Assessment of New and Genome-Reduced Pseudomonas Strains Regarding Their Robustness as Chassis in Biotechnological Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Medium and Culture Conditions
2.3. Extraction of Membrane Lipids
2.4. Determination of Fatty Acid Composition
2.5. Growth in in a Second Phase System of 1-octanol and 1-decanol
2.6. Statistical Analysis
3. Results and Discussion
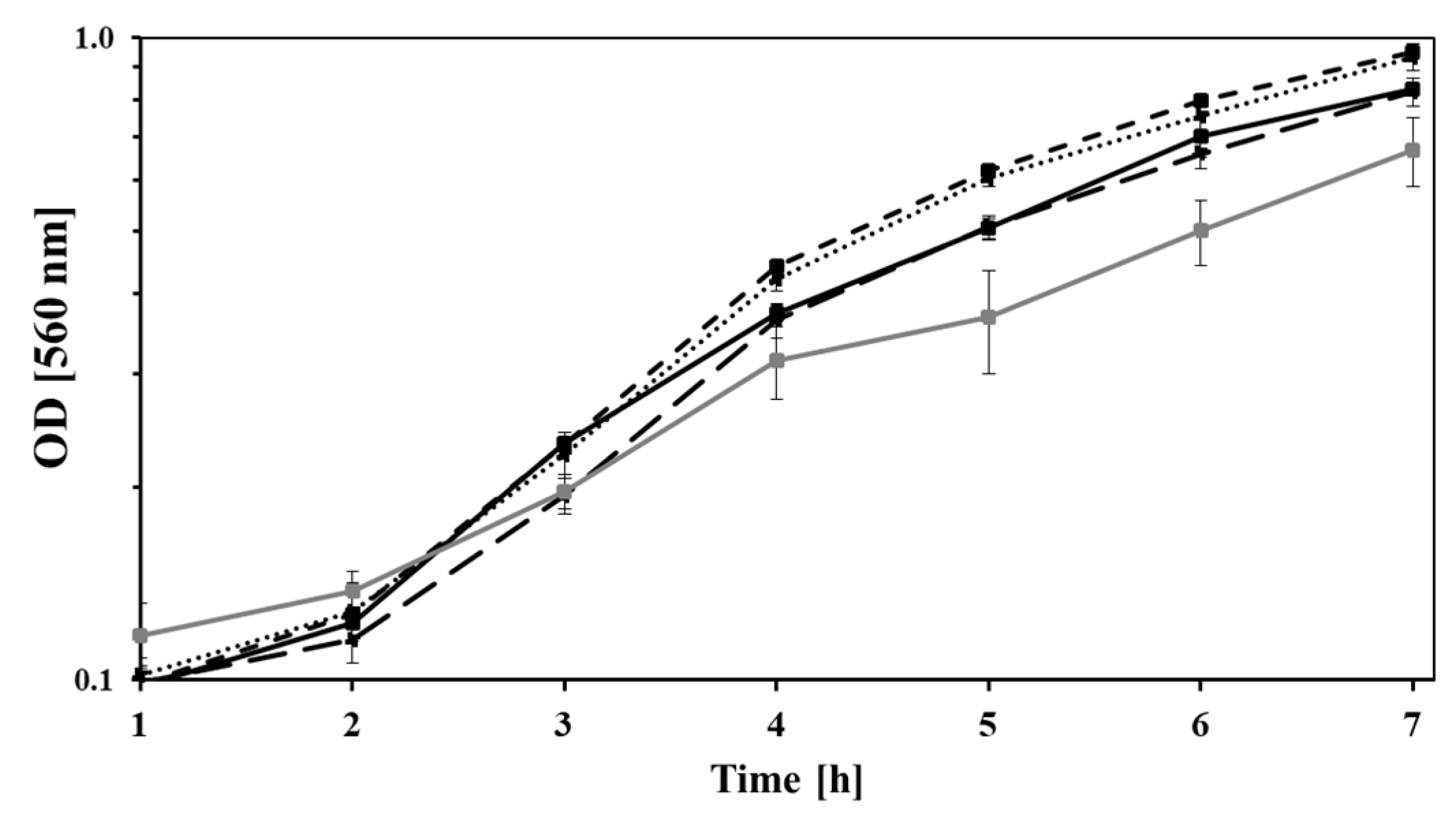
3.1. Growth Kinetics
3.2. Effects of n-alkanols on Bacterial Growth
3.3. Two-Phase Adaptation of 1-octanol and 1-decanol in P. taiwanensis VLB120, GRC1, GRC2, GR3, and P. capeferrum TDA1
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schmid, A.; Dordick, J.; Hauer, B.; Kiener, A.; Wubbolts, M.; Witholt, B. Industrial biocatalysis today and tomorrow. Nature 2001, 409, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Kusumawardhani, H.; Hosseini, R.; de Winde, J.H. Solvent tolerance in bacteria: Fulfilling the promise of the biotech era? Trends Biotechnol. 2018, 36, 1025–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitzenhofer, N.L.; Kruse, L.; Thies, S.; Wynands, B.; Lechtenberg, T.; Ronitz, J.; Kozaeva, E.; Wirth, N.T.; Eberlein, C.; Jaeger, K.E.; et al. Towards robust Pseudomonas cell factories to harbour novel biosynthetic pathways. Essays Biochem. 2021, 65, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Kabelitz, N.; Zehnsdorf, A.; Miltner, A.; Lippold, H.; Meyer, D.; Schmid, A.; Heipieper, H.J. Prediction of the adaptability of Pseudomonas putida DOT-T1E to a second phase of a solvent for economically sound two-phase biotransformations. Appl. Environ. Microbiol. 2005, 71, 6606–6612. [Google Scholar] [CrossRef] [Green Version]
- Blank, L.M.; Ionidis, G.; Ebert, B.E.; Bühler, B.; Schmid, A. Metabolic response of Pseudomonas putida during redox biocatalysis in the presence of a second octanol phase. FEBS J. 2008, 275, 5173–5190. [Google Scholar] [CrossRef]
- Domínguez de María, P.; Hollmann, F. On the (un) greenness of biocatalysis: Some challenging figures and some promising options. Front. Microbiol. 2015, 6, 1257. [Google Scholar] [CrossRef] [Green Version]
- Heipieper, H.J.; Diefenbach, R.; Keweloh, H. Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl. Environ. Microbiol. 1992, 58, 1847–1852. [Google Scholar] [CrossRef] [Green Version]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Eberlein, C.; Baumgarten, T.; Starke, S.; Heipieper, H.J. Immediate response mechanisms of Gram-negative solvent-tolerant bacteria to cope with environmental stress: Cis-trans isomerization of unsaturated fatty acids and outer membrane vesicle secretion. Appl. Microbiol. Biotechnol. 2018, 102, 2583–2593. [Google Scholar] [CrossRef] [Green Version]
