Microorganisms for Bioremediation of Soils Contaminated with Heavy Metals
Abstract
:1. Introduction
2. Materials and Methods
- Seeds were soaked in a consortium of different concentrations, followed by watering;
- Seeds were soaked in water, then watered with a consortium with different concentrations.
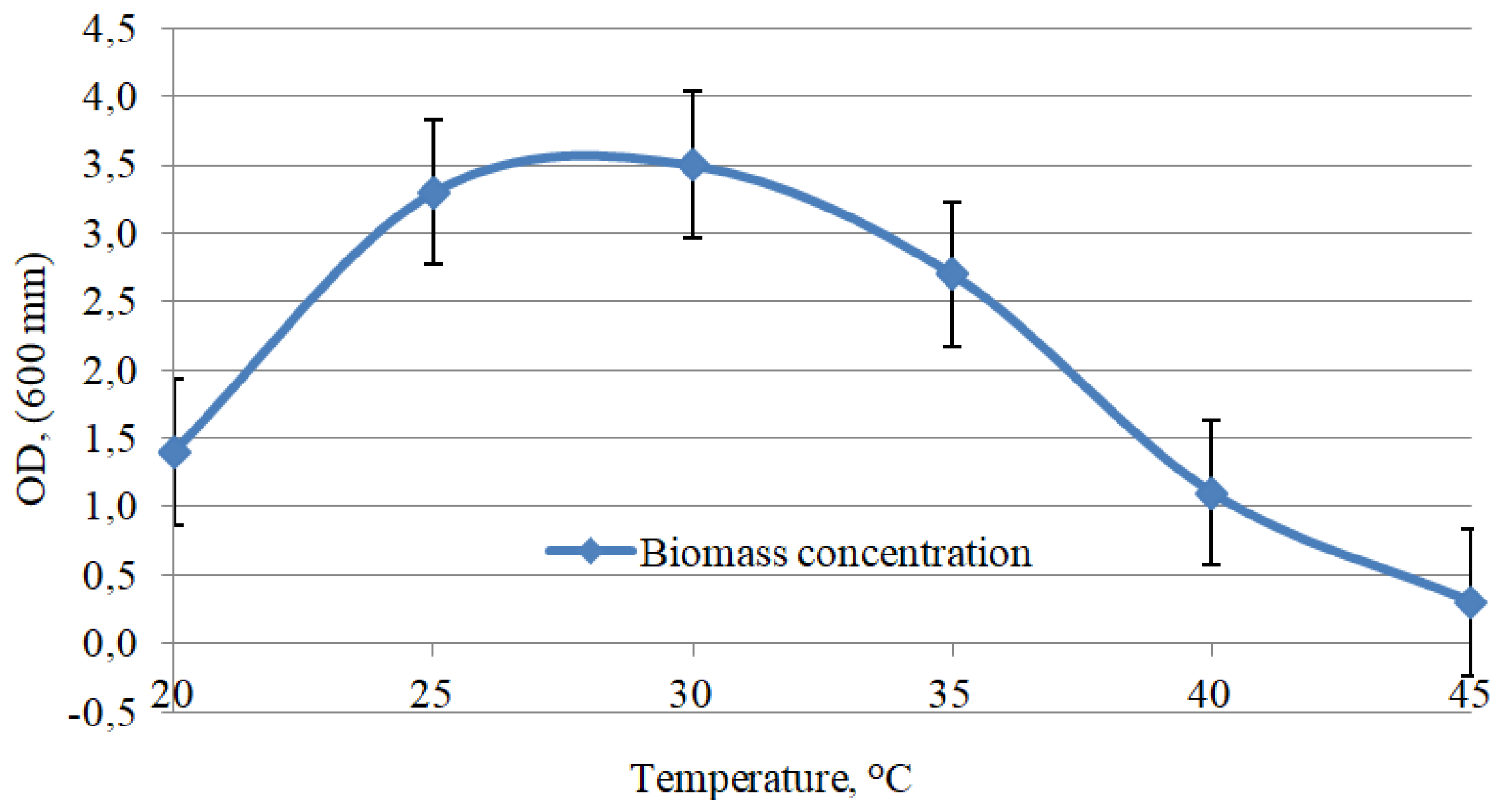
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaashikaa, P.R.; Kumar, P.S. Bioremediation of hazardous pollutants from agricultural soils: A sustainable approach for waste management towards urban sustainability. Environ. Pollut. 2022, 312, 120031. [Google Scholar] [CrossRef] [PubMed]
- Midov, A.Z.; Gavrilina, D.N.; Prosekov, A.Y. Strategizing of food security of Kuzbass. Russ. J. Ind. Econ. 2020, 13, 389–398. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ryo, M.; Lehmann, A.; Aguilar-trigueros, C.A.; Buchertanja, S.; Wulf, A.; Iwasaki, A.; Roy, J.; Yang, G. The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 2019, 366, 886–890. [Google Scholar] [CrossRef]
- Asyakina, L.K.; Dyshlyuk, L.S.; Prosekov, A.Y. Reclamation of Post-Technological Landscapes: International Experience. Food Process. Tech. Technol. 2021, 51, 805–818. [Google Scholar] [CrossRef]
- Debonne, N.; Vliet, J.; Metternicht, G.; Verburg, P. Agency shifts in agricultural land governance and their implications for land degradation neutrality. Glob. Environ. Chang. 2021, 66, 102221. [Google Scholar] [CrossRef]
- Nizamutdinov, T.I.; Suleymanov, A.R.; Morgun, E.N.; Dinkelaker, N.V.; Abakumov, E.V. Ecotoxicological analysis of fallow soils at the yamal experimental agricultural station. Food Process. Tech. Technol. 2022, 52, 350–360. [Google Scholar] [CrossRef]
- Dudley, N.; Alexander, S. Agriculture and biodiversity: A review. Biodiversity 2017, 18, 45–49. [Google Scholar] [CrossRef]
- Website of the United Nations (UN). Available online: https://sdgs.un.org/ru/goals (accessed on 2 February 2023).
