Isolation, Identification, and Characterization of Phosphate-Solubilizing Bacteria from Tunisian Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples
2.1.1. Site Description
2.1.2. Sampling Design
2.1.3. Determination of Soil Physical and Chemical Parameters
2.2. Isolation and Purification of Phosphate Solubilizing Bacteria
2.3. Identification of the Studied Isolates
2.4. Solubilization of Phosphates
2.5. Determination of the Production of Indole Acetic Acid
2.6. Data Analysis
3. Results and Discussion
3.1. Physical and Chemical Characteristics of Soil Samples
3.2. Characterization of the Isolates
3.3. Molecular Identification and Phylogenetic Analyses
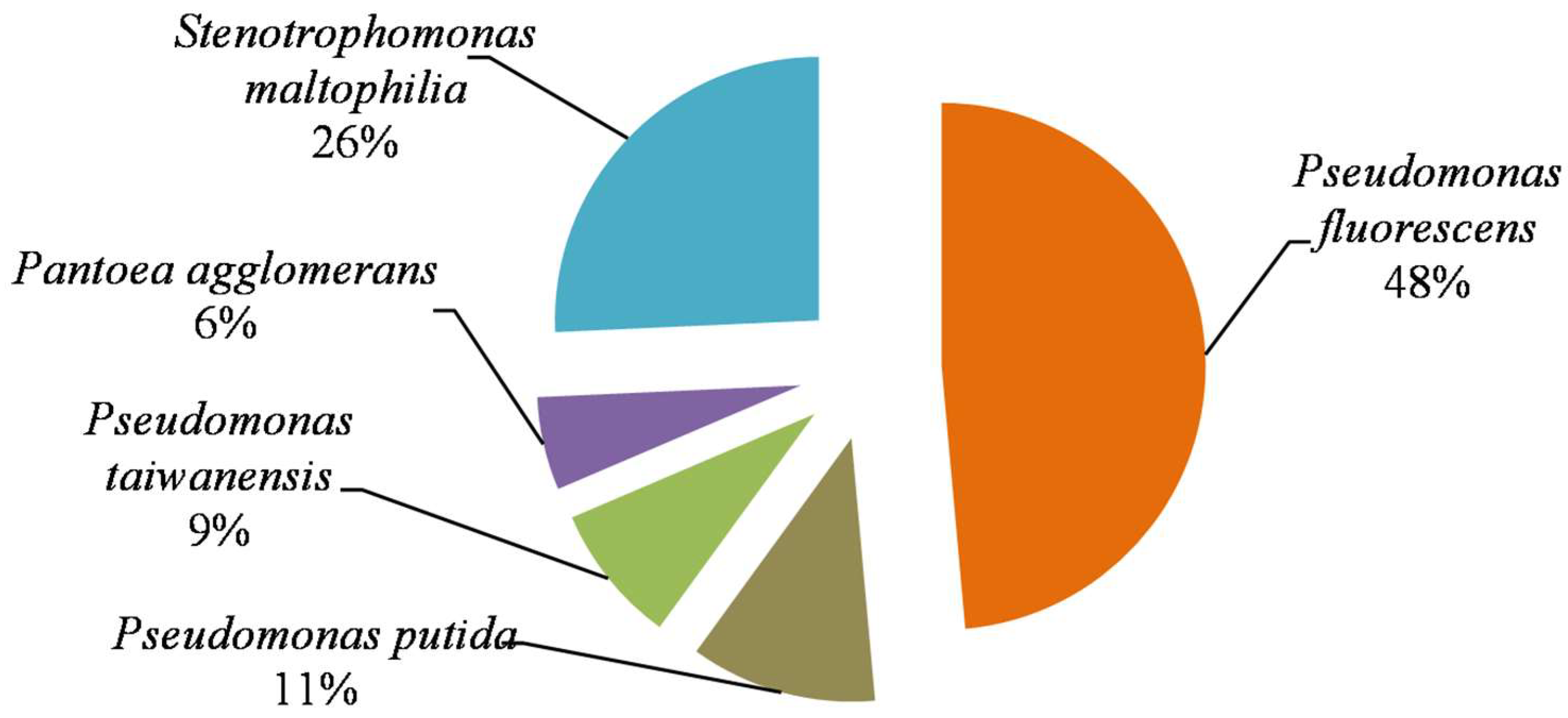
3.4. Composition of PSB Community
3.5. Calculation of the Phosphate Solubilization Index
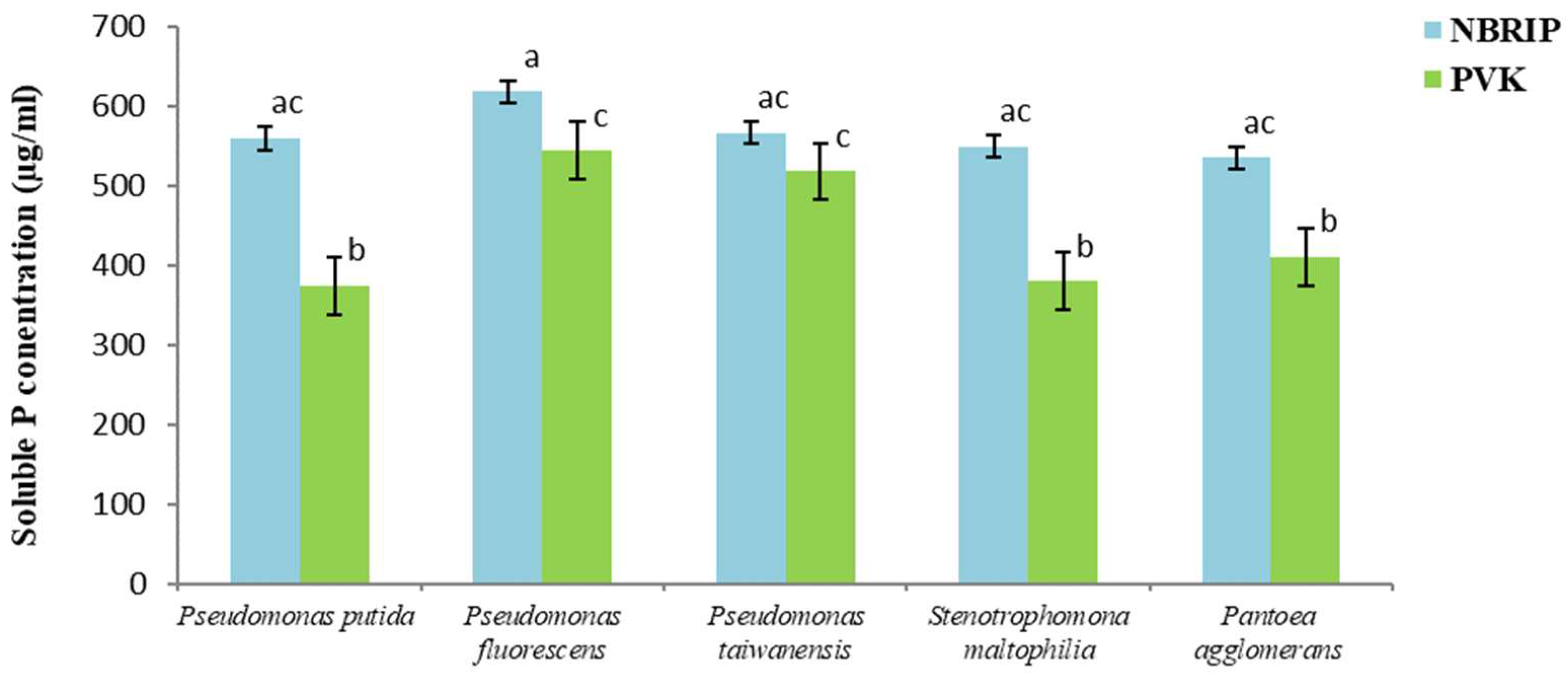
3.6. Evaluation of the Phosphate Solubilization in Liquid Media
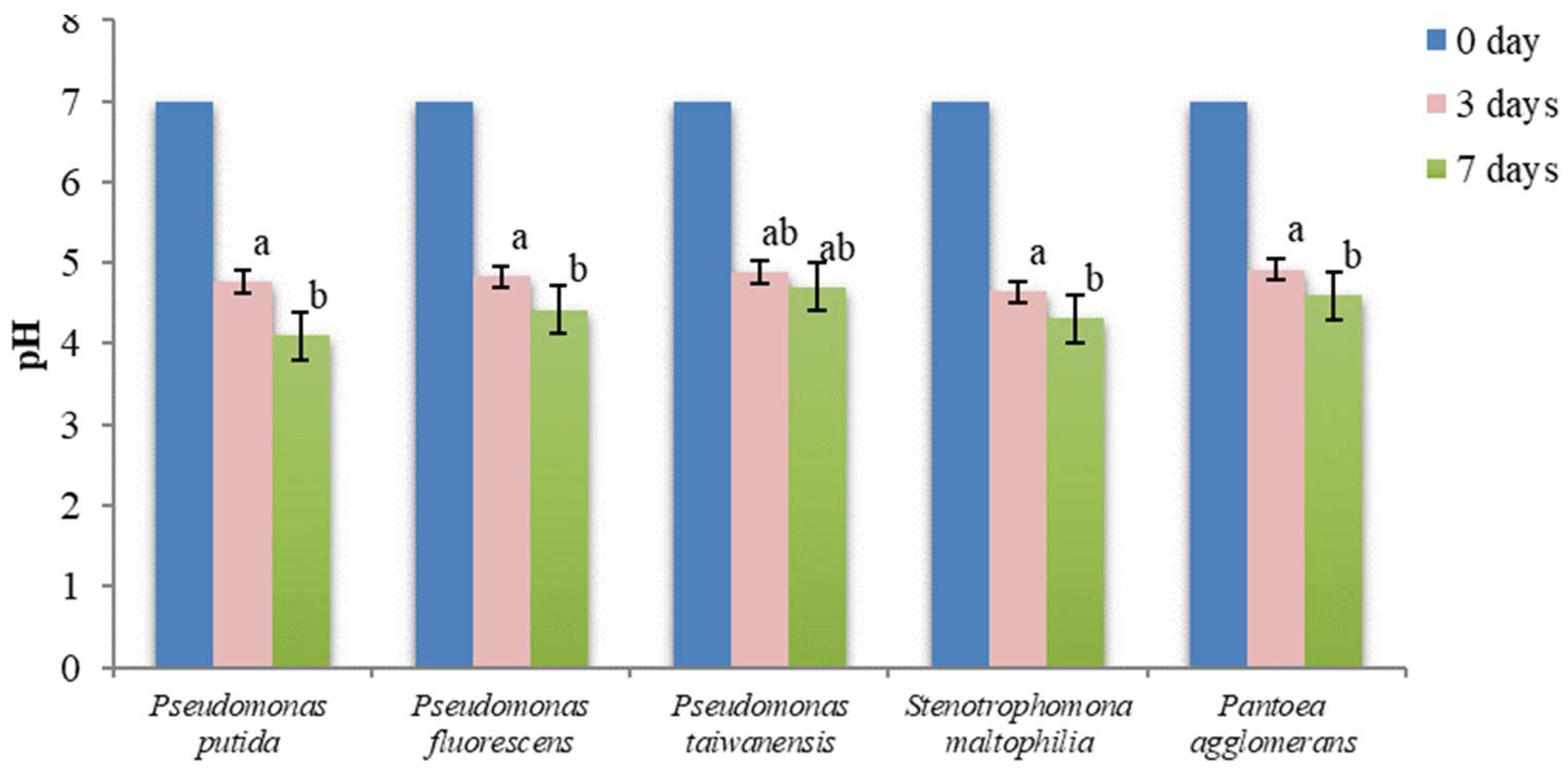
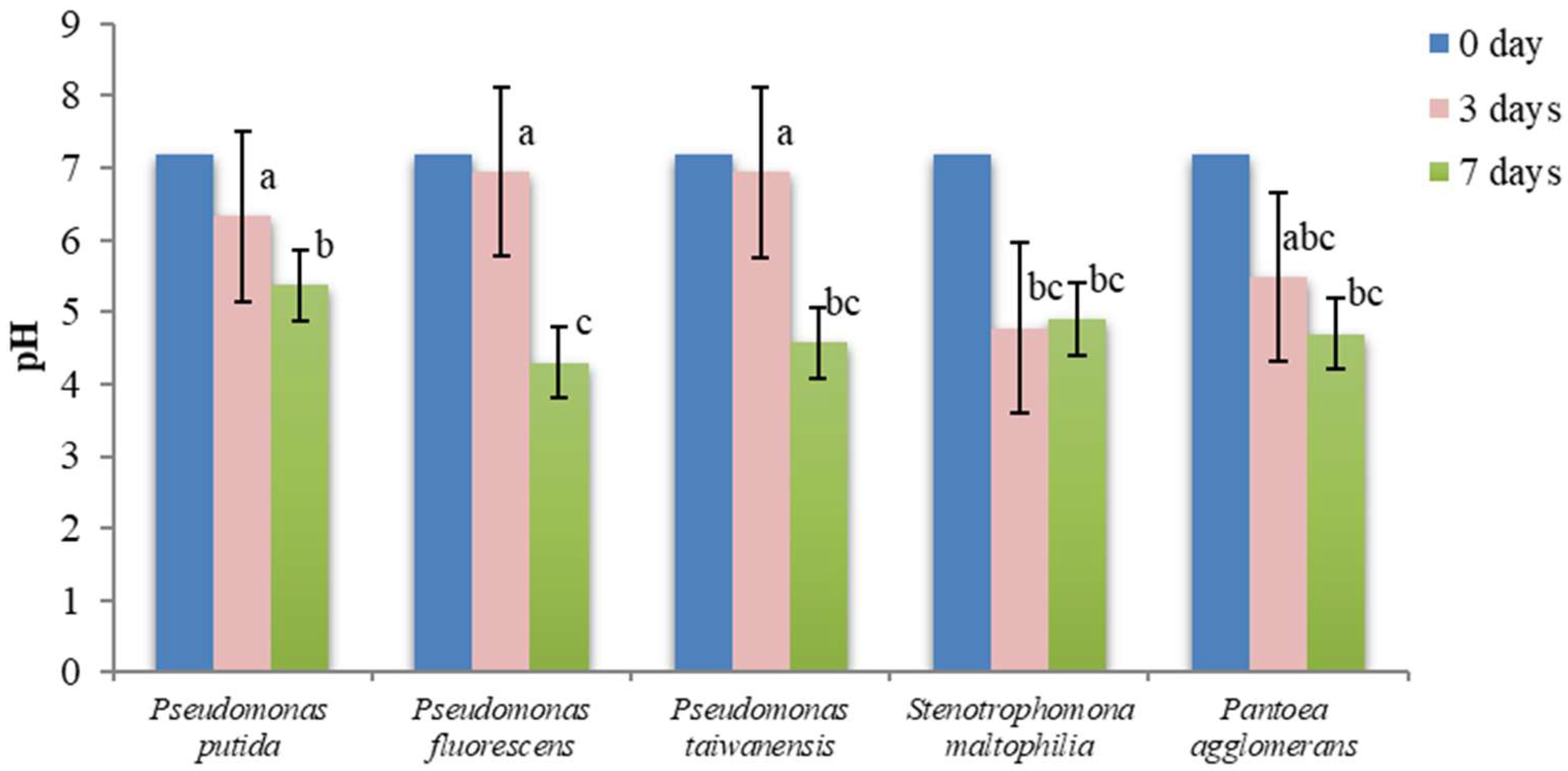
3.7. pH Changes in NBRIP and PVK Liquid Media
3.8. Determination of Indole Acetic Acid Production
3.9. Correlation with Soil Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anand, K.; Kumari, B.; Mallick, M.A. Phosphate solubilizing microbes: An effective and alternative approach as biofertilizers. J. Pharm. Sci. 2016, 8, 37–40. [Google Scholar]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [Green Version]
- Fernández, V.; Guzmán, P.; Peirce, C.A.; McBeath, T.M.; Khayet, M.; McLaughlin, M.J. Effect of wheat phosphorus status on leaf surface properties and permeability to foliar-applied phosphorus. Plant Soil 2014, 384, 7–20. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Shastri, B. Role of phosphate-solubilising microorganisms in sustainable agricultural development. In Agro-Environmental Sustainability; Singh, J., Seneviratne, G., Eds.; Springer: Cham, Switzerland, 2017; pp. 271–303. [Google Scholar]