- Heipieper, H.J.; Neumann, G.; Cornelissen, S.; Meinhardt, F. Solvent-tolerant bacteria for biotransformations in two-phase fermentation systems. Appl. Microbiol. Biotechnol. 2007, 74, 961–973. [Google Scholar] [CrossRef]
- Rojas, A.; Duque, E.; Mosqueda, G.; Golden, G.; Hurtado, A.; Ramos, J.L.; Segura, A. Three efflux pumps are required to provide efficient tolerance to toluene in Pseudomonas putida DOT-T1E. J. Bacteriol. 2001, 183, 3967–3973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillet, S.; Daniels, C.; Pini, C.; Krell, T.; Duque, E.; Bernal, P.; Segura, A.; Lu, D.; Zhang, X.; Ramos, J.L. Transcriptional control of the main aromatic hydrocarbon efflux pump in Pseudomonas. Environ. Microbiol. Rep. 2012, 4, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.L.; Duque, E.; Gallegos, M.-T.; Godoy, P. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 2002, 56, 743–768. [Google Scholar] [CrossRef]
- Vasylkivska, M.; Patakova, P. Role of efflux in enhancing butanol tolerance of bacteria. J. Biotechnol. 2020, 320, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Krell, T.; Lacal, J.; Guazzaroni, M.E.; Busch, A.; Silva-Jiménez, H.; Fillet, S.; Reyes-Darías, J.A.; Muñoz-Martínez, F.; Rico-Jiménez, M.; García-Fontana, C. Responses of Pseudomonas putida to toxic aromatic carbon sources. J. Biotechnol. 2012, 160, 25–32. [Google Scholar] [CrossRef]
- Mukhopadhyay, A. Tolerance engineering in bacteria for the production of advanced biofuels and chemicals. Trends Microbiol. 2015, 23, 498–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieder, S.; Nikel, P.I.; de Lorenzo, V.; Takors, R. Genome reduction boosts heterologous gene expression in Pseudomonas putida. Microb. Cell Factories 2015, 14, 23. [Google Scholar] [CrossRef] [Green Version]
- Kampers, L.F.C.; Volkers, R.J.M.; Martins Dos Santos, V.A.P. Pseudomonas putida KT2440 is HV1 certified, not GRAS. Microb. Biotechnol. 2019, 12, 845–848. [Google Scholar] [CrossRef] [Green Version]
- Keshavarz-Tohid, V.; Vacheron, J.; Dubost, A.; Prigent-Combaret, C.; Taheri, P.; Tarighi, S.; Taghavi, S.M.; Moenne-Loccoz, Y.; Muller, D. Genomic, phylogenetic and catabolic re-assessment of the Pseudomonas putida clade supports the delineation of Pseudomonas alloputida sp. nov., Pseudomonas inefficax sp. nov., Pseudomonas persica sp. nov., and Pseudomonas shirazica sp. nov. Syst. Appl. Microbiol. 2019, 42, 468–480. [Google Scholar] [CrossRef]
- Wynands, B.; Otto, M.; Runge, N.; Preckel, S.; Polen, T.; Blank, L.M.; Wierckx, N. Streamlined Pseudomonas taiwanensis VLB120 chassis strains with improved bioprocess features. ACS Synth. Biol. 2019, 8, 2036–2050. [Google Scholar] [CrossRef]
