Anti-Pseudomonas aeruginosa Vaccines and Therapies: An Assessment of Clinical Trials
Abstract
1. Introduction
1.1. Ventilator-Associated Pneumonia due to P. aeruginosa Infection
1.2. Cystic Fibrosis (CF) and Pneumonia Secondary to P. aeruginosa Infection
1.3. Pseudomonas aeruginosa Burn Wound Infections
2. P. aeruginosa Pathogenesis and Virulence Factors
3. Clinical Trials to Assess the Effectiveness of Anti-Pseudomonas aeruginosa Treatments
3.1. Antibiotics
3.1.1. New Antibiotics
3.1.2. Polymyxin B Derivatives
3.1.3. Beta-Lactamase Inhibitors
3.2. Bacteriophages
3.3. Strategies Targeting P. aeruginosa Virulence (Biofilm, Quorum Sensing, Type III Secretion System, and Antimicrobial Peptides)
3.3.1. Quorum Sensing
3.3.2. Antibiofilm Agent
3.3.3. Antimicrobial Peptides
3.3.4. Type III Secretion System
3.4. P. aeruginosa Virulence Factor Passive Immunotherapy
3.5. P. aeruginosa Outer Membrane Proteins as a Vaccine
3.6. Strategies Targeting P. aeruginosa Iron Acquisition Systems
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Hasan, M.N.; Wilson, J.W.; Lahr, B.D.; Eckel-Passow, J.E.; Baddour, L.M. Incidence of Pseudomonas aeruginosa bacteremia: A population-based study. Am. J. Med. 2008, 121, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Telling, K.; Laht, M.; Brauer, A.; Remm, M.; Kisand, V.; Maimets, M.; Tenson, T.; Lutsar, I. Multidrug resistant Pseudomonas aeruginosa in Estonian hospitals. BMC Infect. Dis. 2018, 18, 513. [Google Scholar] [CrossRef] [PubMed]
- Fridkin, S.K.; Welbel, S.F.; Weinstein, R.A. Magnitude and prevention of nosocomial infections in the intensive care unit. Infect. Dis. Clin. N. Am. 1997, 11, 479–496. [Google Scholar] [CrossRef] [PubMed]
- National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1997, issued May 1997. A report from the NNIS System. Am. J. Infect Control. 1997, 25, 477–487. [CrossRef]
- Gaynes, R.; Edwards, J.R.; National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 2005, 41, 848–854. [Google Scholar] [CrossRef]
- Litwin, A.; Rojek, S.; Gozdzik, W.; Duszynska, W. Pseudomonas aeruginosa device associated—Healthcare associated infections and its multidrug resistance at intensive care unit of University Hospital: Polish, 8.5-year, prospective, single-centre study. BMC Infect. Dis. 2021, 21, 180. [Google Scholar] [CrossRef]
- Kepenekli, E.; Soysal, A.; Yalindag-Ozturk, N.; Ozgur, O.; Ozcan, I.; Devrim, I.; Akar, S.; Bakir, M.; Turkish, P.-H.S.G. Healthcare-associated infections in pediatric intensive care units in Turkey: A national point-prevalence survey. Jpn. J. Infect. Dis. 2015, 68, 381–386. [Google Scholar] [CrossRef]
- Weiner, L.M.; Fridkin, S.K.; Aponte-Torres, Z.; Avery, L.; Coffin, N.; Dudeck, M.A.; Edwards, J.R.; Jernigan, J.A.; Konnor, R.; Soe, M.M.; et al. Vital Signs: Preventing antibiotic-resistant infections in hospitals—United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 235–241. [Google Scholar] [CrossRef]
- Afhami, S.; Seifi, A.; Hajiabdolbaghi, M.; Bazaz, N.E.; Hadadi, A.; Hasibi, M.; Rezaie, P.; Mohamadnejad, E.; Ghahan, A.; Hajinoori, M.; et al. Assessment of device-associated infection rates in teaching hospitals in Islamic Republic of Iran. East Mediterr. Health J. 2019, 25, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Yallew, W.W.; Kumie, A.; Yehuala, F.M. Point prevalence of hospital-acquired infections in two teaching hospitals of Amhara region in Ethiopia. Drug Healthc. Patient Saf. 2016, 8, 71–76. [Google Scholar] [CrossRef]
- Kelly, D.C.; Rizzo, J.; Yun, H.C.; Blyth, D.M. Microbiology and clinical characteristics of industrial oil burns. Burns 2020, 46, 711–717. [Google Scholar] [CrossRef]
- Wardhana, A.; Djan, R.; Halim, Z. Bacterial and antimicrobial susceptibility profile and the prevalence of sepsis among burn patients at the burn unit of Cipto Mangunkusumo Hospital. Ann. Burns Fire Disasters 2017, 30, 107–115. [Google Scholar]
- European Centre for Disease Prevention and Control. Healthcare-Associated Infections Acquired in Intensive Care Units; ECDC: Stockholm, Sweden, 2018.
- European Centre for Disease Prevention and Control. Healthcare-Associated Infections Acquired in Intensive Care Units; ECDC: Stockholm, Sweden, 2019.
- Li, J.; Long, D.; Wu, S.; Wu, X.; Wei, B.; Chen, D.; Shao, Y.; Wang, H.; Cui, L.; Chen, X.; et al. Association of CFH polymorphism with susceptibility to sepsis caused by Pseudomonas aeruginosa in Chinese Han populations: A multi-center study. Gene 2020, 722, 144127. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Lee, K.M.; Kang, H.J.; Shin, D.H.; Kim, D.K. Microbiologic aspects of predominant bacteria isolated from the burn patients in Korea. Burns 2001, 27, 136–139. [Google Scholar] [CrossRef]
- Yildirim, S.; Nursal, T.Z.; Tarim, A.; Torer, N.; Noyan, T.; Demiroglu, Y.Z.; Moray, G.; Haberal, M. Bacteriological profile and antibiotic resistance: Comparison of findings in a burn intensive care unit, other intensive care units, and the hospital services unit of a single center. J. Burn Care Rehabil. 2005, 26, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Oncul, O.; Oksuz, S.; Acar, A.; Ulkur, E.; Turhan, V.; Uygur, F.; Ulcay, A.; Erdem, H.; Ozyurt, M.; Gorenek, L. Nosocomial infection characteristics in a burn intensive care unit: Analysis of an eleven-year active surveillance. Burns 2014, 40, 835–841. [Google Scholar] [CrossRef]
- Garcia Bernal, F.J.; Torrero, V.; Regalado, J.; Gabilondo, F.J. Bacteriology in burn patients undergoing mechanical ventilation. Burns 2000, 26, 731–736. [Google Scholar] [CrossRef]
- Micek, S.T.; Chew, B.; Hampton, N.; Kollef, M.H. A case-control study assessing the impact of nonventilated hospital-acquired pneumonia on patient outcomes. Chest 2016, 150, 1008–1014. [Google Scholar] [CrossRef]
- Restrepo, M.I.; Babu, B.L.; Reyes, L.F.; Chalmers, J.D.; Soni, N.J.; Sibila, O.; Faverio, P.; Cilloniz, C.; Rodriguez-Cintron, W.; Aliberti, S.; et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: A multinational point prevalence study of hospitalised patients. Eur. Respir. J. 2018, 52, 1701190. [Google Scholar] [CrossRef]
- Vesteinsdottir, E.; Helgason, K.O.; Sverrisson, K.O.; Gudlaugsson, O.; Karason, S. Infections and outcomes after cardiac surgery-The impact of outbreaks traced to transesophageal echocardiography probes. Acta Anaesthesiol. Scand 2019, 63, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, D.; Lenney, W.; Alcock, P.; Campbell, C.A.; Gray, J.; Pantin, C. Pseudomonas aeruginosa in cystic fibrosis: Cross-infection and the need for segregation. Respir. Med. 2001, 95, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.C.; Waters, V.J. Microbiology of cystic fibrosis airway disease. Semin. Respir. Crit. Care Med. 2019, 40, 727–736. [Google Scholar] [CrossRef]