- Azzi, V.; Kanso, A.; Kazpard, V.; Kobeissi, A.; Lartiges, B.; EI Samrani, A. Lactuca sativa growth in compacted and non-compacted semi-arid alkaline soil under phosphate fertilizer treatment and cadmium contamination. Soil Tillage Res. 2017, 165, 1–10. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef] [PubMed]
- El Kateb, A.; Stalder, C.; Rüggeberg, A.; Neururer, C.; Spangenberg, J.; Spezzaferri, S. Impact of industrial phosphate waste discharge on the marine environment in the Gulf of Gabes (Tunisia). PLoS ONE 2018, 13, e0197731. [Google Scholar] [CrossRef] [Green Version]
- Wendimu, A.; Yoseph, T.; Ayalew, T. Ditching Phosphatic Fertilizers for Phosphate-Solubilizing Biofertilizers: A Step towards Sustainable Agriculture and Environmental Health. Sustainability 2023, 15, 1713. [Google Scholar] [CrossRef]
- Paul, D.; Sinha, S.N. Phosphate solubilization potential and phosphatase activity of some bacterial strains isolated from thermal power plant effluent exposed water of river Ganga. CIBTech J. Microbiol. 2013, 2, 1–7. [Google Scholar]
- Puri, A.; Padda, K.P.; Chanway, C.P. In vitro and in vivo analyses of plant-growth-promoting potential of bacteria naturally associated with spruce trees growing on nutrient-poor soils. Appl. Soil Ecol. 2020, 149, 103538. [Google Scholar] [CrossRef]
- Rawat, P.; Shankhdhar, D.; Shankhdhar, S.C. Plant growth-promoting rhizobacteria: A booster for ameliorating soil health and agriculture production. In Soil Health; Giri, B., Verma, A., Eds.; Springer: Cham, Switzerland, 2020; pp. 47–68. [Google Scholar]
- Zaheer, A.; Malik, A.; Sher, A.; Qaisrani, M.M.; Mehmood, A.; Khan, S.U.; Ashraf, M.; Mirza, Z.; Karim, S.; Rasool, M. Isolation, characterization, and effect of phosphate-zinc-solubilizing bacterial strains on chickpea (Cicer arietinum L.) growth. Saudi J. Biol. Sci. 2019, 26, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Adnan, M.; Iqbal, S.; Fahad, S.; Saeed, M.; Mian, I.A.; Andaleeb, S. Combining phosphorus (P) with phosphate solubilizing bacteria (PSB) improved wheat yield and P uptake in alkaline soil. Pure Appl. Biol. 2019, 8, 1809–1817. [Google Scholar] [CrossRef]
