan, C.; Chen, M. Widespread Existence of Quorum Sensing Inhibitors in Marine Bacteria: Potential Drugs to Combat Pathogens with Novel Strategies. Mar. Drugs 2019, 17, 275. [Google Scholar] [CrossRef] [Green Version]
- Teasdale, M.E.; Donovan, K.A.; Forschner-Dancause, S.R.; Rowley, D.C. Gram-Positive Marine Bacteria as a Potential Resource for the Discovery of Quorum Sensing Inhibitors. Mar. Biotechnol. 2011, 13, 722–732. [Google Scholar] [CrossRef]
- Reina, J.C.; Pérez, P.; Llamas, I. Quorum Quenching Strains Isolated from the Microbiota of Sea Anemones and Holothurians Attenuate Vibriocorallilyticus Virulence Factors and Reduce Mortality in Artemiasalina. Microorganisms 2022, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.W.; Lindow, S.E. Two Dissimilar N -Acyl-Homoserine Lactone Acylases of Pseudomonas Syringae Influence Colony and Biofilm Morphology. Appl. Environ. Microbiol. 2009, 75, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Czajkowski, R.; Krzyżanowska, D.; Karczewska, J.; Atkinson, S.; Przysowa, J.; Lojkowska, E.; Williams, P.; Jafra, S. Inactivation of AHLs by Ochrobactrum Sp. A44 Depends on the Activity of a Novel Class of AHL Acylase: An Ochrobactrum sp. A44 AHL Acylase. Environ. Microbiol. Rep. 2011, 3, 59–68. [Google Scholar] [CrossRef]
- Torres, M.; Rubio-Portillo, E.; Antón, J.; Ramos-Esplá, A.A.; Quesada, E.; Llamas, I. Selection of the N-Acylhomoserine Lactone-Degrading Bacterium Alteromonas stellipolaris PQQ-42 and of Its Potential for Biocontrol in Aquaculture. Front. Microbiol. 2016, 7, 646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tait, K.; Williamson, H.; Atkinson, S.; Williams, P.; Cámara, M.; Joint, I. Turnover of Quorum Sensing Signal Molecules Modulates Cross-Kingdom Signalling. Environ. Microbiol. 2009, 11, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T.; Boon, N.; Bossier, P.; Verstraete, W. Disruption of Bacterial Quorum Sensing: An Unexplored Strategy to Fight Infections in Aquaculture. Aquaculture 2004, 240, 69–88. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Givskov, M. Quorum Sensing Inhibitors: A Bargain of Effects. Microbiology 2006, 152, 895–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and Promise of Bacterial Quorum Sensing Research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupke, A. Quorum Sensing Plays a Complex Role in Regulating the Enzyme Hydrolysis Activity of Microbes Associated with Sinking Particles in the Ocean. Front. Mar. Sci. 2016, 3, 55. [Google Scholar] [CrossRef] [Green Version]

| Strain | Phylum | Class | Order | Species | Source * |
|---|---|---|---|---|---|
| LLJ1022 | Proteobacteria | Alphaproteobacteria | Hyphomicrobiales | Martelella mediterranea | SD |
| LLJ939 | Proteobacteria | Alphaproteobacteria | Hyphomicrobiales | Nitratireductor indicus | SW |
| SCR2 | Proteobacteria | Alphaproteobacteria | Hyphomicrobiales | Pseudohoeflea suaedae | SG |
| CCR3 | Proteobacteria | Alphaproteobacteria | Rhodobacterales | Roseovarius indicus | CG |
| CJG283 | Proteobacteria | Alphaproteobacteria | Rhodobacterales | Paracoccus rhizosphaerae | SW |
| RWAS1 | Proteobacteria | Alphaproteobacteria | Rhodobacterales | Thalassococcus profundi | SW |
| YESM7 | Proteobacteria | Alphaproteobacteria | Rhodobacterales | Yoonia rosea | SD |
| LLJ869 | Proteobacteria | Alphaproteobacteria | Sphingomonadales | Stakelama pacifica | SW |
