Co-Occurrence of Taste and Odor Compounds and Cyanotoxins in Cyanobacterial Blooms: Emerging Risks to Human Health?
Abstract
:1. Introduction
2. Cyanotoxins (CTXs) and Taste and Odor (T&O) Compounds: Characteristics and Toxicity
2.1. CTXs
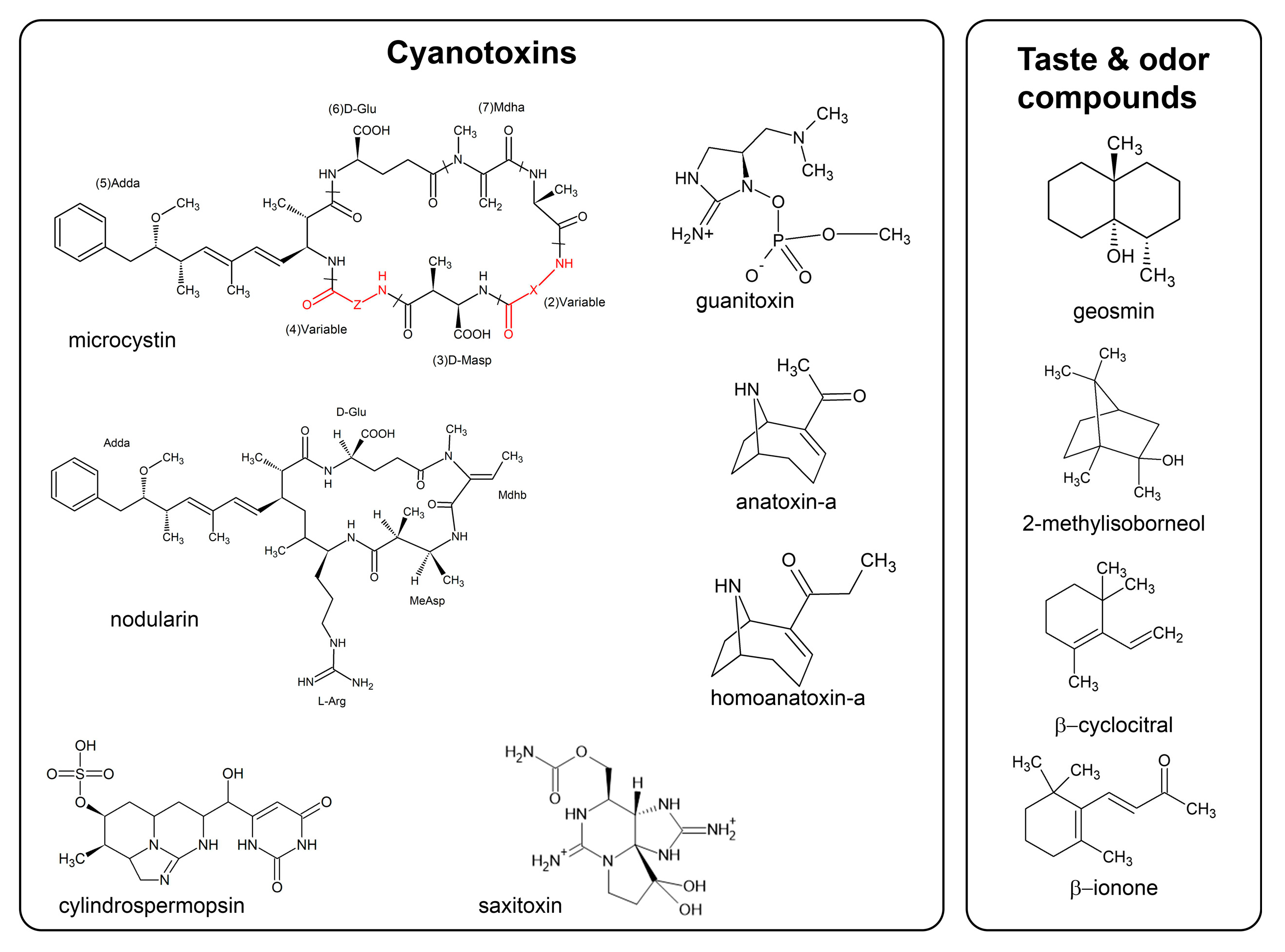
2.1.1. Microcystins and Nodularins
2.1.2. Cylindrospermopsins
2.1.3. Anatoxins and Saxitoxins
2.1.4. Other Bioactive Peptides and CTX Biosynthesis
2.2. T&O Compounds
2.2.1. Geosmin (GEO) and 2-Methylisoborneol (MIB)
2.2.2. β-Ionone and β-Cyclocitral
2.3. Toxicity of T&O Compounds
2.3.1. GEO and MIB
2.3.2. β-Ionone and β-Cyclocitral
3. Environmental Evidence of Co-Occurrence of T&O and CTX
4. Molecular Studies: T&O and CTX Producers
5. Environmental Roles and Parameters Affecting T&O and CTX Production
6. Conclusions and Gaps in Knowledge
Funding
Acknowledgments
Conflicts of Interest
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.S.; Zimba, P.V. Cyanobacterial bioactive metabolites—A review of their chemistry and biology. Harmful Algae 2019, 86, 139–209. [Google Scholar] [CrossRef]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, E.D.; Beasley, V.R. One Health and Cyanobacteria in Freshwater Systems: Animal Illnesses and Deaths Are Sentinel Events for Human Health Risks. Toxins 2015, 7, 1374–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chorus, I.; Welker, M. (Eds.) Toxic Cyanobacteria in Water, 2nd ed.; CRC Press: Boca Raton, FL, USA; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- WHO; HEP; ECH; WSH. Cyanobacterial toxins: Anatoxin-a and analogues. In Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO; HEP; ECH; WSH. Cyanobacterial toxins: Cylindrospermopsins. In Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO; HEP; ECH; WSH. Cyanobacterial toxins: Microcystins. In Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO; HEP; ECH; WSH. Cyanobacterial toxins: Saxitoxins. In Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Akcaalan, R.; Devesa-Garriga, R.; Dietrich, A.; Steinhaus, M.; Dunkel, A.; Mall, V.; Manganelli, M.; Scardala, S.; Testai, E.; Codd, G.A.; et al. Water taste and odor (T&O): Challenges, gaps and solutions from a perspective of the WaterTOP network. Chem. Eng. J. Adv. 2022, 12, 100409. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Lindholm-Lehto, P.; Koskela, J.; Kaseva, J.; Vielma, J. Accumulation of geosmin and 2-methylisoborneol in European whitefish Coregonus lavaretus and rainbow trout Oncorhynchus mykiss in RAS. Fishes 2020, 5, 13. [Google Scholar] [CrossRef]
- Shang, L.; Feng, M.; Xu, X.; Liu, F.; Ke, F.; Li, W. Co-Occurrence of microcystins and taste-and-odor compounds in drinking water source and their Removal in a full-scale drinking water treatment plant. Toxins 2018, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Zamyadi, A.; Glover, C.M.; Yasir, A.; Stuetz, R.; Newcombe, G.; Crosbie, N.D.; Lin, T.F.; Henderson, R. Toxic cyanobacteria in water supply systems: Data analysis to map global challenges and demonstrate the benefits of multi-barrier treatment approaches. H2Open J. 2021, 4, 47–62. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Codd, G.A. Cyanotoxins. In Ecology of Cyanobacteria II—Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 651–675. [Google Scholar]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action, and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Codd, G.A.; Testai, E.; Funari, E.; Svirčev, Z. Cyanobacteria, Cyanotoxins, and Human Health. In Water Treatment for Purification from Cyanobacteria and Cyanotoxins; Hiskia, A.E., Triantis, T.M., Antoniou, M.G., Kaloudis, T., Dionysiou, D.D., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 37–68. [Google Scholar]
- Zhou, W.; Li, X.; Wang, Y.; Wang, J.; Zhang, J.; Wei, H.; Peng, C.; Wang, Z.; Li, G.; Li, D. Physiological and transcriptomic changes of zebrafish (Danio rerio) embryos-larvae in response to 2-MIB exposure. J. Hazard. Mater. 2021, 416, 126142. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, J.; Zhang, J.; Peng, C.; Li, G.; Li, D. Environmentally relevant concentrations of geosmin affect the development, oxidative stress, apoptosis and endocrine disruption of embryo-larval zebrafish. Sci. Total Environ. 2020, 735, 139373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, Y.; Wang, J.; Peng, C.; Wang, Z.; Qin, H.; Li, G.; Li, D. β-Ionone causes endocrine disruption, hyperpigmentation and hypoactivity in zebrafish early life stages. Sci. Total Environ. 2022, 834, 155433. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.B.; Jüttner, F. Chapter 3. Biological production of taste and odour compounds. In Taste and Odour in Source and Drinking Water: Causes, Controls, and Consequences; Lin, T.-F., Watson, S., Dietrich, A., Suffet, I.H.M., Eds.; IWA Publishing: London, UK, 2019. [Google Scholar]
- Watson, S.B.; Monis, P.; Baker, P.; Giglio, S. Biochemistry and genetics of taste- and odor-producing cyanobacteria. Harmful Algae 2016, 54, 112–127. [Google Scholar] [CrossRef]
- Lee, J.; Rai, P.K.; Jeon, Y.J.; Kim, K.-H.; Kwon, E.E. The role of algae and cyanobacteria in the production and release of odorants in water. Environ. Pollut. 2017, 227, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Almuhtaram, H.; Kibuye, F.A.; Ajjampur, S.; Glover, C.M.; Hofmann, R.; Gaget, V.; Owen, C.; Wert, E.C.; Zamyadi, A. State of knowledge on early warning tools for cyanobacteria detection. Ecol. Indic. 2021, 133, 108442. [Google Scholar] [CrossRef]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Testai, E.; Scardala, S.; Vichi, S.; Buratti, F.M.; Funari, E. Risk to human health associated with the environmental occurrence of cyanobacterial neurotoxic alkaloids anatoxins and saxitoxins. Crit. Rev. Toxicol. 2016, 46, 385–419. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Ballot, A.; Thomazeau, S.; Maloufi, S.; Furey, A.; Mankiewicz-Boczek, J.; Pawlik-Skowrońska, B.; Capelli, C.; Salmaso, N. Appendix 2: Cyanobacteria Associated With the Production of Cyanotoxins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G., Eds.; Wiley: Chichester, UK, 2017; pp. 501–525. [Google Scholar]
- Turco, L.; Santori, N.; Buratti, F.M.; Dorne, J.-L.; Testai, E. Congeners-Specific Intestinal Absorption Of Microcystins In An In Vitro 3D Human Intestinal Epithelium: The Role Of Influx/Efflux Transporters. Front. Toxicol. 2022, 4, 883063. [Google Scholar] [CrossRef]