- Martínez-García, E.; Nikel, P.I.; Aparicio, T.; de Lorenzo, V. Pseudomonas 2.0: Genetic upgrading of P. putida KT2440 as an enhanced host for heterologous gene expression. Microb. Cell Factories 2014, 13, 159. [Google Scholar] [CrossRef]
- Puiggené, Ò.; Espinosa, M.J.C.; Schlosser, D.; Thies, S.; Jehmlich, N.; Kappelmeyer, U.; Schreiber, S.; Wibberg, D.; Kalinowski, J.; Harms, H. Extracellular degradation of a polyurethane oligomer involving outer membrane vesicles and further insights on the degradation of 2, 4-diaminotoluene in Pseudomonas capeferrum TDA1. Sci. Rep. 2022, 12, 2666. [Google Scholar] [CrossRef]
- Espinosa, M.J.C.; Blanco, A.C.; Schmidgall, T.; Atanasoff-Kardjalieff, A.K.; Kappelmeyer, U.; Tischler, D.; Pieper, D.H.; Heipieper, H.J.; Eberlein, C. Toward biorecycling: Isolation of a soil bacterium that grows on a polyurethane oligomer and monomer. Front. Microbiol. 2020, 11, 404. [Google Scholar] [CrossRef] [Green Version]
- Utomo, R.N.C.; Li, W.J.; Tiso, T.; Eberlein, C.; Doeker, M.; Heipieper, H.J.; Jupke, A.; Wierckx, N.; Blank, L.M. Defined Microbial Mixed Culture for Utilization of Polyurethane Monomers. ACS Sustain. Chem. Eng. 2020, 8, 17466–17474. [Google Scholar] [CrossRef]
- Hartmans, S.; Smits, J.; Van der Werf, M.; Volkering, F.; De Bont, J. Metabolism of styrene oxide and 2-phenylethanol in the styrene-degrading Xanthobacter strain 124X. Appl. Environ. Microbiol. 1989, 55, 2850–2855. [Google Scholar] [CrossRef] [Green Version]
- Heipieper, H.J.; Loffeld, B.; Keweloh, H.; Debont, J.A.M. The Cis/Trans Isomerization of Unsaturated Fatty-Acids in Pseudomonas putida S12—An Indicator for Environmental-Stress Due to Organic-Compounds. Chemosphere 1995, 30, 1041–1051. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Heipieper, H.; Meinhardt, F.; Segura, A. The cis–trans isomerase of unsaturated fatty acids in Pseudomonas and Vibrio: Biochemistry, molecular biology and physiological function of a unique stress adaptive mechanism. FEMS Microbiol. Lett. 2003, 229, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Concha, E.; Heipieper, H.J.; Wick, L.Y.; Navia, R. Effects of limonene, n-decane and n-decanol on growth and membrane fatty acid composition of the microalga Botryococcus braunii. AMB Express 2018, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Basler, G.; Thompson, M.; Tullman-Ercek, D.; Keasling, J. A Pseudomonas putida efflux pump acts on short-chain alcohols. Biotechnol. Biofuels 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wynands, B.N.D.; Wierckx, N.; Blank, L.M. Engineering of Pseudomonas taiwanensis VLB120 for the sustainable production of hydroxylated aromatics; Fachgruppe Biologie: Aachen, Germany, 2019. [Google Scholar]