- Amy, C.; Avice, J.-C.; Laval, K.; Bressan, M. Are native phosphate-solubilizing bacteria a relevant alternative to mineral fertilizations for crops? Part II: PSB inoculation enables a halving of P input and improves the microbial community in the rapeseed rhizosphere. Rhizosphere 2022, 21, 100480. [Google Scholar] [CrossRef]
- Martínez, E.; Fuentes, J.; Acevedo, E. Carbono orgánico y propiedades del suelo. R. C. Suelo Nutr. Veg. 2008, 8, 68–96. [Google Scholar] [CrossRef]
- Mwendwa, S. Revisiting soil texture analysis: Practices towards a more accurate Bouyoucos method. Heliyon 2022, 8, e09395. [Google Scholar] [CrossRef]
- Hii, Y.; San, C.; Lau, S.; Danquah, M. Isolation and characterisation of phosphate solubilizing microorganisms from peat. Biocatal. Agric. Biotechnol. 2020, 26, 101643. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Gupta, R.; Singal, R.; Shankar, A.; Kuhad, R.C.; Saxena, R.K. A modified plate assay for screening phosphate solubilizing microorganisms. J. Gen. Appl. Microbiol. 1994, 40, 255–260. [Google Scholar] [CrossRef]
- Berza, B.; Sekar, J.; Vaiyapuri, P.; Pagano, M.; Assefa, F. Evaluation of inorganic phosphate solubilizing efficiency and multiple plant growth promoting properties of endophytic bacteria isolated from root nodules Erythrina brucei. BMC Microbiol. 2022, 22, 276. [Google Scholar] [CrossRef] [PubMed]
- Edi-Premono, M.; Moawad, A.M.; Vleck, P.L.G. Effect of phosphate solubilizing Pseudomonas Putida on the growth of maize and its survival in the rhizosphere. Indones. J. Crop Sci. 1996, 11, 13–23. [Google Scholar]
- Rfaki, A.; Nassiri, L.; Ibijbijen, J. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of Faba bean (Vicia faba L.) in Meknes Region, Morocco. Br. Microbiol. Res. J. 2015, 6, 247–254. [Google Scholar] [CrossRef]