- Kaur, G.; Fahrner, R.; Wittmann, V.; Stieger, B.; Dietrich, D.R. Human MRP2 exports MC-LR but not the glutathione conjugate. Chem.-Biol. Interact. 2019, 311, 108761. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Gehringer, M.M. Microcystin-LR and okadaic acid-induced cellular effects: A dualistic response. FEBS Lett. 2004, 557, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Vichi, S.; Buratti, F.M.; Testai, E. Microcystins: Toxicological profile. In Marine and Freshwater Toxins; Gopalakrishnakone, P., Haddad, V., Kem, W., Tubaro, A., Kim, E., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2016; pp. 219–238. [Google Scholar]
- Chernoff, N.; Hill, D.; Lang, J.; Schmid, J.; Le, T.; Farthing, A.; Huang, H. The Comparative Toxicity of 10 Microcystin Congeners Administered Orally to Mice: Clinical Effects and Organ Toxicity. Toxins 2020, 12, 403. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, N.; Hill, D.; Lang, J.; Schmid, J.; Farthing, A.; Huang, H. Dose-Response Study of Microcystin Congeners MCLA, MCLR, MCLY, MCRR, and MCYR Administered Orally to Mice. Toxins 2021, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Funari, E.; Testai, E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Santori, N.; Buratti, F.M.; Scardala, S.; Dorne, J.-L.C.M.; Testai, E. In vitro detoxication of microcystins in human samples: Variability among variants with different hydrophilicity and structure. Toxicol. Lett. 2020, 322, 131–139. [Google Scholar] [CrossRef]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.-C.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef] [PubMed]
- Bagu, J.R.; Sykes, B.D.; Craig, M.M.; Holmes, C.B. A molecular basis for different interactions of marine toxins with protein phosphatase-1: Molecular models for bound motuporin, microcystins, okadaic acid, and calyculin A. J. Biol. Chem. 1997, 272, 5087–5097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2010; pp. v–vii, 1–412. [Google Scholar]
- Harada, K. Chemistry and detection of microcystins. In Toxic Microcystis; Watanabe, M., Harada, K.-I., Carmichael, W., Fujiki, H., Eds.; CRC Press: Boca Raton, FL, USA, 1996; Volume 45, pp. 103–148. [Google Scholar]
- Codd, G.; Bell, S. The Occurrence and Fate of Blue-Green Algal Toxins in Freshwaters; National Rivers Authority: London, UK, 1996; p. 30. [Google Scholar]
- Runnegar, M.T.; Kong, S.-M.; Zhong, Y.-Z.; Lu, S.C. Inhibition of reduced glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem. Pharmacol. 1995, 49, 219–225. [Google Scholar] [CrossRef]
- Froscio, S.M.; Humpage, A.R.; Burcham, P.C.; Falconer, I.R. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. Int. J. 2003, 18, 243–251. [Google Scholar] [CrossRef]
- Meriluoto, J.; Spoof, L.; Codd, G.A. (Eds.) Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Wiley: Colchester, UK, 2017; p. 548. [Google Scholar]
- Testai, E. Anatoxin-a and analogues. In Toxic Cyanobacteria in Water, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA; World Health Organization: Geneva, Switzerland, 2021; pp. 72–93. [Google Scholar]
- Codd, G.A.; Edwards, C.; Beattie, K.A.; Barr, W.M.; Gunn, G.J. Fatal attraction to cyanobacteria? Nature 1992, 359, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Beltran, E.C.; Neilan, B.A. Geographical segregation of the neurotoxin-producing cyanobacterium Anabaena circinalis. Appl. Environ. Microbiol. 2000, 66, 4468–4474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testai, E. Saxitoxins or paralytic shellfish poisons. In Toxic Cyanobacteria in Water, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA; World Health Organization: Geneva, Switzerland, 2021; pp. 94–108. [Google Scholar]
- Fiore, M.F.; de Lima, S.T.; Carmichael, W.W.; McKinnie, S.M.; Chekan, J.R.; Moore, B.S. Guanitoxin, re-naming a cyanobacterial organophosphate toxin. Harmful Algae 2020, 92, 101737. [Google Scholar] [CrossRef] [PubMed]
- Ziemert, N.; Ishida, K.; Liaimer, A.; Hertweck, C.; Dittmann, E. Ribosomal Synthesis of Tricyclic Depsipeptides in Bloom-Forming Cyanobacteria. Angew. Chem. Int. Ed. 2008, 47, 7756–7759. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural Product Biosynthetic Diversity and Comparative Genomics of the Cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef]
- Catherine, A.; Bernard, C.; Spoof, L.; Bruno, M. Microcystins and Nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; Wiley: Chichester, UK, 2017; pp. 109–126. [Google Scholar]
- Agha, R.; Quesada, A. Oligopeptides as Biomarkers of Cyanobacterial Subpopulations. Toward an Understanding of Their Biological Role. Toxins 2014, 6, 1929–1950. [Google Scholar] [CrossRef] [Green Version]
- Janssen, E.M.L. Cyanobacterial peptides beyond microcystins—A review on co-occurrence, toxicity, and challenges for risk assessment. Water Res. 2019, 151, 488–499. [Google Scholar] [CrossRef]
- Rohrlack, T.; Christiansen, G.; Kurmayer, R. Putative antiparasite defensive system involving ribosomal and nonribosomal oligopeptides in cyanobacteria of the genus Planktothrix. Appl. Environ. Microbiol. 2013, 79, 2642–2647. [Google Scholar] [CrossRef] [Green Version]
- Lezcano, M.Á.; Agha, R.; Cirés, S.; Quesada, A. Spatial-temporal survey of Microcystis oligopeptide chemotypes in reservoirs with dissimilar waterbody features and their relation to genetic variation. Harmful Algae 2019, 81, 77–85. [Google Scholar] [CrossRef]
- Kyle, M.; Haande, S.; Ostermaier, V.; Rohrlack, T. The Red Queen Race between Parasitic Chytrids and Their Host, Planktothrix: A Test Using a Time Series Reconstructed from Sediment DNA. PLoS ONE 2015, 10, e0118738. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.A.; Rueckert, A.; Hamilton, D.P.; Cary, S.C.; Dietrich, D.R. Switching toxin production on and off: Intermittent microcystin synthesis in a Microcystis bloom. Environ. Microbiol. Rep. 2011, 3, 118–124. [Google Scholar] [CrossRef]
- Jüttner, F. Physiology and biochemistry of odorous compounds from freshwater cyanobacteria and algae. Water Sci. Technol. 1995, 31, 69–78. [Google Scholar] [CrossRef]