- Rojas, A.; Duque, E.; Schmid, A.; Hurtado, A.; Ramos, J.-L.; Segura, A. Biotransformation in double-phase systems: Physiological responses of Pseudomonas putida DOT-T1E to a double phase made of aliphatic alcohols and biosynthesis of substituted catechols. Appl. Environ. Microbiol. 2004, 70, 3637–3643. [Google Scholar] [CrossRef] [Green Version]
- Otto, M.; Wynands, B.; Marienhagen, J.; Blank, L.M.; Wierckx, N. Benzoate Synthesis from Glucose or Glycerol Using Engineered Pseudomonas taiwanensis. Biotechnol. J. 2020, 15, e2000211. [Google Scholar] [CrossRef]
- Sayqal, A.; Xu, Y.; Trivedi, D.K.; AlMasoud, N.; Ellis, D.I.; Rattray, N.J.; Goodacre, R. Metabolomics analysis reveals the participation of efflux pumps and ornithine in the response of Pseudomonas putida DOT-T1E cells to challenge with propranolol. PLoS ONE 2016, 11, e0156509. [Google Scholar] [CrossRef] [Green Version]
- Isken, S.; Derks, A.; Wolffs, P.F.; de Bont, J.A. Effect of organic solvents on the yield of solvent-tolerant Pseudomonas putida S12. Appl. Environ. Microbiol. 1999, 65, 2631–2635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volmer, J.; Neumann, C.; Bühler, B.; Schmid, A. Engineering of Pseudomonas taiwanensis VLB120 for constitutive solvent tolerance and increased specific styrene epoxidation activity. Appl. Environ. Microbiol. 2014, 80, 6539–6548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, J.M.; Rodríguez-Solana, R.; Curiel, J.A.; de Las Rivas, B.; Munoz, R.; Domínguez, J.M. Bioproduction of 4-vinylphenol from corn cob alkaline hydrolyzate in two-phase extractive fermentation using free or immobilized recombinant E. coli expressing pad gene. Enzym. Microb. Technol. 2014, 58, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Calero, P.; Nikel, P.I. Chasing bacterial chassis for metabolic engineering: A perspective review from classical to non-traditional microorganisms. Microb. Biotechnol. 2019, 12, 98–124. [Google Scholar] [CrossRef]
- De Bont, J.A. Solvent-tolerant bacteria in biocatalysis. Trends Biotechnol. 1998, 16, 493–499. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Martínez, P. Toxicity of hydrocarbons to microorganisms. In Cellular Ecophysiology of Microbe: Hydrocarbon and Lipid Interactions. Handbook of Hydrocarbon and Lipid Microbiology; Springer: Cham, Switzerland, 2018; Volume 335. [Google Scholar]
- Eberlein, C.; Starke, S.; Doncel, A.E.; Scarabotti, F.; Heipieper, H.J. Quantification of outer membrane vesicles: A potential tool to compare response in Pseudomonas putida KT2440 to stress caused by alkanols. Appl. Microbiol. Biotechnol. 2019, 103, 4193–4201. [Google Scholar] [CrossRef]