- Dipak, P.; Sankar, N.S. Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river Ganga, India. Ann. Agrar. Sci. 2017, 15, 130–136. [Google Scholar]
- Kumar, N.P.; Audipudi, A.V. Exploration of a novel plant growth promoting bacteria Stenotrophomonas maltophilia AVP27 isolated from the chilli rhizosphere soil. Int. J. Eng. Res. Generic Sci. 2015, 3, 265–273. [Google Scholar]
- Meireles, C.; Costa, G.; Guinote, I.; Albuquerque, T.; Botelho, A.; Cordeiro, C.; Freire, P. Pseudomonas putida are environmental reservoirs of antimicrobial resistance to β-lactamic antibiotics. World J. Microbiol. Biotechnol. 2013, 29, 1317–1325. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [Green Version]
- Biswas, J.K.; Banerjee, A.; Rai, M.; Naidu, R.; Biswas, B.; Vithanage, M.; Meers, E. Potential application of selected metal resistant phosphate solubilizing bacteria isolated from the gut of earthworm (Metaphire posthuma) in plant growth promotion. Geoderma 2018, 330, 117–124. [Google Scholar] [CrossRef]
- Sharon, J.A.; Hathwaik, G.M.; Glenn, S.H.; Imam, S.H.; Lee, C.C. Isolation of efficient phosphate solubilizing bacteria capable of enhancing tomato plant growth. J. Soil Sci. Plant Nutr. 2016, 16, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Mou, L.; Yi, J.; Wang, J.; Liu, A.; Yu, J. The Eno gene of Burkholderia cenocepacia strain 71-2 is involved in phosphate solubilization. Curr. Microbiol. 2019, 76, 495–502. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, J.; Feng, G.; Declerck, S. The arbuscular mycorrhizal fungus Rhizophagus irregularis MUCL 43194 induces the gene expression of citrate synthase in the tricarboxylic acid cycle of the phosphate-solubilizing bacterium Rahnella aquatilis HX2. Mycorrhiza 2019, 29, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Jha, P.N. The PGPR Stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front. Microbiol. 