- Lebecque, P.; Leal, T.; Zylberberg, K.; Reychler, G.; Bossuyt, X.; Godding, V. Towards zero prevalence of chronic Pseudomonas aeruginosa infection in children with cystic fibrosis. J. Cyst. Fibros. 2006, 5, 237–244. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation. Patient Registry 2020 Annual Data Report; Cystic Fibrosis Foundation: Seattle, WA, USA, 2021. [Google Scholar]
- Paixao, V.A.; Barros, T.F.; Mota, C.M.; Moreira, T.F.; Santana, M.A.; Reis, J.N. Prevalence and antimicrobial susceptibility of respiratory pathogens in patients with cystic fibrosis. Braz. J. Infect. Dis. 2010, 14, 406–409. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation. Patient Registry 2021 Annual Data Report; Cystic Fibrosis Foundation: Seattle, WA, USA, 2022. [Google Scholar]
- Psoter, K.J.; De Roos, A.J.; Wakefield, J.; Mayer, J.; Rosenfeld, M. Season is associated with Pseudomonas aeruginosa acquisition in young children with cystic fibrosis. Clin. Microbiol. Infect. 2013, 19, E483–E489. [Google Scholar] [CrossRef]
- Pittman, J.E.; Noah, H.; Calloway, H.E.; Davis, S.D.; Leigh, M.W.; Drumm, M.; Sagel, S.D.; Accurso, F.J.; Knowles, M.R.; Sontag, M.K. Early childhood lung function is a stronger predictor of adolescent lung function in cystic fibrosis than early Pseudomonas aeruginosa infection. PLoS ONE 2017, 12, e0177215. [Google Scholar] [CrossRef]
- Keating, C.; Poor, A.D.; Liu, X.; Chiuzan, C.; Backenroth, D.; Zhang, Y.; DiMango, E. Reduced survival in adult cystic fibrosis despite attenuated lung function decline. J. Cyst. Fibros. 2017, 16, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, H.G.; Benedetti, A.; Landry, J.S.; Bernier, J.; Matouk, E.; Radzioch, D.; Lands, L.C.; Rousseau, S.; Nguyen, D. Clinical outcomes associated with Staphylococcus aureus and Pseudomonas aeruginosa airway infections in adult cystic fibrosis patients. BMC Pulm. Med. 2015, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.E.; Schultz, H.H.L.; Johansen, H.K.; Pressler, T.; Lund, T.K.; Iversen, M.; Perch, M. Bacterial re-colonization occurs early after lung transplantation in cystic fibrosis patients. J. Clin. Med. 2021, 10, 1275. [Google Scholar] [CrossRef] [PubMed]
- Erfanimanesh, S.; Emaneini, M.; Modaresi, M.R.; Feizabadi, M.M.; Halimi, S.; Beigverdi, R.; Nikbin, V.S.; Jabalameli, F. Distribution and characteristics of bacteria isolated from cystic fibrosis patients with pulmonary exacerbation. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 5831139. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, A.N.; Wilcox, P.G.; Quon, B.S. Cystic fibrosis respiratory microbiology monitoring during a global pandemic: Lessons learned from a shift to telehealth. Ann. Am. Thorac. Soc. 2022, 19, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Ferriere, K.; Hugonnet, S.; Ricou, B.; Suter, P.; Pittet, D. Epidemiology and prognostic determinants of bloodstream infections in surgical intensive care. Arch. Surg. 2002, 137, 1353–1359, discussion 1359. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Cornely, O.A.; Moser, S.; Bethe, U.; Stutzer, H.; Salzberger, B.; Fatkenheuer, G.; Seifert, H. Outcomes of nosocomial bloodstream infections in adult neutropenic patients: A prospective cohort and matched case-control study. Infect. Control. Hosp. Epidemiol. 2003, 24, 905–911. [Google Scholar] [CrossRef]
- Williams, F.N.; Herndon, D.N.; Hawkins, H.K.; Lee, J.O.; Cox, R.A.; Kulp, G.A.; Finnerty, C.C.; Chinkes, D.L.; Jeschke, M.G. The leading causes of death after burn injury in a single pediatric burn center. Crit. Care 2009, 13, R183. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin. Infect. Dis. 2003, 36, 1103–1110. [Google Scholar] [CrossRef]
- Osmon, S.; Ward, S.; Fraser, V.J.; Kollef, M.H. Hospital mortality for patients with bacteremia due to Staphylococcus aureus or Pseudomonas aeruginosa. Chest 2004, 125, 607–616. [Google Scholar] [CrossRef]
- Mikulska, M.; Del Bono, V.; Raiola, A.M.; Bruno, B.; Gualandi, F.; Occhini, D.; di Grazia, C.; Frassoni, F.; Bacigalupo, A.; Viscoli, C. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: Reemergence of Gram-negative rods and increasing antibiotic resistance. Biol. Blood Marrow. Transplant. 2009, 15, 47–53. [Google Scholar] [CrossRef]
- Vitkauskiene, A.; Skrodeniene, E.; Dambrauskiene, A.; Macas, A.; Sakalauskas, R. Pseudomonas aeruginosa bacteremia: Resistance to antibiotics, risk factors, and patient mortality. Medicina 2010, 46, 490–495. [Google Scholar] [CrossRef]
- Satlin, M.J.; Soave, R.; Racanelli, A.C.; Shore, T.B.; van Besien, K.; Jenkins, S.G.; Walsh, T.J. The emergence of vancomycin-resistant enterococcal bacteremia in hematopoietic stem cell transplant recipients. Leuk Lymphoma 2014, 55, 2858–2865. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.K.; Xiao, K.; Huang, Y.T.; Jongwutiwes, U.; Chung, D.; Maloy, M.; Giralt, S.; Barker, J.N.; Jakubowski, A.A.; Papanicolaou, G.A. Impact of peri-transplant vancomycin and fluoroquinolone administration on rates of bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients: A 12-year single institution study. J. Infect. 2014, 69, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Magret, M.; Lisboa, T.; Martin-Loeches, I.; Manez, R.; Nauwynck, M.; Wrigge, H.; Cardellino, S.; Diaz, E.; Koulenti, D.; Rello, J. Bacteremia is an independent risk factor for mortality in nosocomial pneumonia: A prospective and observational multicenter study. Crit. Care 2011, 15, R62. [Google Scholar] [CrossRef] [PubMed]
- Trecarichi, E.M.; Pagano, L.; Candoni, A.; Pastore, D.; Cattaneo, C.; Fanci, R.; Nosari, A.; Caira, M.; Spadea, A.; Busca, A.; et al. Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: An Italian multicentre prospective survey. Clin. Microbiol. Infect. 2015, 21, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.C.; Wang, S.M.; Shen, C.F.; Ma, Y.J.; Ho, T.S.; Chen, J.S.; Cheng, C.N.; Liu, C.C. Bloodstream infections in pediatric patients with acute leukemia: Emphasis on gram-negative bacteria infections. J. Microbiol. Immunol. Infect. 2017, 50, 507–513. [Google Scholar] [CrossRef]
- Kikuchi, M.; Akahoshi, Y.; Nakano, H.; Ugai, T.; Wada, H.; Yamasaki, R.; Sakamoto, K.; Kawamura, K.; Ishihara, Y.; Sato, M.; et al. Risk factors for pre- and post-engraftment bloodstream infections after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2015, 17, 56–65. [Google Scholar] [CrossRef]
- Calvo-Lon, J.; Landaverde, D.U.; Ramos-Esquivel, A.; Villalobos-Vindas, J.M. Epidemiology and outcomes of bloodstream infections in patients with solid tumors in a Central American population at Mexico Hospital, San Jose, Costa Rica. J. Glob. Oncol. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Marin, M.; Gudiol, C.; Garcia-Vidal, C.; Ardanuy, C.; Carratala, J. Bloodstream infections in patients with solid tumors: Epidemiology, antibiotic therapy, and outcomes in 528 episodes in a single cancer center. Medicine (Baltimore) 2014, 93, 143–149. [Google Scholar] [CrossRef]