- Watson, S.B.; Jüttner, F. Malodorous volatile organic sulfur compounds: Sources, sinks and significance in inland waters. Crit. Rev. Microbiol. 2017, 43, 210–237. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yu, J.; Su, M.; Wang, C.; Yang, M.; Cao, N.; Zhao, Y.; Xia, P. Synergistic effect of musty odorants on septic odor: Verification in Huangpu River source water. Sci. Total Environ. 2019, 653, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.B.; Ridal, J.; Boyer, G.L. Taste and odour and cyanobacterial toxins: Impairment, prediction, and management in the Great Lakes. Can. J. Fish. Aquat. Sci. 2008, 65, 1779–1796. [Google Scholar] [CrossRef]
- Watson, S.B. Cyanobacterial and eukaryotic algal odour compounds: Signals or by-products? A review of their biological activity. Phycologia 2003, 42, 332–350. [Google Scholar] [CrossRef]
- Kaloudis, T. Cyanobacterial taste and odour compounds in water. In Toxic Cyanobacteria in Water, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA; World Health Organization: Geneva, Switzerland, 2021; pp. 149–155. [Google Scholar]
- Lukassen, M.B.; de Jonge, N.; Bjerregaard, S.M.; Podduturi, R.; Jørgensen, N.O.; Petersen, M.A.; David, G.S.; da Silva, R.J.; Nielsen, J.L. Microbial Production of the Off-Flavor Geosmin in Tilapia Production in Brazilian Water Reservoirs: Importance of Bacteria in the Intestine and Other Fish-Associated Environments. Front. Microbiol. 2019, 10, 2447. [Google Scholar] [CrossRef] [Green Version]
- Jüttner, F.; Watson, S.B. Biochemical and Ecological Control of Geosmin and 2-Methylisoborneol in Source Waters. Appl. Environ. Microbiol. 2007, 73, 4395–4406. [Google Scholar] [CrossRef] [Green Version]
- Watson, S.B. Aquatic taste and odor: A primary signal of drinking-water integrity. J. Toxicol. Environ. Health Part A 2004, 67, 1779–1795. [Google Scholar] [CrossRef]
- Cane, D.E.; He, X.; Kobayashi, S.; Ōmura, S.; Ikeda, H. Geosmin Biosynthesis in Streptomyces avermitilis. Molecular Cloning, Expression, and Mechanistic Study of the Germacradienol/Geosmin Synthase. J. Antibiot. 2006, 59, 471–479. [Google Scholar] [CrossRef]
- Giglio, S.; Jiang, J.; Saint, C.P.; Cane, D.E.; Monis, P.T. Isolation and characterization of the gene associated with geosmin production in cyanobacteria. Environ. Sci. Technol. 2008, 42, 8027–8032. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, F.; Medger, A.; Börnick, H.; Opitz, M.; Lang, K.; Göttfert, M.; Röske, I. Identification and expression analyses of putative sesquiterpene synthase genes in Phormidium sp. and prevalence of geoA-like genes in a drinking water reservoir. Appl. Environ. Microbiol. 2007, 73, 6988–6993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Song, G.; Shao, J.; Tan, W.; Li, Y.; Li, R. Establishment and field applications of real-time PCR methods for the quantification of potential MIB-producing cyanobacteria in aquatic systems. J. Appl. Phycol. 2016, 28, 325–333. [Google Scholar] [CrossRef]
- Gaget, V.; Almuhtaram, H.; Kibuye, F.; Hobson, P.; Zamyadi, A.; Wert, E.; Brookes, J.D. Benthic cyanobacteria: A utility-centred field study. Harmful Algae 2022, 113, 102185. [Google Scholar] [CrossRef]
- Gaget, V.; Hobson, P.; Keulen, A.; Newton, K.; Monis, P.; Humpage, A.R.; Weyrich, L.S.; Brookes, J.D. Toolbox for the sampling and monitoring of benthic cyanobacteria. Water Res. 2020, 169, 115222. [Google Scholar] [CrossRef]
- Devi, A.; Chiu, Y.-T.; Hsueh, H.-T.; Lin, T.-F. Quantitative PCR based detection system for cyanobacterial geosmin/2-methylisoborneol (2-MIB) events in drinking water sources: Current status and challenges. Water Res. 2021, 188, 116478. [Google Scholar] [CrossRef] [PubMed]
- Churro, C.; Semedo-Aguiar, A.P.; Silva, A.D.; Pereira-Leal, J.B.; Leite, R.B. A novel cyanobacterial geosmin producer, revising geoA distribution and dispersion patterns in bacteria. Sci. Rep. 2020, 10, 8679. [Google Scholar] [CrossRef] [PubMed]
- Godo, T.; Saki, Y.; Nojiri, Y.; Tsujitani, M.; Sugahara, S.; Hayashi, S.; Kamiya, H.; Ohtani, S.; Seike, Y. Geosmin-producing Species of Coelosphaerium (Synechococcales, Cyanobacteria) in Lake Shinji, Japan. Sci. Rep. 2017, 7, 41928. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, S.; Ohtani, S.; Godo, T.; Nojiri, Y.; Saki, Y.; Esumi, T.; Kamiya, H. Identification of geosmin biosynthetic gene in geosmin-producing colonial cyanobacteria Coelosphaerium sp. and isolation of geosmin non-producing Coelosphaerium sp. from brackish Lake Shinji in Japan. Harmful Algae 2019, 84, 19–26. [Google Scholar] [CrossRef]
- Suurnäkki, S.; Gomez-Saez, G.V.; Rantala-Ylinen, A.; Jokela, J.; Fewer, D.P.; Sivonen, K. Identification of geosmin and 2-methylisoborneol in cyanobacteria and molecular detection methods for the producers of these compounds. Water Res. 2015, 68, 56–66. [Google Scholar] [CrossRef]
- Wang, Z.; Song, G.; Li, Y.; Yu, G.; Hou, X.; Gan, Z.; Li, R. The diversity, origin, and evolutionary analysis of geosmin synthase gene in cyanobacteria. Sci. Total Environ. 2019, 689, 789–796. [Google Scholar] [CrossRef]
- Huang, W.-J.; Lai, C.-H.; Cheng, Y.-L. Evaluation of extracellular products and mutagenicity in cyanobacteria cultures separated from a eutrophic reservoir. Sci. Total Environ. 2007, 377, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, C.; Yoon, Y.; Hwang, S.-J. Harmful Cyanobacterial Material Production in the North Han River (South Korea): Genetic Potential and Temperature-Dependent Properties. Int. J. Environ. Res. Public Health 2018, 15, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, H.-S.; Lee, C.S.; Srivastava, A.; Oh, H.-M. Effects of Environmental Factors on Cyanobacterial Production of Odorous Compounds: Geosmin and 2-Methylisoborneol. J. Microbiol. Biotechnol. 2017, 27, 1316–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.; Su, M.; Liu, T.; Guo, Q.; Wang, Q.; Burch, M.; Yu, J.; Yang, M. Light as a possible regulator of MIB-producing Planktothrix in source water reservoir, mechanism and in-situ verification. Harmful Algae 2019, 88, 101658. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.B.; Meyer, K.A.; Šulčius, S.; Brown, N.M.; Dick, G.J.; Cao, H.; Gasiūnas, G.; Timinskas, A.; Yin, Y.; Landry, Z.C.; et al. A closely-related clade of globally distributed bloom-forming cyanobacteria within the Nostocales. Harmful Algae 2018, 77, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Dreher, T.W.; Davis, E.W.; Mueller, R.S.; Otten, T.G. Comparative genomics of the ADA clade within the Nostocales. Harmful Algae 2021, 104, 102037. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Shimizu, K.; Miao, H.; Tsukino, S.; Utsumi, M.; Lei, Z.; Zhang, Z.; Nishimura, O.; Asada, Y.; Fujimoto, N.; et al. Effects of elevated nitrogen on the growth and geosmin productivity of Dolichospermum smithii. Environ. Sci. Pollut. Res. 2021, 28, 177–184. [Google Scholar] [CrossRef]