2017, 8, 1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishore, N.; Pindi, P.K.; Reddy, S.R. Phosphate-solubilizing microorganisms: A critical review. In Plant Biology and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K., Eds.; Springer: New Delhi, India, 2015; pp. 307–333. [Google Scholar]
- Ramos, P.L.; Van Trappen, S.; Thompson, F.L.; Rocha, R.C.; Barbosa, H.R.; De Vos, P.; Moreira-Filho, C.A. Screening for endophytic nitrogen-fixing bacteria in Brazilian sugar cane varieties used in organic farming and description of Stenotrophomonas pavanii sp. nov. Int. J. Syst. Evol. Microbiol. 2011, 61, 926–931. [Google Scholar] [CrossRef] [Green Version]
- Son, H.J.; Park, G.T.; Cha, M.S.; Heo, M.S. Solubilization of insoluble inorganic phosphates by a novel salt-and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresour. Technol. 2006, 97, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S.; Bhadauria, S.; Kumar, P.; Lal, H.; Mondal, R.; Verma, D. Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol. Lett. 2000, 182, 291–296. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Silini, A.; Ghoul, M.; Yahiaoui, B.; Arif, F. Solubilization of phosphate by the Bacillus under salt stress and in the presence of osmoprotectant compounds. Afr. J. Microbiol. Res. 2013, 7, 4562–4571. [Google Scholar]
- Chibani, H.R.; Bellahcene, M.; Djibaoui, R.; Bouznad, A.; Hamoum, H. Optimization of inorganic phosphate solubilization by Pseudomonas fluorescens and Bacillus sp. isolated from wheat rhizospheric soil. Int. J. Biosci. 2017, 10, 142–150. [Google Scholar]
- Gupta, A.; Meyer, J.M.; Goel, R. Development of heavy growth promotion by cold tolerant mutants of Pseudomonas fluorescens. Microbiol. Res. 2002, 158, 163–168. [Google Scholar]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef]