- Sousa, D.; Ceniceros, A.; Galeiras, R.; Pertega-Diaz, S.; Gutierrez-Urbon, J.M.; Rodriguez-Mayo, M.; Lopez-Suso, E.; Mourelo-Farina, M.; Llinares, P. Microbiology in burns patients with blood stream infections: Trends over time and during the course of hospitalization. Infect. Dis. 2018, 50, 289–296. [Google Scholar] [CrossRef]
- Buetti, N.; Lo Priore, E.; Sommerstein, R.; Atkinson, A.; Kronenberg, A.; Marschall, J.; Swiss Centre for Antibiotic resistance (ANRESIS). Epidemiology of subsequent bloodstream infections in the ICU. Crit. Care 2018, 22, 259. [Google Scholar] [CrossRef]
- Thaden, J.T.; Park, L.P.; Maskarinec, S.A.; Ruffin, F.; Fowler, V.G., Jr.; van Duin, D. Results from a 13-year prospective cohort study show increased mortality associated with bloodstream infections caused by Pseudomonas aeruginosa compared to other bacteria. Antimicrob. Agents Chemother. 2017, 61, e02671-16. [Google Scholar] [CrossRef] [PubMed]
- Stoma, I.; Karpov, I.; Milanovich, N.; Uss, A.; Iskrov, I. Risk factors for mortality in patients with bloodstream infections during the pre-engraftment period after hematopoietic stem cell transplantation. Blood Res. 2016, 51, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Pfaller, M.A.; Jones, R.N.; Doern, G.V.; Winokur, P.L.; Gales, A.C.; Sader, H.S.; Kugler, K.; Beach, M. Survey of bloodstream infections due to gram-negative bacilli: Frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin. Infect. Dis. 1999, 29, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Cardozo-Espinola, C.; Puerta-Alcalde, P.; Marco, F.; Tellez, A.; Aguero, D.; Romero-Santana, F.; Diaz-Beya, M.; Gine, E.; Morata, L.; et al. Risk factors for mortality in patients with acute leukemia and bloodstream infections in the era of multiresistance. PLoS ONE 2018, 13, e0199531. [Google Scholar] [CrossRef]
- Fily, F.; Ronat, J.B.; Malou, N.; Kanapathipillai, R.; Seguin, C.; Hussein, N.; Fakhri, R.M.; Langendorf, C. Post-traumatic osteomyelitis in Middle East war-wounded civilians: Resistance to first-line antibiotics in selected bacteria over the decade 2006-2016. BMC Infect. Dis. 2019, 19, 103. [Google Scholar] [CrossRef]
- Djuric, O.; Markovic-Denic, L.; Jovanovic, B.; Bumbasirevic, V. High incidence of multiresistant bacterial isolates from bloodstream infections in trauma emergency department and intensive care unit in Serbia. Acta Microbiol. Immunol. Hung. 2019, 66, 307–325. [Google Scholar] [CrossRef]
- Lari, A.R.; Alaghehbandan, R. Nosocomial infections in an Iranian burn care center. Burns 2000, 26, 737–740. [Google Scholar] [CrossRef]
- Park, J.J.; Seo, Y.B.; Choi, Y.K.; Kym, D.; Lee, J. Changes in the prevalence of causative pathogens isolated from severe burn patients from 2012 to 2017. Burns 2020, 46, 695–701. [Google Scholar] [CrossRef]
- Rello, J.; Jubert, P.; Vallés, J.; Artigas, A.; Rué, M.; Niederman, M.S. Evaluation of outcome for intubated patients with pneumonia due to Pseudomonas aeruginosa. Clin. Infect. Dis. 1996, 23, 973–978. [Google Scholar] [CrossRef]
- Kollef, M.H.; Chastre, J.; Fagon, J.Y.; François, B.; Niederman, M.S.; Rello, J.; Torres, A.; Vincent, J.L.; Wunderink, R.G.; Go, K.W.; et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit. Care Med. 2014, 42, 2178–2187. [Google Scholar] [CrossRef]
- Chastre, J.; Fagon, J.Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef]
- Rello, J.; Borgatta, B.; Lagunes, L. Management of Pseudomonas aeruginosa pneumonia: One size does not fit all. Crit. Care 2014, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Lisboa, T.; Koulenti, D. Respiratory infections in patients undergoing mechanical ventilation. Lancet Respir. Med. 2014, 2, 764–774. [Google Scholar] [CrossRef]
- Rello, J.; Ausina, V.; Ricart, M.; Puzo, C.; Quintana, E.; Net, A.; Prats, G. Risk factors for infection by Pseudomonas aeruginosa in patients with ventilator-associated pneumonia. Intensive Care Med. 1994, 20, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Mariscal, D.; March, F.; Jubert, P.; Sanchez, F.; Valles, J.; Coll, P. Recurrent Pseudomonas aeruginosa pneumonia in ventilated patients: Relapse or reinfection? Am. J. Respir. Crit. Care Med. 1998, 157, 912–916. [Google Scholar] [CrossRef]
- Vallés, J.; Mesalles, E.; Mariscal, D.; del Mar Fernández, M.; Peña, R.; Jiménez, J.L.; Rello, J. A 7-year study of severe hospital-acquired pneumonia requiring ICU admission. Intensive Care Med. 2003, 29, 1981–1988. [Google Scholar] [CrossRef]
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [CrossRef] [PubMed]
- Garnacho-Montero, J.; Sa-Borges, M.; Sole-Violan, J.; Barcenilla, F.; Escoresca-Ortega, A.; Ochoa, M.; Cayuela, A.; Rello, J. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: An observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit. Care Med. 2007, 35, 1888–1895. [Google Scholar] [CrossRef]
- Safdar, N.; Handelsman, J.; Maki, D.G. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect. Dis. 2004, 4, 519–527. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Corcia-Palomo, Y.; Amaya-Villar, R.; Martin-Villen, L. How to treat VAP due to MDR pathogens in ICU patients. BMC Infect. Dis. 2014, 14, 135. [Google Scholar] [CrossRef]
- Ramsey, B.W.; Dorkin, H.L.; Eisenberg, J.D.; Gibson, R.L.; Harwood, I.R.; Kravitz, R.M.; Schidlow, D.V.; Wilmott, R.W.; Astley, S.J.; McBurnie, M.A.; et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N. Engl. J. Med. 1993, 328, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Miller, S.; Sorscher, E.J. Cystic fibrosis. N. Engl. J. Med. 2005, 352, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Rogan, M.P.; Stoltz, D.A.; Hornick, D.B. Cystic fibrosis transmembrane conductance regulator intracellular processing, trafficking, and opportunities for mutation-specific treatment. Chest 2011, 139, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Mogayzel, P.J., Jr.; Naureckas, E.T.; Robinson, K.A.; Brady, C.; Guill, M.; Lahiri, T.; Lubsch, L.; Matsui, J.; Oermann, C.M.; Ratjen, F.; et al. Cystic Fibrosis Foundation pulmonary guideline. pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann. Am. Thorac. Soc. 2014, 11, 1640–1650. [Google Scholar] [CrossRef]
- Saint-Criq, V.; Gray, M.A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci. 2017, 74, 93–115. [Google Scholar] [CrossRef]
- Döring, G.; Flume, P.; Heijerman, H.; Elborn, J.S. Treatment of lung infection in patients with cystic fibrosis: Current and future strategies. J. Cyst. Fibros. 2012, 11, 461–479. [Google Scholar] [CrossRef]
- Lipuma, J.J. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010, 23, 299–323. [Google Scholar] [CrossRef]
- Esposito, A.; Pompilio, A.; Bettua, C.; Crocetta, V.; Giacobazzi, E.; Fiscarelli, E.; Jousson, O.; Di Bonaventura, G. Evolution of Stenotrophomonas maltophilia in Cystic Fibrosis Lung over Chronic Infection: A Genomic and Phenotypic Population Study. Front. Microbiol. 2017, 8, 1590. [Google Scholar] [CrossRef]