- Uchimiya, M.; Bannon, D.; Nakanishi, H.; McBride, M.B.; Williams, M.A.; Yoshihara, T. Chemical speciation, plant uptake, and toxicity of heavy metals in agricultural soils. J. Agric. Food Chem. 2020, 68, 12856–12869. [Google Scholar] [CrossRef]
- Jiang, H.-H.; Cai, L.-M.; Wen, H.-H.; Hu, G.-C.; Chen, L.-G.; Luo, J. An integrated approach to quantifying ecological and human health risks from different sources of soil heavy metals. Sci. Total Environ. 2020, 701, 134466. [Google Scholar] [CrossRef]
- Guan, Q.; Liu, Z.; Shao, W.; Tian, J.; Luo, H.; Ni, F.; Shan, Y. Probabilistic risk assessment of heavy metals in urban farmland soils of a typical oasis city in northwest China. Sci. Total Environ. 2022, 833, 155096. [Google Scholar] [CrossRef]
- Prosekov, A.Y. Migration of mercury in the food chains of the beloosipovo biocenosis (part 1). Foods Raw Mater. 2021, 9, 324–334. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Kapusta-Duch, J.; Leszczyńska, T.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Comparison of lead and cadmium contents in cruciferous vegetables grown under diversified ecological conditions: Cracow region of Poland. Ecol. Food Nutr. 2011, 50, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Gonga, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef]
- Sun, R.; Yang, J.; Xia, P.; Wu, S.; Lin, T.; Yi, Y. Contamination features and ecological risks of heavy metals in the farmland along shoreline of Caohai plateau wetland, China. Chemosphere 2020, 254, 126828. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Drozdova, M.Y.; Pozdnyakova, A.V.; Osintseva, M.A.; Burova, N.V.; Minina, V.I. The microorganism-plant system for remediation of soil exposed to coal mining. Foods Raw Mater. 2021, 9, 406–418. [Google Scholar] [CrossRef]
- Twarog, A.; Mamak, M.; Sechman, H.; Rusiniak, P.; Kasprzak, E.; Stanek, K. Impact of the landfill of ashes from the smelter on the soil environment: Case study from the South Poland, Europe. Environ. Geochem. Health 2020, 42, 1453–1467. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Jiang, D.; Ding, D.; Wu, Y.; Wei, J.; Kong, L.; Long, T.; Fan, T.; Deng, S. Ecological-health risks assessment and source apportionment of heavy metals in agricultural soils around a super-sized lead-zinc smelter with a long production history, in China. Environ. Pollut. 2022, 307, 119487. [Google Scholar] [CrossRef]
- Guan, D.-X.; Sun, F.-S.; Yu, G.-H.; Polizzotto, M.L.; Liu, Y.-G. Total and available metal concentrations in soils from six long-term fertilization sites across China. Environ. Sci. Pollut. Res. Int. 2018, 25, 31666–31678. [Google Scholar] [CrossRef]
- Defarge, N.; de Vendômois, J.S.; Seralini, G.E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.Y.; Zeng, X.B.; Su, S.M.; Duan, R.; Wang, Y.N.; Gao, X. Heavy metal accumulation and source analysis in greenhouse soils of Wuwei District, Gansu Province, China. Environ. Sci. Pollut. Res. Int. 2015, 22, 5359–5369. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Rahman, Z.; Singh, V.P. Bioremediation of toxic heavy metals (THMs) contaminated sites: Concepts, applications and challenges. Environ. Sci. Pollut. Res. Int. 2020, 27, 27563–27581. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Fajardo, C.; Costa, G.; Nande, M.; Botías, P.; García-Cantalejo, J.; Martin, M. Pb, Cd, and Zn soil contamination: Monitoring functional and structural impacts on the microbiome. Appl. Soil Ecol. 2019, 135, 56–64. [Google Scholar] [CrossRef]