- Alghanmi, H.A.; Alkam, F.a.M.; Al-Taee, M.M. Effect of light and temperature on new cyanobacteria producers for geosmin and 2-methylisoborneol. J. Appl. Phycol. 2018, 30, 319–328. [Google Scholar] [CrossRef]
- Lu, J.; Su, M.; Su, Y.; Wu, B.; Cao, T.; Fang, J.; Yu, J.; Zhang, H.; Yang, M. Driving forces for the growth of MIB-producing Planktothricoides raciborskii in a low-latitude reservoir. Water Res. 2022, 220, 118670. [Google Scholar] [CrossRef]
- Cai, F.; Yu, G.; Zhang, K.; Chen, Y.; Li, Q.; Yang, Y.; Xie, J.; Wang, Y.; Li, R. Geosmin production and polyphasic characterization of Oscillatoria limosa Agardh ex Gomont isolated from the open canal of a large drinking water system in Tianjin City, China. Harmful Algae 2017, 69, 28–37. [Google Scholar] [CrossRef]
- Gaget, V.; Humpage, A.R.; Huang, Q.; Monis, P.; Brookes, J.D. Benthic cyanobacteria: A source of cylindrospermopsin and microcystin in Australian drinking water reservoirs. Water Res. 2017, 124, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-T.; Yen, H.-K.; Lin, T.-F. An alternative method to quantify 2-MIB producing cyanobacteria in drinking water reservoirs: Method development and field applications. Environ. Res. 2016, 151, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pan, R.; Luo, Z.; Zhang, T.; Fan, J. Interspecific competition between Microcystis aeruginosa and Pseudanadaena and their production of T&O compounds. Chemosphere 2020, 252, 126509. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, P.; Ma, Z.; Niu, Y.; Tao, M.; Deng, X.; Wang, Q. A systematic study on spatial and seasonal patterns of eight taste and odor compounds with relation to various biotic and abiotic parameters in Gonghu Bay of Lake Taihu, China. Sci. Total Environ. 2010, 409, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, J.; Yang, M.; Zhang, J.; Burch, M.D.; Han, W. Cyanobacterial population and harmful metabolites dynamics during a bloom in Yanghe Reservoir, North China. Harmful Algae 2010, 9, 481–488. [Google Scholar] [CrossRef]
- Li, H.; Gu, X.; Chen, H.; Mao, Z.; Shen, R.; Zeng, Q.; Ge, Y. Co-occurrence of multiple cyanotoxins and taste-and-odor compounds in the large eutrophic Lake Taihu, China: Dynamics, driving factors, and challenges for risk assessment. Environ. Pollut. 2022, 294, 118594. [Google Scholar] [CrossRef]
- Peter, A.; Köster, O.; Schildknecht, A.; von Gunten, U. Occurrence of dissolved and particle-bound taste and odor compounds in Swiss lake waters. Water Res. 2009, 43, 2191–2200. [Google Scholar] [CrossRef]
- Otten, T.G.; Graham, J.L.; Harris, T.D.; Dreher, T.W.; Voordouw, G. Elucidation of Taste- and Odor-Producing Bacteria and Toxigenic Cyanobacteria in a Midwestern Drinking Water Supply Reservoir by Shotgun Metagenomic Analysis. Appl. Environ. Microbiol. 2016, 82, 5410–5420. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.L.; Foster, G.M.; Williams, T.J.; Kramer, A.R.; Harris, T.D. Occurrence of Cyanobacteria, Microcystin, and Taste-and-Odor Compounds in Cheney Reservoir, Kansas, 2001–2016: U.S. Geological Survey Scientific Investigations Report 2017–5016; USGS: Reston, VA, USA, 2017; p. 68. [Google Scholar] [CrossRef]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin Mixtures and Taste-and-Odor Compounds in Cyanobacterial Blooms from the Midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef]
- Yen, H.; Lin, T.; Tseng, I.; Tung, S.; Hsu, M. Correlating 2-MIB and microcystin concentrations with environmental parameters in two reservoirs in south Taiwan. Water Sci. Technol. 2007, 55, 33–41. [Google Scholar] [CrossRef]
- Xuwei, D.; Min, Q.; Ren, R.; Jiarui, L.; Xiaoxue, S.; Ping, X.; Jun, C. The relationships between odors and environmental factors at bloom and non-bloom area in Lake Taihu, China. Chemosphere 2019, 218, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Asquith, E.; Evans, C.; Dunstan, R.H.; Geary, P.; Cole, B. Distribution, abundance and activity of geosmin and 2-methylisoborneol-producing Streptomyces in drinking water reservoirs. Water Res. 2018, 145, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Clercin, N.A.; Druschel, G.K. Influence of Environmental Factors on the Production of MIB and Geosmin Metabolites by Bacteria in a Eutrophic Reservoir. Water Resour. Res. 2019, 55, 5413–5430. [Google Scholar] [CrossRef]
- Clercin, N.A.; Koltsidou, I.; Picard, C.J.; Druschel, G.K. Prevalence of Actinobacteria in the production of 2-methylisoborneol and geosmin, over Cyanobacteria in a temperate eutrophic reservoir. Chem. Eng. J. Adv. 2022, 9, 100226. [Google Scholar] [CrossRef]
- Havaux, M. β-Cyclocitral and derivatives: Emerging molecular signals serving multiple biological functions. Plant Physiol. Biochem. 2020, 155, 35–41. [Google Scholar] [CrossRef]
- Jüttner, F.; Watson, S.B.; von Elert, E.; Köster, O. β-cyclocitral, a grazer defence signal unique to the cyanobacterium Microcystis. J. Chem. Ecol. 2010, 36, 1387–1397. [Google Scholar] [CrossRef]
- Arii, S.; Tsuji, K.; Tomita, K.; Hasegawa, M.; Bober, B.; Harada, K.-I. Cyanobacterial Blue Color Formation during Lysis under Natural Conditions. Appl. Environ. Microbiol. 2015, 81, 2667–2675. [Google Scholar] [CrossRef] [Green Version]
- Harada, K.-I.; Ozaki, K.; Tsuzuki, S.; Kato, H.; Hasegawa, M.; Kuroda, E.K.; Arii, S.; Tsuji, K. Blue color formation of cyanobacteria with β-cyclocitral. J. Chem. Ecol. 2009, 35, 1295–1301. [Google Scholar] [CrossRef]
- Tomita, K.; Hasegawa, M.; Arii, S.; Tsuji, K.; Bober, B.; Harada, K.-I. Characteristic oxidation behavior of β-cyclocitral from the cyanobacterium Microcystis. Environ. Sci. Pollut. Res. 2016, 23, 11998–12006. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Hou, D.; Teng, F.; Cai, Z.; Tao, Y. Production of β-Cyclocitral and Its Precursor β-Carotene in Microcystis aeruginosa: Variation at Population and Single-Cell Levels. Toxins 2022, 14, 201. [Google Scholar] [CrossRef]