- Ratjen, F.; Brockhaus, F.; Angyalosi, G. Aminoglycoside therapy against Pseudomonas aeruginosa in cystic fibrosis: A review. J. Cyst. Fibros. 2009, 8, 361–369. [Google Scholar] [CrossRef]
- Stephenson, A.L.; Bell, S.C. The Cystic Fibrosis Foundation Patient Registry. Design and Methods of a National Observational Disease Registry. Ann. Am. Thorac. Soc. 2016, 13, 1014–1015. [Google Scholar] [CrossRef]
- Kenna, D.T.; Doherty, C.J.; Foweraker, J.; Macaskill, L.; Barcus, V.A.; Govan, J.R.W. Hypermutability in environmental Pseudomonas aeruginosa and in populations causing pulmonary infection in individuals with cystic fibrosis. Microbiology 2007, 153, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Mandsberg, L.F.; Wang, H.; Høiby, N. Phenotypes selected during chronic lung infection in cystic fibrosis patients: Implications for the treatment of Pseudomonas aeruginosa biofilm infections. FEMS Immunol. Med. Microbiol. 2012, 65, 215–225. [Google Scholar] [CrossRef]
- Drenkard, E.; Ausubel, F.M. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 2002, 416, 740–743. [Google Scholar] [CrossRef] [PubMed]
- LiPuma, J.J. Microbiological and immunologic considerations with aerosolized drug delivery. Chest 2001, 120, 118s–123s. [Google Scholar] [CrossRef] [PubMed]
- Flume, P.A.; VanDevanter, D.R. Clinical applications of pulmonary delivery of antibiotics. Adv. Drug Deliv. Rev. 2015, 85, 1–6. [Google Scholar] [CrossRef]
- Máiz, L.; Girón, R.M.; Olveira, C.; Quintana, E.; Lamas, A.; Pastor, D.; Cantón, R.; Mensa, J. Inhaled antibiotics for the treatment of chronic bronchopulmonary Pseudomonas aeruginosa infection in cystic fibrosis: Systematic review of randomised controlled trials. Expert Opin. Pharmacother. 2013, 14, 1135–1149. [Google Scholar] [CrossRef]
- Sanders, D.B.; Hoffman, L.R.; Emerson, J.; Gibson, R.L.; Rosenfeld, M.; Redding, G.J.; Goss, C.H. Return of FEV1 after pulmonary exacerbation in children with cystic fibrosis. Pediatr. Pulmonol. 2010, 45, 127–134. [Google Scholar] [CrossRef]
- McManus, A.T.; Mason, A.D., Jr.; McManus, W.F.; Pruitt, B.A., Jr. Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur. J. Clin. Microbiol. 1985, 4, 219–223. [Google Scholar] [CrossRef]
- Ranjbar, R.; Owlia, P.; Saderi, H.; Mansouri, S.; Jonaidi-Jafari, N.; Izadi, M.; Farshad, S.; Arjomandzadegan, M. Characterization of Pseudomonas aeruginosa strains isolated from burned patients hospitalized in a major burn center in Tehran, Iran. Acta Med. Iran. 2011, 49, 675–679. [Google Scholar]
- de Almeida Silva, K.C.F.; Calomino, M.A.; Deutsch, G.; de Castilho, S.R.; de Paula, G.R.; Esper, L.M.R.; Teixeira, L.A. Molecular characterization of multidrug-resistant (MDR) Pseudomonas aeruginosa isolated in a burn center. Burns 2017, 43, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Fransén, J.; Huss, F.R.; Nilsson, L.E.; Rydell, U.; Sjöberg, F.; Hanberger, H. Surveillance of antibiotic susceptibility in a Swedish Burn Center 1994-2012. Burns 2016, 42, 1295–1303. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Mathee, K.; Narasimhan, G.; Valdes, C.; Qiu, X.; Matewish, J.M.; Koehrsen, M.; Rokas, A.; Yandava, C.N.; Engels, R.; Zeng, E.; et al. Dynamics of Pseudomonas aeruginosa genome evolution. Proc. Natl. Acad. Sci. USA 2008, 105, 3100–3105. [Google Scholar] [CrossRef]
- Passador, L.; Cook, J.M.; Gambello, M.J.; Rust, L.; Iglewski, B.H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 1993, 260, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Pesci, E.C.; Milbank, J.B.; Pearson, J.P.; McKnight, S.; Kende, A.S.; Greenberg, E.P.; Iglewski, B.H. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1999, 96, 11229–11234. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.P.; Passador, L.; Iglewski, B.H.; Greenberg, E.P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1995, 92, 1490–1494. [Google Scholar] [CrossRef]
- Pearson, J.P.; Gray, K.M.; Passador, L.; Tucker, K.D.; Eberhard, A.; Iglewski, B.H.; Greenberg, E.P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 1994, 91, 197–201. [Google Scholar] [CrossRef]
- Bucior, I.; Pielage, J.F.; Engel, J.N. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathog. 2012, 8, e1002616. [Google Scholar] [CrossRef]
- van Delden, C. Virulence Factors in Pseudomonas Aeruginosa. In Virulence and Gene Regulation; Ramos, J.-L., Ed.; Springer: Boston, MA, USA, 2004; pp. 3–45. [Google Scholar]
- Leid, J.G.; Willson, C.J.; Shirtliff, M.E.; Hassett, D.J.; Parsek, M.R.; Jeffers, A.K. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J. Immunol. 2005, 175, 7512–7518. [Google Scholar] [CrossRef]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef]
- Hentzer, M.; Teitzel, G.M.; Balzer, G.J.; Heydorn, A.; Molin, S.; Givskov, M.; Parsek, M.R. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 2001, 183, 5395–5401. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, D.M.; Wozniak, D.J. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol. 2005, 56, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.F.; Sa-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Huszczynski, S.M.; Lam, J.S.; Khursigara, C.M. The Role of Pseudomonas aeruginosa Lipopolysaccharide in Bacterial Pathogenesis and Physiology. Pathogens 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kirienko, D.R.; Webster, P.; Fisher, A.L.; Kirienko, N.V. Pyoverdine, a siderophore from Pseudomonas aeruginosa, translocates into C. elegans, removes iron, and activates a distinct host response. Virulence 2018, 9, 804–817. [Google Scholar] [CrossRef]
- Lau, G.W.; Hassett, D.J.; Ran, H.; Kong, F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004, 10, 599–606. [Google Scholar] [CrossRef]
- Laarman, A.J.; Bardoel, B.W.; Ruyken, M.; Fernie, J.; Milder, F.J.; van Strijp, J.A.; Rooijakkers, S.H. Pseudomonas aeruginosa alkaline protease blocks complement activation via the classical and lectin pathways. J. Immunol. 2012, 188, 386–393. [Google Scholar] [CrossRef]
- Bleves, S.; Viarre, V.; Salacha, R.; Michel, G.P.; Filloux, A.; Voulhoux, R. Protein secretion systems in Pseudomonas aeruginosa: A wealth of pathogenic weapons. Int. J. Med. Microbiol. 2010, 300, 534–543. [Google Scholar] [CrossRef]
- Newman, J.W.; Floyd, R.V.; Fothergill, J.L. The contribution of Pseudomonas aeruginosa virulence factors and host factors in the establishment of urinary tract infections. FEMS Microbiol. Lett. 2017, 364, fnx124. [Google Scholar] [CrossRef]
- Engel, L.S.; Hill, J.M.; Caballero, A.R.; Green, L.C.; O’Callaghan, R.J. Protease IV, a unique extracellular protease and virulence factor from Pseudomonas aeruginosa. J. Biol. Chem. 1998, 273, 16792–16797. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cai, X.; Harris, T.L.; Gooyit, M.; Wood, M.; Lardy, M.; Janda, K.D. Disarming Pseudomonas aeruginosa virulence factor LasB by leveraging a Caenorhabditis elegans infection model. Chem. Biol. 2015, 22, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Tielen, P.; Rosenau, F.; Wilhelm, S.; Jaeger, K.E.; Flemming, H.C.; Wingender, J. Extracellular enzymes affect biofilm formation of mucoid Pseudomonas aeruginosa. Microbiology 2010, 156, 2239–2252. [Google Scholar] [CrossRef] [PubMed]