- Haque, S.; Srivastava, N.; Pal, D.B.; Alkhanani, M.F.; Almalki, A.H.; Areeshi, M.Y.; Naidu, R.; Gupta, V.K. Functional microbiome strategies for the bioremediation of petroleum-hydrocarbon and heavy metal contaminated soils: A review. Sci. Total Environ. 2022, 833, 155222. [Google Scholar] [CrossRef]
- Faskhudinova, E.R.; Osinceva, M.A.; Neverova, O.A. Prospects of Using Soil Microbiome of Mine Tips for Remediation of Anthropogenically Disturbed Ecosystems. Food Process. Tech. Technol. 2021, 51, 883–904. [Google Scholar] [CrossRef]
- Yang, X.; Qin, X.; Xie, J.; Li, X.; Xu, H.; Zhao, Y. Study on the effect of Cr(VI) removal by stimulating indigenous microorganisms using molasses. Chemosphere 2022, 308, 136229. [Google Scholar] [CrossRef]
- Martis, B.S.; Mohan, A.K.; Chiplunkar, S.; Kamath, S.; Goveas, L.C.; Rao, C.V. Bacterium isolated from coffee waste pulp biosorps lead: Investigation of EPS mediated mechanism. Curr. Res. Microb. Sci. 2021, 2, 100029. [Google Scholar] [CrossRef]
- Pressler, Y.; Zhou, J.; He, Z.; van Nostrand, J.D.; Smith, A.P. Post-agricultural tropical forest regeneration shifts soil microbial functional potential for carbon and nutrient cycling. Soil Biol. Biochem. 2020, 145, 107784. [Google Scholar] [CrossRef]
- Wu, M.-H.; Chen, S.-Y.; Chen, J.-W.; Xue, K.; Chen, S.-L.; Wang, X.-M.; Chen, T.; Kang, S.-C.; Rui, J.-P.; Thies, J.E.; et al. Reduced microbial stability in the active layer is associated with carbon loss under alpine permafrost degradation. Proc. Natl. Acad. Sci. USA 2020, 118, e2025321118. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Zhu, Y.-G.; Chu, H. Microbial resistance promotes plant production in a four-decade nutrient fertilization experiment. Soil Biol. Biochem. 2020, 141, 107679. [Google Scholar] [CrossRef]
- Khan, I.; Aftab, M.; Shakir, S.; Ali, M.; Qayyum, S.; Rehman, M.U.; Haleem, K.S.; Touseef, I. Mycoremediation of heavy metal (Cd and Cr)–polluted soil through indigenous metallotolerant fungal isolates. Environ. Monit. Assess. 2019, 191, 585. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-C.; Chen, J.-X.; Wang, Y.-G.; Leng, F.-F.; Zhao, J.; Chen, K.; Zhang, Q.-C. Molecular mechanisms of heavy metals resistance of Stenotrophomonas rhizophila JC1 by whole genome sequencing. Arch. Microbiol. 2021, 203, 2699–2709. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Chen, Q.; Gu, Y.; Zhao, K.; Xiang, Q.; Zou, L.; Ma, M. The potential of cadmium ion-immobilized Rhizobium pusense KG2 to prevent soybean root from absorbing cadmium in cadmium-contaminated soil. J. Appl. Microbiol. 2019, 126, 919–930. [Google Scholar] [CrossRef]
- Mo, F.; Li, H.; Li, Y.; Cui, W.; Wang, M.; Li, Z.; Chai, R.; Wang, H. Toxicity of Ag+ on microstructure, biochemical activities and genic material of Trifolium pratense L. seedlings with special reference to phytoremediation. Ecotoxicol. Environ. Saf. 2020, 195, 110499. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, H.; Lu, J.; Chen, Q.; Li, W.; Wu, L.; Tang, J.; Ma, L. The Immobilization of Soil Cadmium by the Combined Amendment of Bacteria and Hydroxyapatite. Sci. Rep. 2020, 10, 2189. [Google Scholar] [CrossRef] [Green Version]
- GOST 17.4.4.02–2017. Protection of Nature. Soils. Methods for Taking and Preparing Samples for Chemical, Bacteriological, Helminthological Analysis. Available online: https://files.stroyinf.ru/Data2/1/4293737/4293737734.pdf (accessed on 3 February 2023).