- Young, W.F.; Horth, H.; Crane, R.; Ogden, T.; Arnott, M. Taste and odour threshold concentrations of potential potable water contaminants. Water Res. 1996, 30, 331–340. [Google Scholar] [CrossRef]
- Kaloudis, T.; Triantis, T.M.; Hiskia, A. Taste and Odour Compounds Produced by Cyanobacteria. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 196–201. [Google Scholar]
- Sugiura, N.; Utsumi, M.; Wei, B.; Iwami, N.; Okano, K.; Kawauchi, Y.; Maekawa, T. Assessment for the complicated occurrence of nuisance odours from phytoplankton and environmental factors in a eutrophic lake. Lakes Reserv. Sci. Policy Manag. Sustain. Use 2004, 9, 195–201. [Google Scholar] [CrossRef]
- Izaguirre, G.; Taylor, W.D. Geosmin and MIB events in a new reservoir in southern California. Water Sci. Technol. 2007, 55, 9–14. [Google Scholar] [CrossRef]
- Tung, S.-C.; Lin, T.-F.; Yang, F.-C.; Liu, C.-L. Seasonal change and correlation with environmental parameters for 2-MIB in Feng-Shen Reservoir, Taiwan. Environ. Monit. Assess. 2008, 145, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Mochida, K.Y.O. Evaluation of the Cytotoxicity of Geosmin and 2-Methylisoborneol Using Cultured Human, Monkey, and Dog Cells. Biocontrol Sci. 2009, 14, 35–38. [Google Scholar] [CrossRef] [Green Version]
- Proulx, F.; Rodriguez, M.J.; Sérodes, J.B.; Bouchard, C. Spatio-temporal variability of tastes and odors of drinking water within a distribution system. J. Environ. Manag. 2012, 105, 12–20. [Google Scholar] [CrossRef]
- Qi, M.; Chen, J.; Sun, X.; Deng, X.; Niu, Y.; Xie, P. Development of Models for Predicting the Predominant Taste and Odor Compounds in Taihu Lake, China. PLoS ONE 2012, 7, e51976. [Google Scholar] [CrossRef] [Green Version]
- Burgos, L.; Lehmann, M.; de Andrade, H.H.R.; de Abreu, B.R.R.; de Souza, A.P.; Juliano, V.B.; Dihl, R.R. In vivo and in vitro genotoxicity assessment of 2-methylisoborneol, causal agent of earthy–musty taste and odor in water. Ecotoxicol. Environ. Saf. 2014, 100, 282–286. [Google Scholar] [CrossRef]
- Bláha, L.; Sabater, S.; Babica, P.; Vilalta, E.; Maršáálek, B. Geosmin occurrence in riverine cyanobacterial mats: Is it causing a significant health hazard? Water Sci. Technol. 2004, 49, 307–312. [Google Scholar] [CrossRef]
- Dionigi, C.P.; Lawlor, T.E.; McFarland, J.E.; Johnsen, P.B. Evaluation of geosmin and 2-methylisoborneol on the histidine dependence of TA98 and TA100 Salmonella typhimurium tester strains. Water Res. 1993, 27, 1615–1618. [Google Scholar] [CrossRef]
- Burgos, L.; Lehmann, M.; Simon, D.; de Andrade, H.H.R.; de Abreu, B.R.R.; Nabinger, D.D.; Grivicich, I.; Juliano, V.B.; Dihl, R.R. Agents of earthy-musty taste and odor in water: Evaluation of cytotoxicity, genotoxicity and toxicogenomics. Sci. Total Environ. 2014, 490, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.I.; Di Consiglio, E.; Blaauboer, B.J.; Testai, E. Biokinetics in repeated-dosing in vitro drug toxicity studies. Toxicol. Vitr. 2015, 30, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.B.; Westerfield, M. Zebrafish models in translational research: Tipping the scales toward advancements in human health. Dis. Model. Mech. 2014, 7, 739–743. [Google Scholar] [CrossRef] [Green Version]
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish Embryos and Larvae as Alternative Animal Models for Toxicity Testing. Int. J. Mol. Sci. 2021, 22, 13417. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.C.M.; De-Carvalho, R.R.; De-Oliveira, A.C.A.X.; Delgado, I.F.; Paumgartten, F.J.R. Study on the developmental toxicity of β-ionone in the rat. Regul. Toxicol. Pharmacol. 2018, 97, 110–119. [Google Scholar] [CrossRef]
- OECD. Screening Information Data Set (SIDS)-β-Ionone [(E)-4-(2,6,6-Trimethyl-1-Cyclohexen-1-Yl)-3-Buten-2-One] CAS N 79-77-6; UNEP Publications: Paris, France, 2004. [Google Scholar]
- Lalko, J.; Lapczynski, A.; McGinty, D.; Bhatia, S.; Letizia, C.S.; Api, A.M. Fragrance material review on ionone. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2007, 45 (Suppl. 1), S251–S257. [Google Scholar] [CrossRef]
- IPCS-WHO. WHO FOOD ADDITIVES SERIES: 42—Ionones and Structurally Related Substances. Available online: https://inchem.org/documents/jecfa/jecmono/v042je19.htm (accessed on 22 February 2023).
- Belsito, D.; Bickers, D.; Bruze, M.; Calow, P.; Greim, H.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Tagami, H. A toxicologic and dermatologic assessment of ionones when used as fragrance ingredients. Food Chem. Toxicol. 2007, 45, S130–S167. [Google Scholar] [CrossRef]
- Gomes-Carneiro, M.R.; De-Oliveira, A.C.A.X.; De-Carvalho, R.R.; Araujo, I.B.; Souza, C.A.M.; Kuriyama, S.N.; Paumgartten, F.J.R. Inhibition of cyclophosphamide-induced teratogenesis by β-ionone. Toxicol. Lett. 2003, 138, 205–213. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, L.; Yang, L.; Zhou, B. Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat. Toxicol. 2012, 110–111, 141–148. [Google Scholar] [CrossRef]
- EFSA. Panel on Food Contact Materials, Enzymes, Flavorings Processing Aids—Scientific Opinion on Flavouring Group Evaluation 12, Revision 4 (FGE.12Rev4): Primary saturated or unsaturated alicyclic alcohols, aldehydes, acids and esters from chemical groups 1 and 7. EFSA J. 2013, 11, 3391. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Panel on Additives Products or Substances used in Animal Feed—Safety and efficacy of secondary alicyclic saturated and unsaturated alcohols, ketones, ketals and esters with ketals containing alicyclic alcohols or ketones and esters containing secondary alicyclic alcohols from chemical group 8 when used as flavourings for all animal species. EFSA J. 2016, 14, e04475. [Google Scholar] [CrossRef]
- Paparella, A.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its Occurrence and Biological Function and Metabolic Engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.G.; Chun, Y.-J.; Yun, C.-H.; Moon, C.-K.; Lee, H.-S.; Han, S.S.; Lee, E.-S.; Jeong, T.C. Induction of cytochrome p450 1a and 2b by α-and β-lonone in sprague dawley rats. Arch. Pharmacal Res. 2002, 25, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Xu, H.; Yang, M.; Pan, N.; Zheng, T.; Xu, C.; Li, Y.; Zuo, Z. Toxic mechanism of two cyanobacterial volatiles β-cyclocitral and β-ionone on the photosynthesis in duckweed by altering gene expression. Environ. Pollut. 2022, 308, 119711. [Google Scholar] [CrossRef] [PubMed]