- Park, P.W.; Pier, G.B.; Preston, M.J.; Goldberger, O.; Fitzgerald, M.L.; Bernfield, M. Syndecan-1 shedding is enhanced by LasA, a secreted virulence factor of Pseudomonas aeruginosa. J. Biol. Chem. 2000, 275, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.; Murphy, L.M.; Conners, R.; Sessions, R.B.; Gamblin, S.J. Crystal structure of the LasA virulence factor from Pseudomonas aeruginosa: Substrate specificity and mechanism of M23 metallopeptidases. J. Mol. Biol. 2010, 396, 908–923. [Google Scholar] [CrossRef]
- Kessler, E.; Safrin, M.; Abrams, W.R.; Rosenbloom, J.; Ohman, D.E. Inhibitors and specificity of Pseudomonas aeruginosa LasA. J. Biol. Chem. 1997, 272, 9884–9889. [Google Scholar] [CrossRef]
- Guillon, A.; Brea, D.; Morello, E.; Tang, A.; Jouan, Y.; Ramphal, R.; Korkmaz, B.; Perez-Cruz, M.; Trottein, F.; O’Callaghan, R.J.; et al. Pseudomonas aeruginosa proteolytically alters the interleukin 22-dependent lung mucosal defense. Virulence 2017, 8, 810–820. [Google Scholar] [CrossRef]
- Michalska, M.; Wolf, P. Pseudomonas Exotoxin A: Optimized by evolution for effective killing. Front. Microbiol. 2015, 6, 963. [Google Scholar] [CrossRef]
- Schultz, M.J.; Speelman, P.; Zaat, S.A.; Hack, C.E.; van Deventer, S.J.; van der Poll, T. The effect of pseudomonas exotoxin A on cytokine production in whole blood exposed to Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 2000, 29, 227–232. [Google Scholar] [CrossRef]
- Frank, D.W. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 1997, 26, 621–629. [Google Scholar] [CrossRef]
- Rangel, S.M.; Logan, L.K.; Hauser, A.R. The ADP-ribosyltransferase domain of the effector protein ExoS inhibits phagocytosis of Pseudomonas aeruginosa during pneumonia. mBio 2014, 5, e01080-14. [Google Scholar] [CrossRef] [PubMed]
- Rangel, S.M.; Diaz, M.H.; Knoten, C.A.; Zhang, A.; Hauser, A.R. The Role of ExoS in Dissemination of Pseudomonas aeruginosa during Pneumonia. PLoS Pathog. 2015, 11, e1004945. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.H.; Hauser, A.R. Pseudomonas aeruginosa cytotoxin ExoU is injected into phagocytic cells during acute pneumonia. Infect. Immun. 2010, 78, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.J.; Goldufsky, J.W.; Bello, D.; Masood, S.; Shafikhani, S.H. Pseudomonas aeruginosa ExoT Induces Mitochondrial Apoptosis in Target Host Cells in a Manner That Depends on Its GTPase-activating Protein (GAP) Domain Activity. J. Biol. Chem. 2015, 290, 29063–29073. [Google Scholar] [CrossRef] [PubMed]
- Sayner, S.L.; Frank, D.W.; King, J.; Chen, H.; VandeWaa, J.; Stevens, T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ. Res. 2004, 95, 196–203. [Google Scholar] [CrossRef]
- Chen, L.; Zou, Y.; She, P.; Wu, Y. Composition, function, and regulation of T6SS in Pseudomonas aeruginosa. Microbiol. Res. 2015, 172, 19–25. [Google Scholar] [CrossRef]
- Schiller, N.L.; Hackley, D.R.; Morrison, A. Isolation and characterization of serum-resistant strains ofPseudomonas aeruginosa derived from serum-sensitive parental strains. Curr. Microbiol. 1984, 10, 185–189. [Google Scholar] [CrossRef]
- Vitkauskiene, A.; Scheuss, S.; Sakalauskas, R.; Dudzevicius, V.; Sahly, H. Pseudomonas aeruginosa strains from nosocomial pneumonia are more serum resistant than P. aeruginosa strains from noninfectious respiratory colonization processes. Infection 2005, 33, 356–361. [Google Scholar] [CrossRef]
- Hancock, R.E.; Mutharia, L.M.; Chan, L.; Darveau, R.P.; Speert, D.P.; Pier, G.B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: A class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect. Immun. 1983, 42, 170–177. [Google Scholar] [CrossRef]
- Munguia, J.; LaRock, D.L.; Tsunemoto, H.; Olson, J.; Cornax, I.; Pogliano, J.; Nizet, V. The Mla pathway is critical for Pseudomonas aeruginosa resistance to outer membrane permeabilization and host innate immune clearance. J. Mol. Med. 2017, 95, 1127–1136. [Google Scholar] [CrossRef]
- Dzvova, N.; Colmer-Hamood, J.A.; Griswold, J.A.; Hamood, A.N. Heparinase Is Essential for Pseudomonas aeruginosa Virulence during Thermal Injury and Infection. Infect. Immun. 2018, 86, e00755-17. [Google Scholar] [CrossRef] [PubMed]
- Persyn, E.; Sassi, M.; Aubry, M.; Broly, M.; Delanou, S.; Asehnoune, K.; Caroff, N.; Cremet, L. Rapid genetic and phenotypic changes in Pseudomonas aeruginosa clinical strains during ventilator-associated pneumonia. Sci. Rep. 2019, 9, 4720. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, P.D.; Lam, J.S. WaaL of Pseudomonas aeruginosa utilizes ATP in in vitro ligation of O antigen onto lipid A-core. Mol. Microbiol. 2007, 65, 1345–1359. [Google Scholar] [CrossRef]
- Malekian, A.; Esmaeeli Djavid, G.; Akbarzadeh, K.; Soltandallal, M.; Rassi, Y.; Rafinejad, J.; Rahimi Foroushani, A.; Farhoud, A.; Bakhtiary, R.; Totonchi, M. Efficacy of Maggot Therapy on Staphylococcus aureus and Pseudomonas aeruginosa in Diabetic Foot Ulcers: A Randomized Controlled Trial. J. Wound Ostomy Cont. Nurs. 2019, 46, 25–29. [Google Scholar] [CrossRef]
- Stone, G.G.; Newell, P.; Bradford, P.A. In Vitro Activity of Ceftazidime-Avibactam against Isolates from Patients in a Phase 3 Clinical Trial for Treatment of Complicated Intra-abdominal Infections. Antimicrob. Agents Chemother. 2018, 62, e02584-17. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Hornick, D.; Fedler, J.; Launspach, J.L.; Teresi, M.E.; Santacroce, T.R.; Cavanaugh, J.E.; Horan, R.; Nelson, G.; Starner, T.D.; et al. Randomized controlled study of aerosolized hypertonic xylitol versus hypertonic saline in hospitalized patients with pulmonary exacerbation of cystic fibrosis. J. Cyst. Fibros. 2020, 19, 108–113. [Google Scholar] [CrossRef]
- Ratjen, F.; Moeller, A.; McKinney, M.L.; Asherova, I.; Alon, N.; Maykut, R.; Angyalosi, G. Eradication of early P. aeruginosa infection in children <7 years of age with cystic fibrosis: The early study. J. Cyst. Fibros. 2019, 18, 78–85. [Google Scholar] [CrossRef]
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.; Michels, G.; Kluge, S.; et al. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: A randomized controlled trial. Intensive Care Med. 2022, 48, 311–321. [Google Scholar] [CrossRef]
- Eklöf, J.; Misiakou, M.A.; Sivapalan, P.; Armbruster, K.; Browatzki, A.; Nielsen, T.L.; Lapperre, T.S.; Andreassen, H.F.; Janner, J.; Ulrik, C.S.; et al. Persistence and genetic adaptation of Pseudomonas aeruginosa in patients with chronic obstructive pulmonary disease. Clin. Microbiol. Infect. 2022, 28, 990–995. [Google Scholar] [CrossRef]