- Julia, W.T.; Gunther, A. A Streak Plate Method for determining Growth Curves. J. Lab. Clin. Med. 1947, 32, 11–39. [Google Scholar]
- Wu, B.; Luo, S.; Luo, H.; Huang, H.; Xu, F.; Feng, S.; Xu, H. Improved phytoremediation of heavy metal contaminated soils by Miscanthus floridulus under a varied rhizosphere ecological characteristic. Sci. Total Environ. 2022, 808, 15995. [Google Scholar] [CrossRef]
- Naaz, F.; Bhattacharya, A.; Mathur, M.; Bano, F.; Pant, K.K.; Malik, A. Exploration of heavy metal uptake potential of three algal strains/consortia in suspended and attached growth systems. J. Water Process. Eng. 2021, 43, 102315. [Google Scholar] [CrossRef]
- Voitenkova, E.V.; Matveeva, Z.N.; Makarova, M.A.; Egorova, S.A.; Zabrovskaia, A.V.; Suzhaeva, L.V.; Zueva, E.V.; Kaftyreva, L.A. Difficulties in identification of Comamonas kerstersii strains isolated from intestinal microbiota of residents of republic of Guinea and Russian Federation. Infektsiia Immun. 2018, 8, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Irkitova, A.N.; Kagan, J.R.; Sokolova, G.G. Comparative analysis of the methods to define antagonistic activity of Lactic Bacteria. News Altai State Univ. 2012, 41–44. [Google Scholar]
- Sarmiento-Lopez, L.G.; Lopez-Meyer, M.; Maldonado-Mendoza, I.E.; Quiroz-Figueroa, F.R.; Sepúlveda-Jiménez, G.; Rodríguez-Monroy, M. Production of indole-3-acetic acid by Bacillus circulans E9 in a low-cost medium in a bioreactor. J. Biosci. Bioeng. 2022, 134, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Morales, L.G.; Soto-Urzua, L.; Baca, E.B.; Sa¤nchez-Ahedo, J.A. Indole-3-butyric acid (IBA) production in culture medium by wild strain Azospirillum brasilense. FEMS Microbiol. Lett. 2003, 228, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Talukdar, D.; Jasrotia, T.; Sharma, R.; Jaglan, S.; Kumar, R.; Vats, R.; Kumar, R.; Mahnashi, M.H.; Umar, A. Evaluation of novel indigenous fungal consortium for enhanced bioremediation of heavy metals from contaminated sites. Environ. Technol. Innov. 2020, 20, 101050. [Google Scholar] [CrossRef]
- Liu, C.; Lin, H.; Li, B.; Dong, Y.; Menzembere, E.R.G.Y. Endophyte Pseudomonas putida enhanced Trifolium repens L. growth and heavy metal uptake: A promising in-situ non-soil cover phytoremediation method of nonferrous metallic tailing. Chemosphere 2021, 272, 129816. [Google Scholar] [CrossRef]
- Liu, A.; Wang, W.; Chen, X.; Zheng, X.; Fu, W.; Wang, G.; Ji, J.; Guan, C. Phytoremediation of DEHP and heavy metals co-contaminated soil by rice assisted with a PGPR consortium: Insights into the regulation of ion homeostasis, improvement of photosynthesis and enrichment of beneficial bacteria in rhizosphere soil. Environ. Pollut. 2022, 314, 120303. [Google Scholar] [CrossRef]
- Wang, G.; Yang, Y.; Kong, Y.; Ma, R.; Yuan, J.; Li, G. Rodríguez-Llorente, I.D.; Mateos-Naranjo, E. Key factors affecting seed germination in phytotoxicity tests during sheep manure composting with carbon additives. J. Hazard. Mater. 2022, 421, 126809. [Google Scholar] [CrossRef]
- Paredes-Paliz, K.I.; Pajuelo, E.; Doukkali, B.; Caviedes, M.A. Bacterial inoculants for enhanced seed germination of Spartina densiflora: Implications for restoration of metal polluted areas. Mar. Pollut. Bull. 2016, 110, 396–400. [Google Scholar] [CrossRef]
- Budryn, G.; Gałązka-Czarnecka, I.; Brzozowska, E.; Grzelczyk, J.; Mostowski, R.; Żyżelewicz, D.; José Cerón-Carrasco, P.; Pérez-Sánchez, H. Evaluation of estrogenic activity of red clover (Trifolium pratense L.) sprouts cultivated under different conditions by content of isoflavones, calorimetric study and molecular modeling. Food Chem. 2018, 245, 324–336. [Google Scholar] [CrossRef]