- Verhoef, S.; Wierckx, N.; Westerhof, R.M.; de Winde, J.H.; Ruijssenaars, H.J. Bioproduction of p-hydroxystyrene from glucose by the solvent-tolerant bacterium Pseudomonas putida S12 in a two-phase water-decanol fermentation. Appl. Environ. Microbiol. 2009, 75, 931–936. [Google Scholar] [CrossRef] [Green Version]
- Garikipati, S.J.; Peeples, T.L. Solvent resistance pumps of Pseudomonas putida S12: Applications in 1-naphthol production and biocatalyst engineering. J. Biotechnol. 2015, 210, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Strain | Abbreviation | ttgTGH | ttgVW |
|---|---|---|---|
| P. taiwanensis VLB120 | VLB120 or wildtype | + | + |
| P. taiwanensis VLB120 GRC1 | GRC1 | − | − |
| P.taiwanensis VLB120 GRC2 | GRC2 | + | − |
| P.taiwanensis VLB120 (GRC3) | GRC3 | + | + |
| P. capeferrum TDA1 | TDA1 | − | − |
| n-alkanols | P. putida DOT-T1E [4] | P. taiwanensis GRC3 | P. capeferrum TDA1 |
|---|---|---|---|
| 1-Butanol | 49 | 73 | 123 |
| 1-Hexanol | 6.35 | 6 | 6 |
| 1-Octanol | 0.80 | 1.38 | 1.75 |
| 1-Decanol | 0.11 | 0.25 | 0.26 |
| Strain | 1-Octanol OD | 1-Octanol Adaptation | 1-Decanol OD | 1-Decanol Adaptation |
|---|---|---|---|---|
| P. taiwanensis VLB120 | 0.73 ± 0.02 | + | 1.25 ± 0.31 | ++ |
| P. taiwanensis GRC1 | 1.33 ± 0.09 | ++ | 1.36 ± 0.14 | ++ |
| P. taiwanensis GRC2 | 1.25 ± 0.16 | ++ | 1.47 ± 0.21 | ++ |
| P. taiwanensis GRC3 | 1.07 ± 0.11 | ++ | 1.72 ± 0.34 | ++ |
| P. capeferrum TDA1 | 0.71 ± 0.18 | + | 2.28 ± 0.10 | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas Espinosa, M.J.; Schmidgall, T.; Pohl, J.; Wagner, G.; Wynands, B.; Wierckx, N.; Heipieper, H.J.; Eberlein, C. Assessment of New and Genome-Reduced Pseudomonas Strains Regarding Their Robustness as Chassis in Biotechnological Applications. Microorganisms 2023, 11, 837. https://doi.org/10.3390/microorganisms11040837
Cárdenas Espinosa MJ, Schmidgall T, Pohl J, Wagner G, Wynands B, Wierckx N, Heipieper HJ, Eberlein C. Assessment of New and Genome-Reduced Pseudomonas Strains Regarding Their Robustness as Chassis in Biotechnological Applications. Microorganisms. 2023; 11(4):837. https://doi.org/10.3390/microorganisms11040837
Chicago/Turabian StyleCárdenas Espinosa, María José, Tabea Schmidgall, Jessica Pohl, Georg Wagner, Benedikt Wynands, Nick Wierckx, Hermann J. Heipieper, and Christian Eberlein. 2023. "Assessment of New and Genome-Reduced Pseudomonas Strains Regarding Their Robustness as Chassis in Biotechnological Applications" Microorganisms 11, no. 4: 837. https://doi.org/10.3390/microorganisms11040837