- Patel, T.; Saraf, M. Exploration of novel plant growth promoting bacteria Stenotrophomonas maltophilia MTP42 isolated from the rhizospheric soil of Coleus forskohlii. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 944–955. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.H.; Lee, C.Y.; Son, H.J. Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plant growth-promoting activities. Lett. Appl. Microbiol. 2009, 49, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Muleta, D.; Assefa, F.; Börjesson, E.; Granhall, U. Phosphate-solubilising rhizobacteria associated with Coffea arabica L. in natural coffee forests of south western Ethiopia. J. Saudi Soc. Agric. Sci. 2013, 12, 73–84. [Google Scholar]
- Ghosh, R.; Chatterjee, S.; Mandal, N.C. Stenotrophomonas. In Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Amaresan, N., Kumar, M.S., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 427–442. [Google Scholar]
- Costa, E.; Usall, J.; Teixido, N.; Delgado, J.; Vinas, I. Water activity, temperature, and pH effects on growth of the biocontrol agent Pantoea agglomerans CPA2. Can. J. Microbiol. 2002, 48, 1082–1088. [Google Scholar] [CrossRef]
- Wan, W.; Qin, Y.; Wu, H.; Zuo, W.; He, H.; Tan, J.; Wang, Y.; He, D. Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Front. Microbiol. 2020, 11, 752. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.D.; Zaidi, A.; Ahmad, E. Mechanism of phosphate solubilization and physiological functions of phosphate-solubilizing microorganisms. In Phosphate Solubilizing Microorganisms; Khan, M., Zaidi, A., Musarrat, J., Eds.; Springer: Cham, Switzerland, 2014; pp. 31–62. [Google Scholar]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [Green Version]
- Kadmiri, I.M.; Chaouqui, L.; Azaroual, S.E.; Sijilmassi, B.; Yaakoubi, K.; Wahby, I. Phosphate-solubilizing and auxin-producing rhizobacteria promote plant growth under saline conditions. Arab. J. Sci. Eng. 2018, 43, 3403–3415. [Google Scholar] [CrossRef]