- Harris, A.; Xanthos, S.J.; Galiotos, J.K.; Douvris, C. Investigation of the metal content of sediments around the historically polluted Potomac River basin in Washington D.C., United States by inductively coupled plasma-optical emission spectroscopy (ICP-OES). Microchem. J. 2018, 142, 140–143. [Google Scholar] [CrossRef]
- Xie, Y.; Bu, H.; Feng, Q.; Wassie, M.; Amee, M.; Jiang, Y.; Bi, Y.; Hu, L.; Chen, L. Identification of Cd-resistant microorganisms from heavy metal-contaminated soil and its potential in promoting the growth and Cd accumulation of bermudagrass. Environ. Res. 2021, 200, 111730. [Google Scholar] [CrossRef] [PubMed]
- Anusha, P.; Natarajan, D. Bioremediation potency of multi metal tolerant native bacteria Bacillus cereus isolated from bauxite mines, kolli hills, Tamilnadu—A lab to land approach. Biocatal. Agric. Biotechnol. 2020, 25, 101581. [Google Scholar] [CrossRef]
- Xie, Y.; Luo, H.; Du, Z.; Hu, L.; Fu, J. Identification of cadmium-resistant fungi related to Cd transportation in bermudagrass [Cynodon dactylon (L.) Pers.]. Chemosphere 2014, 117, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.-F.; Xia, J.-J.; Jiang, C.-Y.; He, L.-Y.; Qian, M. Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ. Pollut. 2008, 156, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, S.; Yang, M.; Ning, X.; Nan, Z. Experimental study on treatment of heavy metal–contaminated soil by manganese-oxidizing bacteria. Environ. Sci. Pollut. Res. Int. 2022, 29, 5526–5540. [Google Scholar] [CrossRef]
- Liang, D.H.; Hu, Y. Application of a heavy metal-resistant Achromobacter sp. for the simultaneous immobilization of cadmium and degradation of sulfamethoxazole from wastewater. J. Hazard. Mater. 2021, 402, 124032. [Google Scholar] [CrossRef]
- Ni, L.; Xu, Y.; Chen, L. First experimental evidence for the presence of potentially virulent Klebsiella Oxytoca in 14 species of commonly consumed aquatic animals, and phenotyping and genotyping of K. Oxytoca isolates. Antibiotics 2021, 10, 1235. [Google Scholar] [CrossRef]
- Alboghobeish, H.; Tahmourespour, A.; Doudi, M. The study of Nickel Resistant Bacteria (NiRB) isolated from wastewaters polluted with different industrial sources. J. Environ. Sci. Eng. 2014, 12, 44. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Cui, Y.; Zhou, J.; Penttinen, P.; Liu, J.; Zeng, L.; Chen, Q.; Gu, Y.; Zou, L.; Zhao, K.; et al. Nickel mine soil is a potential source for soybean plant growth promoting and heavy metal tolerant rhizobia. PeerJ 2022, 10, e13215. [Google Scholar] [CrossRef]
- Suliasih; Widawati, S. The application of Klebsiella sp. and Rhizobium Radiobacter as biofertilizer and palm oil mills effluent (POME) as organic fertilizer on growth of Paraserianthes falcataria. IOP Conf. Ser. Earth Environ. Sci. 2018, 308, 152075. [Google Scholar] [CrossRef] [Green Version]
- Mogal, C.S.; Solanki, V.H.; Kansara, R.W.; Jha, S.; Singh, S.; Parekh, V.B.; Rajkumar, B.K. UHPLC-MS/MS and QRT-PCR profiling of PGP agents and Rhizobium spp. of induced phytohormones for growth promotion in mungbean (var. Co4). Helion 2022, 8, e09532. [Google Scholar] [CrossRef]
- Singh, K.; Tripathi, S.; Chandra, R. Bacterial assisted phytoremediation of heavy metals and organic pollutants by Cannabis sativa as accumulator plants growing on distillery sludge for ecorestoration of polluted site. J. Environ. Manag. 2023, 332, 117294. [Google Scholar] [CrossRef]
- Mitra, S.; Pramanik, K.; Ghosh, P.K.; Soren, T.; Sarkar, A.; Dey, R.S.; Pandey, S.; Maiti, T.K. Characterization of Cd-resistant Klebsiella michiganensis MCC3089 and its potential for rice seedling growth promotion under Cd stress. Microbiol. Res. 2018, 210, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, X.; Wang, Y.; Cheng, Y.; Fan, W. Long-term bioremediation of cadmium contaminated sediment using sulfate reducing bacteria: Perspective on different depths of the sediment profile. Chem. Eng. J. 2023, 451, 138697. [Google Scholar] [CrossRef]