- Cogen, J.D.; Onchiri, F.M.; Hamblett, N.M.; Gibson, R.L.; Morgan, W.J.; Rosenfeld, M. Association of Intensity of Antipseudomonal Antibiotic Therapy With Risk of Treatment-Emergent Organisms in Children With Cystic Fibrosis and Newly Acquired Pseudomonas Aeruginosa. Clin. Infect. Dis. 2021, 73, 987–993. [Google Scholar] [CrossRef]
- Slekovec, C.; Robert, J.; Berthelot, P.; van der Mee-Marquet, N.; Rogues, A.M.; Derouin, V.; Cholley, P.; Bertrand, X.; Gbaguidi-Haore, H. Do Contact Precautions Reduce the Incidence of Intensive Care Unit-Acquired Pseudomonas aeruginosa Infections? The DPCPYO (Detection and Contact Precautions for Patients With P. aeruginosa) Cluster-Randomized Crossover Trial. Clin. Infect. Dis. 2021, 73, e2781–e2788. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.G.; Bruno, C.; Castanheira, M.; Yu, B.; Huntington, J.A.; Carmelitano, P.; Rhee, E.G.; De Anda, C.; Motyl, M. Evaluating the emergence of nonsusceptibility among Pseudomonas aeruginosa respiratory isolates from a phase-3 clinical trial for treatment of nosocomial pneumonia (ASPECT-NP). Int. J. Antimicrob. Agents 2021, 57, 106278. [Google Scholar] [CrossRef] [PubMed]
- Portsmouth, S.; van Veenhuyzen, D.; Echols, R.; Machida, M.; Ferreira, J.C.A.; Ariyasu, M.; Tenke, P.; Nagata, T.D. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: A phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect. Dis. 2018, 18, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Bouglé, A.; Tuffet, S.; Federici, L.; Leone, M.; Monsel, A.; Dessalle, T.; Amour, J.; Dahyot-Fizelier, C.; Barbier, F.; Luyt, C.E.; et al. Comparison of 8 versus 15 days of antibiotic therapy for Pseudomonas aeruginosa ventilator-associated pneumonia in adults: A randomized, controlled, open-label trial. Intensive Care Med. 2022, 48, 841–849. [Google Scholar] [CrossRef]
- Fabre, V.; Amoah, J.; Cosgrove, S.E.; Tamma, P.D. Antibiotic Therapy for Pseudomonas aeruginosa Bloodstream Infections: How Long Is Long Enough? Clin. Infect. Dis. 2019, 69, 2011–2014. [Google Scholar] [CrossRef]
- Eklöf, J.; Alispahic, I.A.; Sivapalan, P.; Wilcke, T.; Seersholm, N.; Armbruster, K.; Kjærgaard, J.L.; Saeed, M.I.; Nielsen, T.L.; Browatzki, A.; et al. Targeted AntiBiotics for Chronic pulmonary diseases (TARGET ABC): Can targeted antibiotic therapy improve the prognosis of Pseudomonas aeruginosa-infected patients with chronic pulmonary obstructive disease, non-cystic fibrosis bronchiectasis, and asthma? A multicenter, randomized, controlled, open-label trial. Trials 2022, 23, 817. [Google Scholar] [CrossRef]
- Strouvalis, I.; Routsi, C.; Adamopoulou, M.; Raftogiannis, M.; Renieris, G.; Orfanos, S.E.; Kotanidou, A.; Sabracos, L.; Giamarellos-Bourboulis, E.J. Early increase of VEGF-A is associated with resolution of ventilator-associated pneumonia: Clinical and experimental evidence. Respirology 2018, 23, 942–949. [Google Scholar] [CrossRef]
- Mikamo, H.; Monden, K.; Miyasaka, Y.; Horiuchi, T.; Fujimoto, G.; Fukuhara, T.; Yoshinari, T.; Rhee, E.G.; Shizuya, T. The efficacy and safety of tazobactam/ceftolozane in combination with metronidazole in Japanese patients with complicated intra-abdominal infections. J. Infect. Chemother. 2019, 25, 111–116. [Google Scholar] [CrossRef]
- Wach, A.; Dembowsky, K.; Dale, G.E. Pharmacokinetics and Safety of Intravenous Murepavadin Infusion in Healthy Adult Subjects Administered Single and Multiple Ascending Doses. Antimicrob. Agents Chemother. 2018, 62, e02355-17. [Google Scholar] [CrossRef]
- Cheung, S.W.; Boost, M.V.; Cho, P. Effect of povidone iodine contact lens disinfecting solution on orthokeratology lens and lens case contamination and organisms in the microbiome of the conjunctiva. Cont. Lens. Anterior. Eye 2021, 44, 101412. [Google Scholar] [CrossRef]
- van Duijn, P.J.; Verbrugghe, W.; Jorens, P.G.; Spöhr, F.; Schedler, D.; Deja, M.; Rothbart, A.; Annane, D.; Lawrence, C.; Nguyen Van, J.C.; et al. The effects of antibiotic cycling and mixing on antibiotic resistance in intensive care units: A cluster-randomised crossover trial. Lancet Infect. Dis. 2018, 18, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.Y.; Bo, S.N.; Shen, N.; Zhou, Q.T.; Yin, A.Y.; Lu, W. Pharmacokinetic-pharmacodynamic analysis of ciprofloxacin in elderly Chinese patients with lower respiratory tract infections caused by Gram-negative bacteria. Chin. Med. J. 2019, 132, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Halimeh, A.; Farhad, R.B.; Naseh, S.; Karim, N. Comparative efficacy of honey 12.5% and chlorhexidine 0.2% mouthwashes on the oropharyngeal bacterial colonization in mechanically-ventilated patients: A randomized controlled trial. J. Tradit. Chin. Med. 2020, 40, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Castanheira, M.; Woosley, L.N.; Stone, G.G.; Bradford, P.A.; Flamm, R.K. Characterization of β-Lactamase Content of Ceftazidime-Resistant Pathogens Recovered during the Pathogen-Directed Phase 3 REPRISE Trial for Ceftazidime-Avibactam: Correlation of Efficacy against β-Lactamase Producers. Antimicrob. Agents Chemother. 2019, 63, e02655-18. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Tan, C.M.; Kaelin, D.; Meinke, P.T.; Miesel, L.; Olsen, D.B.; Fukuda, H.; Kishii, R.; Takei, M.; Ohata, K.; et al. Structure activity relationship of N-1 substituted 1,5-naphthyrid-2-one analogs of oxabicyclooctane-linked novel bacterial topoisomerase inhibitors as broad-spectrum antibacterial agents (Part-9). Bioorg. Med. Chem. Lett. 2022, 75, 128808. [Google Scholar] [CrossRef] [PubMed]
- Meesters, K.; Michelet, R.; Mauel, R.; Raes, A.; Van Bocxlaer, J.; Vande Walle, J.; Vermeulen, A. Results of a Multicenter Population Pharmacokinetic Study of Ciprofloxacin in Children with Complicated Urinary Tract Infection. Antimicrob. Agents Chemother. 2018, 62, e00517-18. [Google Scholar] [CrossRef]
- Surapat, B.; Montakantikul, P.; Malathum, K.; Kiertiburanakul, S.; Santanirand, P.; Chindavijak, B. Microbial epidemiology and risk factors for relapse in gram-negative bacteria catheter-related bloodstream infection with a pilot prospective study in patients with catheter removal receiving short-duration of antibiotic therapy. BMC Infect. Dis. 2020, 20, 604. [Google Scholar] [CrossRef]
- Oesterreicher, Z.; Lackner, E.; Jäger, W.; Höferl, M.; Zeitlinger, M. Lack of dermal penetration of topically applied gentamicin as pharmacokinetic evidence indicating insufficient efficacy. J. Antimicrob. Chemother. 2018, 73, 2823–2829. [Google Scholar] [CrossRef]
- Ooi, M.L.; Jothin, A.; Bennett, C.; Ooi, E.H.; Vreugde, S.; Psaltis, A.J.; Wormald, P.J. Manuka honey sinus irrigations in recalcitrant chronic rhinosinusitis: Phase 1 randomized, single-blinded, placebo-controlled trial. Int. Forum. Allergy Rhinol. 2019, 9, 1470–1477. [Google Scholar] [CrossRef]