- Li, L.; Chen, R.; Zuo, Z.; Lv, Z.; Yang, Z.; Mao, W.; Liu, Y.; Zhou, Y.; Huang, J.; Song, Z. Evaluation and improvement of phosphate solubilization by an isolated bacterium Pantoea agglomerans ZB. World J. Microbiol. Biotechnol. 2020, 36, 865–869. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Devi, S.; Patil, S.; Payal, C.; Negi, S. Isolation, screening and characterization of bacteria from rhizospheric soils for different plant growth promotion (PGP) activities: An in vitro study. Recent. Res. Sci. Technol. 2012, 4, 1–5. [Google Scholar]
- Suckstorff, I.; Berg, G. Evidence for dose-dependent effects on plant growth by Stenotrophomonas strains from different origins. J. Appl. Microbiol. 2003, 95, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parnell, J.J.; Berka, R.; Young, H.A.; Sturino, J.M.; Kang, Y.; Barnhart, D.M.; Dileo, M.V. From the lab to the farm: An industrial perspective of plant beneficial microorganisms. Front. Plant. Sci. 2016, 7, 1110. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant. Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scavino, A.F.; Pedraza, R.O. The role of siderophores in plant growth-promoting bacteria. In Bacteria in Agrobiology: Crop Productivity; Maheswari, D., Saraf, M., Aeron, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 265–285. [Google Scholar]


| Site | Location | Climate | Average Temperature | Typical Monthly Precipitation | Main Culture | Type |
|---|---|---|---|---|---|---|
| 1 | N: 36°46′12.0″ E: 008°34′44.5″ | Warm temperate | 18.6 °C | 1–11 mm | Hackberry trees | Forest |
| 2 | N: 36°46′10.7″ E: 008°34′41.2″ | Warm temperate | 18.6 °C | 1–11 mm | Quercus suber | Forest |
| 3 | N: 36°46′12.6″ E: 008°34′42.6″ | Warm temperate | 18.6 °C | 1–11 mm | Hackberry trees | Forest |
| 4 | N: 36°58′13.3″ E: 008°52′34.9″ | Hot | 24.0 °C | 1–6 mm | Olive trees | Agriculture |
| 5 | N: 36°58′12.8″ E: 008°52′34.7″ | Hot | 24.0 °C | 1–6 mm | Pine trees | Forest |