- Peng, W.; Li, X.; Song, J.; Jiang, W.; Liu, Y.; Fan, W. Bioremediation of cadmium- and zinc-contaminated soil using Rhodobacter sphaeroides. Chemosphere 2018, 197, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.H.; Ullah, I.; Dunwell, J.M.; Tibbett, M. Bioremediation potential of Cd by transgenic yeast expressing a metallothionein gene from Populus trichocarpa. Ecotoxicol. Environ. Saf. 2020, 202, 110917. [Google Scholar] [CrossRef]
- Babu, A.G.; Shea, P.J.; Sudhakar, D.; Jung, I.-B.; Oh, B.-T. Potential use of Pseudomonas koreensis AGB-1 in association with Miscanthus sinensis to remediate heavy metal(loid)-contaminated mining site soil. J. Environ. Manag. 2015, 151, 160–166. [Google Scholar] [CrossRef]
- Durand, A.; Piutti, S.; Rue, M.; Morel, J.L.; Echevarria, G.; Benizri, E. Improving nickel phytoextraction by co-cropping hyperaccumulator plants inoculated by plant growth promoting rhizobacteria. Plant Soil 2016, 399, 179–192. [Google Scholar] [CrossRef]



| Microorganism | Cultural and Morphological Features |
|---|---|
| 1 | Mobile, Gram-negative bacilli of 0.5–1.0 × 1.0–3.0 µm that do not form spores; even and round glossy yellow colonies with a flat profile, 1.7–2.1 mm in diameter. |
| 2 | Immobile, Gram-positive diplo-bacilli of 1.1–1.6 × 0.5–0.7 μm that do not form spores but have a capsule; even and round glossy yellow colonies with an elevated profile, 0.9–1.2 mm in diameter. |
| 3 | Mobile, Gram-negative bacilli of 0.3–0.5 × 0.9–1.2 μm that do not form spores; even and round dull colonies, non-pigmented, slightly convex, 1.0–1.5 mm in diameter. |
| 4 | Immobile, Gram-positive bacilli of 0.4–0.7 × 1.5–1.7 µm that do not form spores and have a nucleus; even and round glossy colonies, whitish and flat, 2.1–2.8 mm in diameter. |
| 5 | Mobile, Gram-negative bacilli of 0.4–0.6 × 0.3–0.5 µm that form spores; even and round yellow glossy colonies, with an elevated profile, 0.9–1.2 mm in diameter. |
| 6 | Immotile, Gram-negative bacilli of 1.3–1.5 × 0.6–0.8 µm that form spores and have a capsule; even and round oily colonies of light beige color, convex, 2.0–3.5 mm in diameter. |
| 7 | Mobile, Gram-negative bacilli of 1.5–3.0 × 0.6–1.0 µm that do not form spores; even and round glossy colonies, non-pigmented and convex, 0.9–1.1 mm in diameter. |
| 8 | Mobile, Gram-positive diplococci and streptococci of 0.5–0.6 µm that do not form spores; even and rounded dim colonies, transparent and flat, 1.7–2.1 mm in diameter. |
| 9 | Mobile, Gram-negative cocci and diplococci of 0.4–0.6 µm that form spores; smooth and round orange oily colonies, with an elevated profile, 0.8–1.0 mm in diameter. |
| 10 | Mobile, Gram-negative bacilli of 0.7–0.8 × 2.3–2.8 µm that form spores; even and round glossy colonies, light brown, with an elevated profile, 0.8–1.3 mm in diameter. |
| Isolate | Total Metal Absorption, mg/L | ||||
|---|---|---|---|---|---|
| Pb | As | Hg | Ni | Cd | |
| 1 | 62.15 ± 3.17 | 37.44 ± 1.65 | 45.44 ± 2.23 | 48.10 ± 2.36 | 57.51 ± 2.65 |
| 2 | 46.02 ± 2.25 | 25.76 ± 1.03 | 13.32 ± 0.96 | 47.41 ± 2.04 | 19.14 ± 1.14 |
| 3 | 73.10 ± 2.55 | 40.03 ± 1.60 | 73.08 ± 3.82 | 53.32 ± 2.29 | 62.41 ± 2.99 |
| 4 | 34.76 ± 1.77 | 33.21 ± 1.43 | 41.44 ± 1.70 | 36.08 ± 1.62 | 38.50 ± 1.85 |
| 5 | 36.02 ± 1.66 | 28.47 ± 1.31 | 21.46 ± 1.01 | 39.74 ± 1.22 | 16.72 ± 0.87 |
| 6 | 51.14 ± 2.35 | 44.37 ± 1.86 | 51.33 ± 2.31 | 47.32 ± 2.13 | 78.02 ± 3.67 |
| 7 | 74.26 ± 3.42 | 59.52 ± 2.86 | 61.57 ± 3.16 | 56.96 ± 2.90 | 41.65 ± 2.04 |
| 8 | 25.11 ± 1.16 | 24.57 ± 1.03 | 32.34 ± 1.68 | 48.19 ± 1.97 | 29.97 ± 1.44 |