| RASR5 | Proteobacteria | Alphaproteobacteria | Sphingomonadales | Sphingobium yanoikuyae | SD |
| BODM11 | Proteobacteria | Gammaproteobacteria | Enterobacterales | Klebsiella michiganensis | M |
| BOM1 | Proteobacteria | Gammaproteobacteria | Enterobacterales | Enterobacter hormaechei | M |
| CCM21 | Proteobacteria | Gammaproteobacteria | Enterobacterales | Microvirga calopogonii | CG |
| RWCR7 | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Marinobacter zhanjiangensis | SW |
| BOS2 | Actinobacteria | Actinomycetia | Cellulomonadales | Cellulomonas taurus | M |
| LLJ752 | Actinobacteria | Actinomycetia | Dermabacterales | Brachybacterium muris | SW |
| CCS19 | Bacteroidetes | Cytophagia | Cytophagales | Cyclobacterium marinum | CG |
| CCM2 | Bacteroidetes | Flavobacteriia | Flavobacteriales | Arenibacter palladensis | CG |
| CJG092 | Firmicutes | Bacilli | Bacillales | Neobacillus niacini | SW |
| Phylum | Class | Order | The Number of Test Strains | The Number of Strains Degrading C6-HSL | The Number of Strains Degrading C12-HSL |
|---|---|---|---|---|---|
| Proteobacteria | Alphaproteobacteria | Rhizobiales | 13 | 2 | 6 |
| Rhodobacterales | 29 | 12 | 17 | ||
| Sphingomonadales | 12 | 9 | 5 | ||
| Gammaproteobacteria | Alteromonadales | 11 | 6 | 7 | |
| Pseudomonadales | 10 | 5 | 8 | ||
| Enterobacterales | 4 | 0 | 0 | ||
| Oceanospirillales | 9 | 0 | 5 | ||
| Xanthomonadales | 1 | 0 | 1 | ||
| Vibrionales | 7 | 2 | 2 | ||
| Betaproteobacteria | Burkholderiales | 3 | 1 | 2 | |
| Firmicutes | Bacillales | Bacillaceae | 46 | 15 | 28 |
| Actinobacteria | Actinomycetia | Corynebacteriales | 2 | 2 | 1 |
| Micrococcales | 7 | 4 | 4 | ||
| Bacteroidetes | Flavobacteriales | Flavobacteriales | 4 | 2 | 3 |
| Cytophagia | Cytophagales | 1 | 0 | 0 | |
| Total number | 159 | 60 (37.73%) | 89 (55.97%) |
| Source | Total Number | The Number of Isolates Degrading C6-HSL (%) | The Number of Isolates Degrading C12-HSL (%) |
|---|---|---|---|
| Seawater | 55 | 20 (36.36%) | 29 (52.73%) |
| Sediment | 56 | 23 (41.07%) | 33 (58.93%) |
| Organism | 48 | 17 (35.41%) | 27 (56.25%) |
| Total number | 159 | 60 (38.37%) | 89 (55.98%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, F.; Gao, D.; Yue, L.; Zhang, Y.; Liu, J.; Zhang, X.-H.; Yu, M. Diversity of Bacteria with Quorum Sensing and Quenching Activities from Hydrothermal Vents in the Okinawa Trough. Microorganisms 2023, 11, 748. https://doi.org/10.3390/microorganisms11030748
Yin F, Gao D, Yue L, Zhang Y, Liu J, Zhang X-H, Yu M. Diversity of Bacteria with Quorum Sensing and Quenching Activities from Hydrothermal Vents in the Okinawa Trough. Microorganisms. 2023; 11(3):748. https://doi.org/10.3390/microorganisms11030748
Chicago/Turabian StyleYin, Fu, Di Gao, Li Yue, Yunhui Zhang, Jiwen Liu, Xiao-Hua Zhang, and Min Yu. 2023. "Diversity of Bacteria with Quorum Sensing and Quenching Activities from Hydrothermal Vents in the Okinawa Trough" Microorganisms 11, no. 3: 748. https://doi.org/10.3390/microorganisms11030748
APA StyleYin, F., Gao, D., Yue, L., Zhang, Y., Liu, J., Zhang, X.-H., & Yu, M. (2023). Diversity of Bacteria with Quorum Sensing and Quenching Activities from Hydrothermal Vents in the Okinawa Trough. Microorganisms, 11(3), 748. https://doi.org/10.3390/microorganisms11030748