- EU. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006 No 1272/2008. Off. J. Eur. Union 2008, L353, 1–1357. [Google Scholar]
- More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Hougaard Bennekou, S.; Koutsoumanis, K.P.; EFSA, Scientific Committee. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA J. 2019, 17, e05708. [Google Scholar] [CrossRef] [Green Version]
- Api, A.M.; Belmonte, F.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; et al. RIFM fragrance ingredient safety assessment, isocyclocitral, CAS Registry number 1335-66-6. Food Chem. Toxicol. 2019, 134, 110709. [Google Scholar] [CrossRef]
- Zhang, K.; Lin, T.F.; Zhang, T.; Li, C.; Gao, N. Characterization of typical taste and odor compounds formed by Microcystis aeruginosa. J. Environ. Sci. 2013, 25, 1539–1548. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, M.; Zuo, Z. Toxic mechanism of eucalyptol and β-cyclocitral on Chlamydomonas reinhardtii by inducing programmed cell death. J. Hazard. Mater. 2020, 389, 121910. [Google Scholar] [CrossRef]
- Manganelli, M.; Stefanelli, M.; Vichi, S.; Andreani, P.; Nascetti, G.; Scialanca, F.; Scardala, S.; Testai, E.; Funari, E. Cyanobacteria biennal dynamic in a volcanic mesotrophic lake in central Italy: Strategies to prevent dangerous human exposures to cyanotoxins. Toxicon 2016, 115, 28–40. [Google Scholar] [CrossRef]
- Harris, T.D.; Graham, J.L. Predicting cyanobacterial abundance, microcystin, and geosmin in a eutrophic drinking-water reservoir using a 14-year dataset. Lake Reserv. Manag. 2017, 33, 32–48. [Google Scholar] [CrossRef]
- Codd, G.A.; Lindsay, J.; Young, F.M.; Morrison, L.F.; Metcalf, J.S. HARMFUL CYANOBACTERIA—From mass mortalities to management measures. In Harmful Cyanobacteria; Huisman, J., Matthijs, H., Visser, P., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 1–23. [Google Scholar]
- Zuo, Z.-J.; Zhu, Y.-R.; Bai, Y.-L.; Wang, Y. Volatile communication between Chlamydomonas reinhardtii cells under salt stress. Biochem. Syst. Ecol. 2012, 40, 19–24. [Google Scholar] [CrossRef]
- Becher, P.G.; Verschut, V.; Bibb, M.J.; Bush, M.J.; Molnár, B.P.; Barane, E.; Al-Bassam, M.M.; Chandra, G.; Song, L.; Challis, G.L.; et al. Developmentally regulated volatiles geosmin and 2-methylisoborneol attract a soil arthropod to Streptomyces bacteria promoting spore dispersal. Nat. Microbiol. 2020, 5, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Testai, E.; Buratti, F.M.; Funari, E.; Manganelli, M.; Vichi, S.; Arnich, N.; Biré, R.; Fessard, V.; Sialehaamoa, A. Review and Analysis of Occurrence, Exposure and Toxicity of Cyanobacteria Toxins in Food; EFSA: Parma, Italy, 2016; p. 309. [Google Scholar]
- Zuo, Z. Why Algae Release Volatile Organic Compounds—The Emission and Roles. Front. Microbiol. 2019, 10, 491. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, R. Effects of light and temperature on the odor production of 2-methylisoborneol-producing Pseudanabaena sp. and geosmin-producing Anabaena ucrainica (cyanobacteria). Biochem. Syst. Ecol. 2015, 58, 219–226. [Google Scholar] [CrossRef]
- Saadoun, I.M.; Schrader, K.K.; Blevins, W.T. Environmental and nutritional factors affecting geosmin synthesis by Anabaena sp. Water Res. 2001, 35, 1209–1218. [Google Scholar] [CrossRef]
- Perkins, R.G.; Slavin, E.I.; Andrade, T.M.C.; Blenkinsopp, C.; Pearson, P.; Froggatt, T.; Godwin, G.; Parslow, J.; Hurley, S.; Luckwell, R.; et al. Managing taste and odour metabolite production in drinking water reservoirs: The importance of ammonium as a key nutrient trigger. J. Environ. Manag. 2019, 244, 276–284. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, L.; Yang, W.; Bai, Y.; Hou, P.; Zhao, J.; Zhou, L.; Zuo, Z. Volatile organic compounds released from Microcystis flos-aquae under nitrogen sources and their toxic effects on Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2017, 135, 191–200. [Google Scholar] [CrossRef]
- Zuo, Z.; Yang, Y.; Xu, Q.; Yang, W.; Zhao, J.; Zhou, L. Effects of phosphorus sources on volatile organic compound emissions from Microcystis flos-aquae and their toxic effects on Chlamydomonas reinhardtii. Environ. Geochem. Health 2018, 40, 1283–1298. [Google Scholar] [CrossRef]
- Ozaki, K.; Ohta, A.; Iwata, C.; Horikawa, A.; Tsuji, K.; Ito, E.; Ikai, Y.; Harada, K.-I. Lysis of cyanobacteria with volatile organic compounds. Chemosphere 2008, 71, 1531–1538. [Google Scholar] [CrossRef]
- Omidi, A.; Esterhuizen-Londt, M.; Pflugmacher, S. Still challenging: The ecological function of the cyanobacterial toxin microcystin—What we know so far. Toxin Rev. 2018, 37, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ding, P.; Lu, S.; Wu, P.; Wei, X.; Huang, R.; Kai, T. Cell density-dependent regulation of microcystin synthetase genes (mcy) expression and microcystin-LR production in Microcystis aeruginosa that mimics quorum sensing. Ecotoxicol. Environ. Saf. 2021, 220, 112330. [Google Scholar] [CrossRef] [PubMed]
- Guljamow, A.; Barchewitz, T.; Große, R.; Timm, S.; Hagemann, M.; Dittmann, E. Diel Variations of Extracellular Microcystin Influence the Subcellular Dynamics of RubisCO in Microcystis aeruginosa PCC 7806. Microorganisms 2021, 9, 1265. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.D.; Steffensen, D.A.; Humpage, A.R.; Nicholson, B.C.; Falconer, I.R.; Lanthois, B.; Fergusson, K.M.; Saint, C.P. Preliminary evidence of toxicity associated with the benthic cyanobacterium Phormidium in South Australia. Environ. Toxicol. 2001, 16, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Suffet, I.H.; Khiari, D.; Bruchet, A. The drinking water taste and odor wheel for the millennium: Beyond geosmin and 2-methylisoborneol. Water Sci. Technol. 1999, 40, 1–13. [Google Scholar] [CrossRef]
- EFSA. Outcome of the public consultation on the draft EFSA ‘Guidance Document on Scientific criteria for grouping chemicals into assessment groups for human risk assessment of combined exposure to multiple chemicals’. EFSA Support. Publ. 2021, 18, 7029E. [Google Scholar] [CrossRef]