| 9 | 22.04 ± 1.06 | 10.36 ± 0.41 | 64.38 ± 2.64 | 43.50 ± 2.11 | 11.84 ± 0.50 |
| 10 | 46.36 ± 2.04 | 43.45 ± 2.16 | 56.16 ± 2.75 | 43.32 ± 2.21 | 53.46 ± 2.19 |
| Isolate | Biomass Accumulation, g/L | |||||
|---|---|---|---|---|---|---|
| Pb | As | Hg | Ni | Cd | Control (No Metal) | |
| 1 | 0.88 ± 0.07 | 0.75 ± 0.07 | 0.69 ± 0.06 | 0.81 ± 0.07 | 0.90 ± 0.08 | 0.91 ± 0.07 |
| 2 | 0.82 ± 0.07 | 0.28 ± 0.02 | 0.21 ± 0.02 | 0.85 ± 0.07 | 0.54 ± 0.05 | 0.88 ± 0.05 |
| 3 | 0.85 ± 0.07 | 0.72 ± 0.06 | 0.77 ± 0.07 | 0.90 ± 0.08 | 0.87 ± 0.08 | 0.93 ± 0.05 |
| 4 | 0.63 ± 0.05 | 0.69 ± 0.06 | 0.51 ± 0.04 | 0.55 ± 0.05 | 0.58 ± 0.05 | 0.90 ± 0.06 |
| 5 | 0.50 ± 0.04 | 0.37 ± 0.03 | 0.41 ± 0.04 | 0.44 ± 0.04 | 0.29 ± 0.03 | 0.94 ± 0.08 |
| 6 | 0.68 ± 0.06 | 0.93 ± 0.08 | 0.88 ± 0.08 | 0.78 ± 0.07 | 0.86 ± 0.07 | 0.91 ± 0.06 |
| 7 | 0.81 ± 0.07 | 0.74 ± 0.06 | 0.61 ± 0.05 | 0.89 ± 0.08 | 0.77 ± 0.07 | 0.89 ± 0.07 |
| 8 | 0.33 ± 0.03 | 0.21 ± 0.02 | 0.48 ± 0.04 | 0.57 ± 0.05 | 0.40 ± 0.03 | 0.87 ± 0.04 |
| 9 | 0.35 ± 0.03 | 0.19 ± 0.02 | 0.74 ± 0.06 | 0.59 ± 0.05 | 0.22 ± 0.02 | 0.81 ± 0.05 |
| 10 | 0.82 ± 0.07 | 0.76 ± 0.07 | 0.68 ± 0.06 | 0.91 ± 0.08 | 0.79 ± 0.07 | 0.96 ± 0.08 |
| Substrate | Pantoea sp. | Achromobacter denitrificans | Klebsiella oxytoca | Rhizobium radiobacter | Pseudomonas fluorescens |
|---|---|---|---|---|---|
| Ala-Phe-Pro-arylamidase | − | − | − | − | − |
| Adonitol | − | − | + | + | − |
| L-pyrrolidonyl arylamidase | − | + | + | − | + |
| L-Arabitol | − | − | − | + | − |
| D-Cellobiose | + | − | + | + | − |
| Beta-galactosidase | + | − | + | − | − |
| H2S | − | − | − | − | − |
| Beta-N-acetyl-glucosaminidase | − | − | − | − | − |
| Glutamyl arylamidase pNA | − | − | − | − | − |
| D-glucose | + | − | + | + | + |
| Gamma-glutamyl-transferase | + | − | + | − | − |
| Fermentation/glucose | + | − | + | − | − |
| Beta-glucosidase | − | − | + | + | − |
| D-maltose | + | − | + | − | − |
| D-mannitol | + | − | + | + | − |
| D-mannose | + | − | + | + | + |
| Beta-xylosidase | + | − | + | − | − |
| Beta-alanine arylamidase pNA | − | − | − | − | − |
| L-proline arylamidase | − | − | − | − | + |
| Lipase | − | − | − | − | − |
| Palatinose | − | − | + | + | − |
| Tyrosine arylamidase | − | − | − | + | − |
| Urease | − | − | − | − | − |
| D-sorbitol | + | − | + | + | − |
| Saccharose/sucrose | + | − | + | + | − |
| D-tagatose | − | − | + | + | − |
| D-trehalose | + | − | + | + | − |
| Citrate (sodium) | + | − | + | − | + |
| Malonate | − | − | + | − | − |
| 5-keto-D-gluconate | − | − | + | − | − |
| L-Lactate alkalinization | + | + | + | − | − |
| Alpha-glucosidase | − | − | − | − | − |
| Succinate alkalinization | − | + | + | − | − |
| Beta-N-acetyl-galactosaminidase | − | − | − | − | − |
| Alpha-galactosidase | − | − | + | − | − |
| Phosphatase | + | − | + | − | − |
| Glycine arylamidase | − | − | − | − | + |
| Ornithine decarboxylase | − | − | − | − | − |
| Lysine decarboxylase | − | − | + | − | − |
| L-histidine assimilation | − | + | − | − | − |
| Coumarate | − | − | − | − | + |
| Beta-glucuronidase | − | − | − | − | − |
| O/129 resistance (comp. vibrio) | + | − | + | − | − |
| Glu-Gly-Arg-arylamidase | − | − | − | − | − |
| L-malate assimilation | + | − | − | − | − |
| ELLMAN | + | − | + | − | − |
| L-Lactate assimilation | − | − | − | − | − |
| Microorganism | Pantoea sp. | Achromobacter denitrificans | Klebsiella oxytoca | Rhizobium radiobacter | Pseudomonas fluorescens |
|---|---|---|---|---|---|
| Pantoea sp. | − | + | − | + | |
| Achromobacter denitrificans | − | + | + | − | |
| Klebsiella oxytoca | + | + | + | − | |
| Rhizobium radiobacter | − | + | + | − | |
| Pseudomonas fluorescens | + | − | − | − |