- Shorr, A.F.; Bruno, C.J.; Zhang, Z.; Jensen, E.; Gao, W.; Feng, H.P.; Huntington, J.A.; Yu, B.; Rhee, E.G.; De Anda, C.; et al. Ceftolozane/tazobactam probability of target attainment and outcomes in participants with augmented renal clearance from the randomized phase 3 ASPECT-NP trial. Crit. Care 2021, 25, 354. [Google Scholar] [CrossRef] [PubMed]
- Sagel, S.D.; Khan, U.; Heltshe, S.L.; Clancy, J.P.; Borowitz, D.; Gelfond, D.; Donaldson, S.H.; Moran, A.; Ratjen, F.; VanDalfsen, J.M.; et al. Clinical Effectiveness of Lumacaftor/Ivacaftor in Patients with Cystic Fibrosis Homozygous for F508del-CFTR. A Clinical Trial. Ann. Am. Thorac. Soc. 2021, 18, 75–83. [Google Scholar] [CrossRef]
- Busse, D.; Simon, P.; Schmitt, L.; Petroff, D.; Dorn, C.; Dietrich, A.; Zeitlinger, M.; Huisinga, W.; Michelet, R.; Wrigge, H.; et al. Comparative Plasma and Interstitial Tissue Fluid Pharmacokinetics of Meropenem Demonstrate the Need for Increasing Dose and Infusion Duration in Obese and Non-obese Patients. Clin. Pharm. 2022, 61, 655–672. [Google Scholar] [CrossRef]
- Deeb, M.A.; Alsahhaf, A.; Mubaraki, S.A.; Alhamoudi, N.; Al-Aali, K.A.; Abduljabbar, T. Clinical and microbiological outcomes of photodynamic and systemic antimicrobial therapy in smokers with peri-implant inflammation. Photodiagnosis Photodyn Ther. 2020, 29, 101587. [Google Scholar] [CrossRef]
- van Duijn, P.J.; Verbrugghe, W.; Jorens, P.G.; Spöhr, F.; Schedler, D.; Deja, M.; Rothbart, A.; Annane, D.; Lawrence, C.; Jereb, M.; et al. The effects of antibiotic cycling and mixing on acquisition of antibiotic resistant bacteria in the ICU: A post-hoc individual patient analysis of a prospective cluster-randomized crossover study. PLoS ONE 2022, 17, e0265720. [Google Scholar] [CrossRef]
- Sjövall, F.; Alobaid, A.S.; Wallis, S.C.; Perner, A.; Lipman, J.; Roberts, J.A. Maximally effective dosing regimens of meropenem in patients with septic shock. J. Antimicrob. Chemother. 2018, 73, 191–198. [Google Scholar] [CrossRef]
- Dingemans, J.; Eyns, H.; Willekens, J.; Monsieurs, P.; Van Houdt, R.; Cornelis, P.; Malfroot, A.; Crabbé, A. Intrapulmonary percussive ventilation improves lung function in cystic fibrosis patients chronically colonized with Pseudomonas aeruginosa: A pilot cross-over study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Caceres, S.M.; Sanders, L.A.; Rysavy, N.M.; Poch, K.R.; Jones, C.R.; Pickard, K.; Fingerlin, T.E.; Marcus, R.A.; Malcolm, K.C.; Taylor-Cousar, J.L.; et al. Blood mRNA biomarkers distinguish variable systemic and sputum inflammation at treatment initiation of inhaled antibiotics in cystic fibrosis: A prospective non-randomized trial. PLoS ONE 2022, 17, e0267592. [Google Scholar] [CrossRef] [PubMed]
- Genpeng, L.; Jinen, S.; Tao, W.; Zhihui, L.; Rixiang, G.; Jianyong, L.; Jingqiang, Z. Intraoperative application of inactivated Pseudomonas aeruginosa in patients undergoing lateral neck dissection for metastatic thyroid cancer: A randomized, parallel group, placebo-controlled trial. Surgery 2020, 168, 340–346. [Google Scholar] [CrossRef]
- Chu, L.; Acosta, A.M.; Aazami, H.; Dennis, P.; De Valle, O.; Ehmer, D., Jr.; Hedrick, J.A.; Ansley, J.F. Efficacy and Safety of Ciprofloxacin Plus Fluocinolone Acetonide Among Patients With Acute Otitis Externa: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2221699. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.M.; Yang, Q.Y.; Monsel, A.; Yan, J.Y.; Dai, C.X.; Zhao, J.Y.; Shi, G.C.; Zhou, M.; Zhu, X.M.; Li, S.K.; et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12134. [Google Scholar] [CrossRef] [PubMed]
- Wieërs, G.; Verbelen, V.; Van Den Driessche, M.; Melnik, E.; Vanheule, G.; Marot, J.C.; Cani, P.D. Do Probiotics During In-Hospital Antibiotic Treatment Prevent Colonization of Gut Microbiota With Multi-Drug-Resistant Bacteria? A Randomized Placebo-Controlled Trial Comparing Saccharomyces to a Mixture of Lactobacillus, Bifidobacterium, and Saccharomyces. Front. Public Health 2020, 8, 578089. [Google Scholar] [CrossRef] [PubMed]
- McCallin, S.; Sarker, S.A.; Sultana, S.; Oechslin, F.; Brüssow, H. Metagenome analysis of Russian and Georgian Pyophage cocktails and a placebo-controlled safety trial of single phage versus phage cocktail in healthy Staphylococcus aureus carriers. Environ. Microbiol. 2018, 20, 3278–3293. [Google Scholar] [CrossRef]
- Loose, M.; Naber, K.G.; Shields, P.; Reinhart, H.; Wagenlehner, F.M.E. Urinary concentrations and antimicrobial activity of tobramycin in healthy volunteers receiving a single oral dose of a novel formulation for improved absorption. Int. J. Antimicrob. Agents 2018, 51, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Loebinger, M.R.; Polverino, E.; Chalmers, J.D.; Tiddens, H.; Goossens, H.; Tunney, M.; Ringshausen, F.C.; Hill, A.T.; Pathan, R.; Angyalosi, G.; et al. Efficacy and safety of TOBI Podhaler in Pseudomonas aeruginosa-infected bronchiectasis patients: iBEST study. Eur. Respir. J. 2021, 57, 2001451. [Google Scholar] [CrossRef]
- Langton Hewer, S.C.; Smyth, A.R.; Brown, M.; Jones, A.P.; Hickey, H.; Kenna, D.; Ashby, D.; Thompson, A.; Sutton, L.; Clayton, D.; et al. Intravenous or oral antibiotic treatment in adults and children with cystic fibrosis and Pseudomonas aeruginosa infection: The TORPEDO-CF RCT. Health Technol. Assess 2021, 25, 1–128. [Google Scholar] [CrossRef]
- Durfey, S.L.; Pipavath, S.; Li, A.; Vo, A.T.; Ratjen, A.; Carter, S.; Morgan, S.J.; Radey, M.C.; Grogan, B.; Salipante, S.J.; et al. Combining Ivacaftor and Intensive Antibiotics Achieves Limited Clearance of Cystic Fibrosis Infections. mBio 2021, 12, e0314821. [Google Scholar] [CrossRef]
- Meijer, L.; Hery-Arnaud, G.; Leven, C.; Nowak, E.; Hillion, S.; Renaudineau, Y.; Durieu, I.; Chiron, R.; Prevotat, A.; Fajac, I.; et al. Safety and pharmacokinetics of Roscovitine (Seliciclib) in cystic fibrosis patients chronically infected with Pseudomonas aeruginosa, a randomized, placebo-controlled study. J. Cyst. Fibros. 2022, 21, 529–536. [Google Scholar] [CrossRef]
- Puvvadi, R.; Mikkelsen, H.; McCahon, L.; Grogan, S.; Ditcham, W.; Reid, D.W.; Lamont, I.; Stick, S.M.; Clements, B. Role of Tris-CaEDTA as an adjuvant with nebulised tobramycin in cystic fibrosis patients with Pseudomonas aeruginosa lung infections: A randomised controlled trial. J. Cyst. Fibros. 2021, 20, 316–323. [Google Scholar] [CrossRef]
- Chastre, J.; François, B.; Bourgeois, M.; Komnos, A.; Ferrer, R.; Rahav, G.; De Schryver, N.; Lepape, A.; Koksal, I.; Luyt, C.E.; et al. Safety, efficacy, and pharmacokinetics of gremubamab (MEDI3902), an anti-Pseudomonas aeruginosa bispecific human monoclonal antibody, in P. aeruginosa-colonised, mechanically ventilated intensive care unit patients: A randomised controlled trial. Crit. Care 2022, 26, 355. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- NLM. ClinicalTrials.gov—A Database of Privately and Publicaly Funded Clinical Studies Conducted Around the World. Available online: https://clinicaltrials.gov/ct2/home (accessed on 10 March 2023).