| Site | Type | Texture | pH | Organic Matter (%) | Total Carbon (%) | Total Nitrogen (mg/L) | Total Phosphorous (ppm) |
|---|---|---|---|---|---|---|---|
| 1 | Forest | Sandy clay | 7.05 | 13.21 | 7.65 | 0.52 | 67.19 |
| 2 | Forest | Sandy loam | 6.50 | 7.02 | 4.06 | 0.39 | 72.51 |
| 3 | Forest | Sandy loam | 6.56 | 11.27 | 6.49 | 0.47 | 68.86 |
| 4 | Agriculture | Sandy loam | 6.90 | 4.38 | 2.53 | 0.30 | 66.12 |
| 5 | Forest | Sandy | 6.60 | 1.07 | 0.62 | 0.28 | 66.69 |
| PSB | SI in NBRIP | SI in PVK |
|---|---|---|
| Pseudomonas putida | 3.53 ± 0.05 a | 2.14 ± 0.03 e |
| Pseudomonas fluorescens | 3.82 ± 0.05 c | 3.51 ± 0.05 a |
| Pseudomonas taiwanensis | 3.64 ± 0.05 a | 3.12 ± 0.03 f |
| Stenotrophomonas maltophilia | 2.83 ± 0.02 d | 2.31 ± 0.02 b |
| Pantoea agglomerans | 2.35 ± 0.02 b | 2.98 ± 0.03 g |
| Soil Property | Correlation Coefficient | p-Value |
|---|---|---|
| pH | −0.759 | 0.137 |
| Organic Matter (%) | 0.210 | 0.734 |
| Total Carbon (%) | 0.208 | 0.737 |
| Total Nitrogen (mg/L) | 0.236 | 0.703 |
| Total Phosphorous (ppm) | 0.954 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amri, M.; Rjeibi, M.R.; Gatrouni, M.; Mateus, D.M.R.; Asses, N.; Pinho, H.J.O.; Abbes, C. Isolation, Identification, and Characterization of Phosphate-Solubilizing Bacteria from Tunisian Soils. Microorganisms 2023, 11, 783. https://doi.org/10.3390/microorganisms11030783
Amri M, Rjeibi MR, Gatrouni M, Mateus DMR, Asses N, Pinho HJO, Abbes C. Isolation, Identification, and Characterization of Phosphate-Solubilizing Bacteria from Tunisian Soils. Microorganisms. 2023; 11(3):783. https://doi.org/10.3390/microorganisms11030783
Chicago/Turabian StyleAmri, Marwa, Mohamed Ridha Rjeibi, Marwa Gatrouni, Dina M. R. Mateus, Nedra Asses, Henrique J. O. Pinho, and Chaabane Abbes. 2023. "Isolation, Identification, and Characterization of Phosphate-Solubilizing Bacteria from Tunisian Soils" Microorganisms 11, no. 3: 783. https://doi.org/10.3390/microorganisms11030783