| Consortium | Total Metal Absorption, mg/L | ||||
|---|---|---|---|---|---|
| Pb | As | Hg | Ni | Cd | |
| A | 63.50 ± 3.11 | 45.08 ± 2.21 | 35.73 ± 1.75 | 54.46 ± 2.67 | 47.13 ± 2.31 |
| B | 82.84 ± 4.06 | 38.46 ± 1.89 | 63.90 ± 3.04 | 76.53 ± 3.75 | 78.60 ± 3.86 |
| C | 58.18 ± 2.85 | 49.42 ± 2.42 | 54.41 ± 2.67 | 51.98 ± 2.55 | 94.50 ± 4.64 |
| D | 91.13 ± 4.47 | 61.17 ± 3.00 | 58.03 ± 2.85 | 98.22 ± 4.82 | 56.39 ± 2.77 |
| Consortium | Biomass Accumulation, g/L | |||||
|---|---|---|---|---|---|---|
| Pb | As | Hg | Ni | Cd | Control (No Metal) | |
| A | 0.87 ± 0.08 | 0.71 ± 0.06 | 0.55 ± 0.06 | 0.94 ± 0.08 | 0.78 ± 0.07 | 0.92 ± 0.08 |
| B | 0.77 ± 0.07 | 0.72 ± 0.06 | 0.74 ± 0.07 | 0.85 ± 0.07 | 0.90 ± 0.08 | 0.91 ± 0.08 |
| C | 0.69 ± 0.06 | 0.90 ± 0.08 | 0.73 ± 0.06 | 0.67 ± 0.06 | 0.92 ± 0.08 | 0.91 ± 0.06 |
| D | 0.91 ± 0.08 | 0.74 ± 0.06 | 0.48 ± 0.05 | 0.71 ± 0.06 | 0.77 ± 0.07 | 0.93 ± 0.07 |
| Consortium | Total Metal Absorption, mg/L | Biomass Accumulation, g/L | |||||
|---|---|---|---|---|---|---|---|
| Pb | As | Hg | Ni | Cd | Composite Pollutant | Control | |
| A | 58.48 ± 2.87 | 41.91 ± 2.06 | 19.03 ± 0.93 | 42.81 ± 2.10 | 38.10 ± 1.87 | 0.87 ± 0.07 | 0.90 ± 0.08 |
| B | 39.83 ± 3.72 | 48.01 ± 2.60 | 23.41 ± 2.33 | 44.40 ± 4.04 | 18.95 ± 4.12 | 0.83 ± 0.08 | 0.91 ± 0.08 |
| C | 46.60 ± 2.29 | 26.19 ± 1.28 | 14.65 ± 0.72 | 21.73 ± 1.07 | 58.08 ± 2.85 | 0.85 ± 0.07 | 0.88 ± 0.08 |
| D | 75.07 ± 1.92 | 53.72 ± 2.39 | 47.33 ± 1.14 | 82.95 ± 2.20 | 83.26 ± 0.90 | 0.96 ± 0.07 | 0.90 ± 0.08 |
| Consortium | Indole-3-Acetic Acid, μg/mL of Nutrient Medium | Indole-3-Butyric Acid, μg/mL of Nutrient Medium |
|---|---|---|
| A | 14.63 ± 0.63 | 1.93 ± 0.06 |
| B | 12.32 ± 0.52 | 0.63 ± 0.03 |
| C | 10.12 ± 0.47 | 0.97 ± 0.04 |
| D | 18.03 ± 0.92 | 2.02 ± 0.07 |
| Seed Soaking | Watering | Residual Content Metal in Soil, % | Average Survival Rate of Seedlings, pcs | ||||
|---|---|---|---|---|---|---|---|
| Pb | As | Hg | Ni | Cd | |||
| Consortium D 1.5 × 10−2, McFarland standard | Water | 77.35 ± 3.01 | 87.47 ± 2.31 | 89.66 ± 2.27 | 76.95 ± 2.21 | 79.36 ± 2.08 | 8 ± 1 |
| Consortium D 2.5 × 10−2, McFarland standard | 68.14 ± 2.96 | 85.36 ± 1.84 | 87.21 ± 2.73 | 69.11 ± 2.85 | 75.51 ± 2.16 | 9 ± 2 | |
| Water | Consortium D 1.5 × 10−2, McFarland standard | 79.67 ± 2.71 | 93.87 ± 2.98 | 94.47 ± 1.32 | 79.12 ± 3.03 | 84.68 ± 2.48 | 7 ± 2 |
| Consortium D 2.5 × 10−2, McFarland standard | 76.33 ± 1.99 | 89.24 ± 2.04 | 91,36 ± 2.62 | 81,87 ± 2.28 | 83.74 ± 1.74 | 9 ± 1 | |
| Water | Water | 84.32 ± 2.21 | 95.47 ± 1.72 | 98.36 ± 2.14 | 86.47 ± 2.55 | 92.84 ± 1.89 | 5 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atuchin, V.V.; Asyakina, L.K.; Serazetdinova, Y.R.; Frolova, A.S.; Velichkovich, N.S.; Prosekov, A.Y. Microorganisms for Bioremediation of Soils Contaminated with Heavy Metals. Microorganisms 2023, 11, 864. https://doi.org/10.3390/microorganisms11040864
Atuchin VV, Asyakina LK, Serazetdinova YR, Frolova AS, Velichkovich NS, Prosekov AY. Microorganisms for Bioremediation of Soils Contaminated with Heavy Metals. Microorganisms. 2023; 11(4):864. https://doi.org/10.3390/microorganisms11040864
Chicago/Turabian StyleAtuchin, Victor V., Lyudmila K. Asyakina, Yulia R. Serazetdinova, Anna S. Frolova, Natalia S. Velichkovich, and Alexander Yu. Prosekov. 2023. "Microorganisms for Bioremediation of Soils Contaminated with Heavy Metals" Microorganisms 11, no. 4: 864. https://doi.org/10.3390/microorganisms11040864
APA StyleAtuchin, V. V., Asyakina, L. K., Serazetdinova, Y. R., Frolova, A. S., Velichkovich, N. S., & Prosekov, A. Y. (2023). Microorganisms for Bioremediation of Soils Contaminated with Heavy Metals. Microorganisms, 11(4), 864. https://doi.org/10.3390/microorganisms11040864