- Žegura, B.; Štraser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res. /Rev. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, Á.; Moreno, F.J.; Cameán, A.M. Biochemical and pathological toxic effects induced by the cyanotoxin cylindrospermopsin on the human cell line Caco-2. Water Res. 2012, 46, 1566–1575. [Google Scholar] [CrossRef]
- SCHER; SCCS; SCENIHR. Opinion on the Toxicity and Assessment of Chemical Mixtures; European Union: Brussels, Belgium, 2012. [Google Scholar]
- Grosh, W. Aroma Compounds. In Food Chemistry; Belitz, H.-D., Grosch, W., Schieberle, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 340–402. [Google Scholar]
- Metcalf, J.S.; Codd, G.A. Co-Occurrence of Cyanobacteria and Cyanotoxins with Other Environmental Health Hazards: Impacts and Implications. Toxins 2020, 12, 629. [Google Scholar] [CrossRef]
| Cyanotoxins | Potential Cyanobacteria Producer | Cyanotoxins Contents in Cultured Strains (Range) | Occurrence in Water Environments |
|---|---|---|---|
| Microcystins | Anabaena sp. Anabaenopsis sp. Annamia toxica Aphanocapsa sp. Arthrospira sp. Calothrix sp. Dolichospermum (Anabaena) sp. Fischerella sp. Haphalosiphon hibernicus Leptolyngbya sp. Merismopedia sp. Microcystis sp. Nostoc sp. Oscillatoria sp. Phormidium sp. Planktothrix agardhii Planktothrix rubescens Plectonema sp. Pseudanabaena sp. Radiocystis sp. Synechococcus sp. | <0.1–700 mg/g dw <0.3–7 μg/mm3 ~2–854 fg/cell | Average in the pelagic water outside scums: <1 up to (not frequently) several tens of μg/L In surface blooms and scums reported maximum values of up 124 mg/L |
| Nodularin | Nodularia spumigena Nodularia sp. Nostoc sp. Iningainema pulvinus | 100–700 mg/g dw | In blooms: from 3.5 to 18 mg/g dw In the open water: a few μg up to 18 mg/L in surface blooms |
| Cylindrospermopsin | Anabaena lapponica Aphanizomenon flosa-quae Aphanizomenon gracile Chrysosporum (Anabaena) bergii Chrysosporum (Aphanizomenon) ovalisporum Dolichospermum spp. Hormoscilla pringsheimii Microseira (Lyngbya) wollei R. curvata R. mediterranea Raphidiopsis (Cylindrospermopsis) raciborskii Umezakia natans | ~1–279 mg/g dw nd—9 μg/mm3 0.3–1.6 fg/cell | <1–10 μg/L, rarely up to 800 μg/L Australia 10–100 up to 800 μg/L Mediterranean regions <10 up to 202 μg/L America and regions in Northern Europe <10 up to 18 μg/L |
| Anatoxin-a | Anabaena mendotae Blennothrix Chrysosporum (Aphanizomenon) ovalisporum Cuspidothrix Cylindrospermum, D. flosaquae D. lemmermannii Dolichospermum (Anabaena) circinale Dolichospermum (Anabaena) flos-aquae Kamptonema Microcoleus Oscillatoria Phormidium Planktothrix Raphidiopsis (Cylindrospermopsis) Tychonema | 0.003–13 mg/g dw 9.4–400 fg/cell | 13–1430 μg/L 0.002–8 mg/g dw USA: nd (most sample) up to 1170 μg/L Europe: range nd-13.1 μg/L max: 444 μg/L Australia: up to 25 μg/L. in bloom: 4.4 mg/g dw Africa: bloom and scum: 1.26 mg/g dw |
| Homoanatoxin-a | Dolichospermum/Anabaena Kamptonema (Oscillatoria) formosum Microcoleus (Phormidium) autumnalis Oscillatoria Raphidiopsis mediterranea | 437 fg/cell; ATXeq Frequently nd | 0.44 μg/g ww 34–2118 μg/L |
| Guanitoxin | Dolichospermum (Anabaena) flos-aquae D. lemmermannii D. spiroides | nd—0.74 mg/g dw | 3.3 mg/g dw |
| Saxitoxins | Aphanizomenon Dolichospermum (Anabaena) Microseira (Lyngbya) wollei Planktothrix Raphidiopsis (Cylindrospermopsis) raciborskii Scytonema Sphaerospermopsis torques-reginae | 0.010–2553 μg/g dw 0.77–34.6 fg/cell | 3.14–1000 μg/L 0.0005–4.47 mg/g dw 0.07–0.17 fg/cell |
| Cyanotoxins | Drinking Water (for Chronic Lifetime Exposure) | Drinking Water (Short-Term Exposure ≈2 Weeks) 3 | Recreational Water (Short-Term Exposure) |
|---|---|---|---|
| Microcystins 1 | 1 | 12 | 24 |
| Cylindrospermopsin 1 | 0.7 | 3 | 6 |
| Anatoxin-a 2 | Insufficient information to develop a long-term health-based GV | 30 | 60 |
| Guanitoxin | No toxicological data available (New Zealand has established a limit as provisional maximum acceptable value of 1 μg/L) | ||
| Saxitoxin | Insufficient information to develop a long-term health-based GV | 3 4 | 30 |
| Habitat | Producing Cyanobacteria | T&O Compounds [Co-Production/Co-Occurrence of Cyanotoxins] | Ref. | A | ||
|---|---|---|---|---|---|---|
| Molecular Identification | Production | |||||
| Cultures | ||||||
| Pl | Dolichospermum circinale straight (LC006113) | GEO | gys | 0.01–0.09 ng/µg Chl-a | [81] | Yes |
| [Total MCs] | mcyA | [0.1–0.2 ngMC/µg Chl-a] | ||||
| Pl | Dolichospermum circinale coiled (LC006112) | GEO | gys | |||
| No mcyA | ||||||
| B | Oscillatoria limosa (LC178838) | MIB | mibC | 0.17 ng/µg Chl-a | ||
| No mcyA | ||||||
| Pl | Coelosphaerium sp. G2 | GEO | Yes | [76] | Yes | |
| Pl | Coelosphaerium sp. G2 | GEO | geoA + | [77] | ||
| Coelosphaerium sp.S3C5 | geoA − | |||||
| G2 and S3C5 are morphologically identical | ||||||
| Pl | Anabaena sp. Chusori | GEO; extracellular | 1–2 pg/cell | [82] | Yes | |
| Anabaena sp. NIER | 7–12 pg/cell | |||||
| Anabaena sp. FACHB-1384 | MIB | |||||
| Planktothrix FACHB-1374 | ~400 pg/cell | |||||
| Pl | Planktothrix FACHB-1375 | MIB | 1.6 pg/cell | [83] | Yes | |
| Pl | Anabaena sp. CRKS33 | GEO | metagenomic analysis | [84] | Yes | |
| [Bacteriocins] | ||||||
| [Aeruginosin] | ||||||
| Pl | Dolichospermum circinale AWQC131C | GEO | ||||
| [Bacteriocins] | ||||||
| [Aeruginosin] | ||||||
| [STX] | ||||||
| Pl | Dolichospermum circinale AWQC310F | GEO | ||||
| [Bacteriocins] | ||||||
| [Aeruginosin] | ||||||
| [Cyanobactin] | ||||||
| Pl | Aphanizomenon flos-aquae NIES-81 | GEO | ||||
| [Anabaenopeptin] | ||||||
| [Bacteriocins] | ||||||
| Pl | Dolichospermum DEX189 | GEO | metagenomic analysis | [85] | Yes | |
| [Anabaenopeptin] | ||||||
| Cylindrospermum stagnale PCC 7417 Oscillatoria sp. 193 Oscillatoria sp. PCC 6506 | GEO | geoA | Yes | [78] | ||
| [ATX] [dihydroanatoxin-a] [homoanatoxin-a] | Data from literature | |||||
| L | Nostoc sp. UK18aI Nostoc sp. UK222II_C Nostoc sp. UKK_S60 | GEO | geoA | Yes | ||
| [MCs] | Data from literature | |||||
| Dolichospermum smithii NIES-824 | GEO | geoA | 0.7 ng/µg Chl-a | [86] | Yes | |
| S | Microcoleus asticus sp. nov. | GEO | geoA | Yes | [75] | Yes |
| S | Microcoleus vaginatus (Axenic, from soil) | MIB | 10–100 ng/L | [87] | Yes | |
| Phormidium retzii (Axenic, from a river) | GEO | 10–140 ng/L | ||||
| Pl | Planktothricoides raciborskii | MIB | 3–52 ng/L | [88] | Yes | |
| B | Oscillatoria limosa CHAB 7000 | GEO | geo | 140 ng/µg Chl-a 1 180 ng/µg Chl-a 2 | [89] | Yes |
| B | Phormidium ambiguum AWQC-PHO021 | GEO | 3.79 × 104 ng extracel/L in stationary phase | [73,90] | Yes | |
| [CYN] [deoCYN] | [739 ng/mg dw] [107 ng/mg dw] | |||||
| B | I018-018 Unidentified | GEO | 8 ng/g ww | [72] | Yes | |
| I018-002 Oscillatoria curviceps | 17 ng/g ww | |||||
| I018-046 Anagnostidinema amphibium | 43 ng/g ww | |||||
| I018-044 Anagnostidinema acutissimum | 85 ng/g ww | |||||
| I018-031 Phormidium papyraceum | 167 ng/g ww | |||||
| I018-030 Leptolyngbya frigida | 211 ng/g ww | |||||
| I018-042 2 filaments—Phormidium and Geitlerinema | 489 ng/g ww | |||||
| I018-001 Anagnostidinema acutissimum | 1.141 ng/g ww | |||||
| I018-028 Nodosilinea bijugata | 4.466 ng/g ww | |||||
| I018-043 Anagnostidinema acutissimum | 6.397 ng/g ww | |||||
| I018-029 2 filaments—Leptolyngbya and Geitlerinema | 16.559 ng/g ww | |||||
| I018-047 Phormidium lividum | 8 ng/g ww | |||||
| I018-033 2 filaments—Phormidium and Leptolyngbya | MIB | 4.471 ng/g ww | ||||
| I018-023 Phormidium lusitanicum | MIB | 223 ng/g ww | ||||
| [ATX] | [0.049 ng/g ww] | |||||
| I018-003 Potamolinea aruginocaerulea | GEO | 8 ng/g ww | ||||
| [ATX] | [0.95 ng/g ww] | |||||
| I018-006 Anagnostidinema pseudacutissimum | MIB | 26 ng/g ww | ||||
| [ATX] | [0.27 ng/g ww] | |||||
| I018-034 Geitlerinema sulphureum | MIB | 75 ng/g ww | ||||
| [ATX] | [0.011 ng/g ww] | |||||
| I018-004 Scytonema crispum | [MCs] | [80.44 ng/g ww] | ||||
| I018-015 Geitlerinema acuminatum | [ATX] | [0.014 ng/g ww] | ||||
| I018-039 Geitlerinema sulphureum | [ATX] | [0.014 ng/g ww] | ||||
| Pl, B | Pseudoanabaena galeata TWNCKU13 Pseudanabaena galeata TWNCKU14 | MIB | mibC | 5.96 to 51.1 (fg/cell) | [91] | Yes |
| Pl | Anabaena ucrainica CHAB 1434 Nostocales | GEO | geo | Yes | [79] | Yes |
| Pl | Anabaena planctonica SDZ-1 Nostocales | |||||
| Pl | Anabaena circinalis CHAB 3585 Nostocales | |||||
| B | Anabaena minutissima FACHB 250 Nostocales | |||||
| Pe | Calothrix sp. CHAB 2384 Nostocales | |||||
| B | Cylindrospermum sp. CHAB 2115 Nostocales | |||||
| Pe, S | Nostoc commune FACHB 261 Nostocales | |||||
| Pl | Nodularia sp. Su-A Nostocales | |||||
| Pl | Aphanizomenon sp. CHAB 1684 Nostocales | |||||
| Pl | Aphanizomenon gracile CHAB 2417 Nostocales | |||||
| Pe, S | Nostoc flagelliforme CHAB 2816 Nostocales | |||||
| Pe | Scytonema sp. CHAB 3651 Nostocales | |||||
| Pe | Tychonema bourrellyi CHAB 663 Oscillatoriales | |||||
| Pe, S | Lyngbya kuetzingii FACHB 388 Oscillatoriales | |||||
| Pe | Phormidium sp. D6 Oscillatoriales | |||||
| Pe, S | Leptolyngbya bijugata A4 Oscillatoriales | GEO & MIB producer | Yes | |||
| Pl | Microcystis aeruginosa FACHB-905 | β-cyclocitral | up to 277.8 µg/L | [92] | ||
| [MC-LR (dissolved)] | [up to 1.7 µg/L] | |||||
| Pseudanabaena sp. FACHB-1277 | MIB | up to 178.9 µg/L | ||||
| Anabaena | GEO | up to 10 ng/L | [80] | Yes | ||
| MIB | up to 12 ng/L | |||||
| Oscillatoria | GEO | up to 7 ng/L | ||||
| MIB | up to 7 ng/L | |||||
| Microcystis | GEO | up to 4 ng/L | ||||
| MIB | up to 4 ng/L | |||||
| [MC-LR] | [2.57–23.71 μg/L] | |||||
| Field studies | ||||||
| Microcystis aeruginosa | β-cyclocitral | 0–538 ng/L | [93] 3 | |||
| β-ionone | 0–50.44 ng/L | |||||
| GEO | 0–11.29 ng/L | |||||
| [MCs] | [0–35.42 μg/L] | |||||
| Anabaena spiroides Microcystis sp. (mainly aeruginosa) | GEO | 7.1 μg/L (0.1 pg/cell average) | [94] | |||
| [MC-RR] | [1.56 μg/L] | |||||
| [MC-LR] | [0.544 μg/L] | |||||
| [MC-YR] | [0.066 μg/L] | |||||
| [ATX] | [0.184 μg/L] | |||||
| Lake Taihu—no analysis of cyanobacteria | GEO | 0–37.9 ng/L | [95] 4 | |||
| MIB | 0–832.9 ng/L | |||||
| β-cyclocitral | 0–1706.9 ng/L | |||||
| β-ionone | 0–255.2 ng/L | |||||
| [MCs] | [up to 8.716 μg/L] | |||||
| [CYNs] | [up to 0.623 μg/L] | |||||
| [STXs] | [0–0.338 μg/L] | |||||
| Microcystis Dolichospermum | β-cyclocitral, β-ionone, MIB, GEO | yearly max 250.7 ng/L | [13] | |||
| [MCs] | [8.86 µg/L] | |||||
| B-Mats | Benthic cyanobacteria | GEO | geoA | [73] | ||
| MIB | MIB synthase gene | |||||
| [CYNs] | [cyrA] | |||||
| [STXs] | [stx] | |||||
| [MCs] | [mcyE] | |||||
| B-Mats | Benthic cyanobacteria from Temperate, Sub-tropical, Tropical regions | GEO | geoA | [72] | ||
| MIB | MIB synthase gene | |||||
| [ATX] | [anaF] | |||||
| [STX] | [stxA] | |||||
| [MC] | [mcyE] | |||||
| [CYN] | [CyrA] | |||||
| Planktothrix rubescens | β-ionone | up to 27 ng/L | [96] 5 | |||
| Anabaena | GEO | geoA | 8.02–84.00 ng/L | [97] | ||
| Microcystis | [MCs] | [mcyE] | [up to 10 µg/L] | |||
| Oscillatoria limosa (tentatively) | MIB | MIB synthase gene | up to 9.7 ng/L | |||
| species or genera not reported | GEO | max 110 ng/L | [98] 6 | |||
| [MCs] | max 7.3 µg/L | |||||
| Anabaena Aphanizomenon Microcystis Cylindrospermopsis | GEO | max 0.86 µg/L | [99] 7 | |||
| MIB | max 0.06 µg/L | |||||
| [MCs] | [max 19,000 µg/L] | |||||
| [ATX] | [max 9.5 µg/L] | |||||
| Microcystis sp. Cylindrospermopsis sp. Anabaena sp. Aphanizomenon Pseudanabaena | GEO, MIB, MCs, CYN, STX | [14] 8 | ||||
| Cyanobacteria genus not reported | MIB | 2–30 ng/L | [100] | |||
| [MCs] | [30–340 ng/L] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manganelli, M.; Testai, E.; Tazart, Z.; Scardala, S.; Codd, G.A. Co-Occurrence of Taste and Odor Compounds and Cyanotoxins in Cyanobacterial Blooms: Emerging Risks to Human Health? Microorganisms 2023, 11, 872. https://doi.org/10.3390/microorganisms11040872
Manganelli M, Testai E, Tazart Z, Scardala S, Codd GA. Co-Occurrence of Taste and Odor Compounds and Cyanotoxins in Cyanobacterial Blooms: Emerging Risks to Human Health? Microorganisms. 2023; 11(4):872. https://doi.org/10.3390/microorganisms11040872
Chicago/Turabian StyleManganelli, Maura, Emanuela Testai, Zakaria Tazart, Simona Scardala, and Geoffrey A. Codd. 2023. "Co-Occurrence of Taste and Odor Compounds and Cyanotoxins in Cyanobacterial Blooms: Emerging Risks to Human Health?" Microorganisms 11, no. 4: 872. https://doi.org/10.3390/microorganisms11040872
APA StyleManganelli, M., Testai, E., Tazart, Z., Scardala, S., & Codd, G. A. (2023). Co-Occurrence of Taste and Odor Compounds and Cyanotoxins in Cyanobacterial Blooms: Emerging Risks to Human Health? Microorganisms, 11(4), 872. https://doi.org/10.3390/microorganisms11040872