- Trapnell, B.C.; McColley, S.A.; Kissner, D.G.; Rolfe, M.W.; Rosen, J.M.; McKevitt, M.; Moorehead, L.; Montgomery, A.B.; Geller, D.E. Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with pseudomonas airway infection. Am. J. Respir. Crit. Care Med. 2013, 185, 171–178. [Google Scholar] [CrossRef]
- MacLeod, D.L.; Barker, L.M.; Sutherland, J.L.; Moss, S.C.; Gurgel, J.L.; Kenney, T.F.; Burns, J.L.; Baker, W.R. Antibacterial activities of a fosfomycin/tobramycin combination: A novel inhaled antibiotic for bronchiectasis. J. Antimicrob. Chemother. 2009, 64, 829–836. [Google Scholar] [CrossRef]
- Safety, Tolerability and PK 3-Period Crossover Study Comparing 2 Single Doses of ZTI-01 and Monurol® in Healthy Subjects. Available online: https://clinicaltrials.gov/ct2/show/NCT02178254 (accessed on 10 March 2023).
- Polverino, E.; Hill, A. Safety, Efficacy and PK/PD of POL7080 in Patients With Exacerbation of Non-Cystic Fibrosis Bronchiectasis. Available online: https://clinicaltrials.gov/ct2/show/NCT02096315 (accessed on 10 March 2023).
- Dale, G.E.; Halabi, A.; Petersen-Sylla, M.; Wach, A.; Zwingelstein, C. Pharmacokinetics, Tolerability, and Safety of Murepavadin, a Novel Antipseudomonal Antibiotic, in Subjects with Mild, Moderate, or Severe Renal Function Impairment. Antimicrob. Agents Chemother. 2019, 62, e00490-18. [Google Scholar] [CrossRef] [PubMed]
- BioSpace. Polyphor Temporarily Halts Enrollment in the Phase III Studies of Murepavadin for the Treatment of Patients with Nosocomial Pneumonia. Available online: https://www.biospace.com/article/polyphor-temporarily-halts-enrollment-in-the-phase-iii-studies-of-murepavadin-for-the-treatment-of-patients-with-nosocomial-pneumonia/ (accessed on 10 March 2023).
- Pivotal Study in Nosocomial Pneumonia Suspected or Confirmed to be Due to Pseudomonas (PRISM-UDR). Available online: https://clinicaltrials.gov/ct2/show/NCT03582007 (accessed on 10 March 2023).
- Recida Therapeutics, Inc. Single and Multiple Dose Escalation Trial of an Intravenous Antibiotic RC-01. Available online: https://clinicaltrials.gov/ct2/show/NCT03832517?term=NCT03832517&draw=2 (accessed on 10 March 2023).
- Koch, A. Phase 1 Study to Evaluate DDI, PK, Safety, Tolerability of SPR741. Available online: https://clinicaltrials.gov/ct2/show/NCT03376529?cond=SPR741&draw=2&rank=1 (accessed on 10 March 2023).
- Farinola, N. A First in Human Study of the Safety and Tolerability of Single and Multiple Doses of SPR741 in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT03022175?cond=SPR741&draw=2&rank=2 (accessed on 10 March 2023).
- Kuo, J. A First in Human Study of the Safety and Tolerability of Single and Multiple Doses of SPR206 in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT03792308 (accessed on 10 March 2023).
- MicuRx; Biomedical Advanced Research and Development Authority; Wellcome Trust. Study of the Safety, Tolerability, and PK of MRX-8 Administered Intravenously to HVs in SAD and MAD Cohorts. Available online: https://clinicaltrials.gov/ct2/show/NCT04649541?term=NCT04649541&draw=2 (accessed on 10 March 2023).
- Merck Sharp & Dohme LLC. Efficacy and Safety of Imipenem+Cilastatin/Relebactam (MK-7655A) Versus Colistimethate Sodium+Imipenem+Cilastatin in Imipenem-Resistant Bacterial Infection (MK-7655A-013) (RESTORE-IMI 1). Available online: https://clinicaltrials.gov/ct2/show/NCT02452047 (accessed on 10 March 2023).
- Motsch, J.; Murta de Oliveira, C.; Stus, V.; Köksal, I.; Lyulko, O.; Boucher, H.W.; Kaye, K.S.; File, T.M., Jr.; Brown, M.L.; Khan, I.; et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin. Infect. Dis. 2020, 70, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Titov, I.; Wunderink, R.G.; Roquilly, A.; Rodríguez Gonzalez, D.; David-Wang, A.; Boucher, H.W.; Kaye, K.S.; Losada, M.C.; Du, J.; Tipping, R.; et al. A Randomized, Double-blind, Multicenter Trial Comparing Efficacy and Safety of Imipenem/Cilastatin/Relebactam Versus Piperacillin/Tazobactam in Adults With Hospital-acquired or Ventilator-associated Bacterial Pneumonia (RESTORE-IMI 2 Study). Clin. Infect. Dis. 2021, 73, e4539–e4548. [Google Scholar] [CrossRef]
- Merck Sharp & Dohme LLC. Imipenem/Relebactam/Cilastatin Versus Piperacillin/Tazobactam for Treatment of Participants With Bacterial Pneumonia (MK-7655A-014) (RESTORE-IMI 2). Available online: https://clinicaltrials.gov/ct2/show/NCT02493764 (accessed on 10 March 2023).
- Merck Sharp & Dohme LLC. Imipenem/Cilastatin/Relebactam (MK-7655A) Versus Piperacillin/Tazobactam in Participants With Hospital-Acquired or Ventilator-Associated Bacterial Pneumonia (MK-7655A-016). 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03583333 (accessed on 28 March 2023).
- Kuti, J.L. Imipenem/Cilastatin/Relebactam Pharmacokinetics, Safety, and Outcomes in Adults and Adolescents With Cystic Fibrosis. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05561764 (accessed on 10 March 2023).
- Pan, Y. Evaluation of the Efficacy and Safety of Intravenous Imipenem/Cilastatin/XNW4107 in Comparison With Recarbrio in Adults With HABP/VABP (REITAB-2). Available online: https://clinicaltrials.gov/ct2/show/NCT05204563 (accessed on 10 March 2023).
- Hoffmann-La Roche. A Study to Investigate the Intrapulmonary Lung Penetration of Nacubactam in Healthy Participants. Available online: https://clinicaltrials.gov/ct2/show/NCT03182504 (accessed on 10 March 2023).
- Egawa, M. A Phase I Study to Assess Safety, Tolerability and Pharmacokinetics of OP0595. Available online: https://clinicaltrials.gov/ct2/show/NCT02134834 (accessed on 10 March 2023).
- Hoffmann-La Roche. A Study to Investigate the Safety, Tolerability, and Pharmacokinetics of RO7079901 and the Combination of RO7079901 With Meropenem in Adult Healthy Volunteers. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT02972255 (accessed on 10 March 2023).
- Martin-Loeches, I.; Dale, G.E.; Torres, A. Murepavadin: A new antibiotic class in the pipeline. Expert Rev. Anti Infect. Ther. 2018, 16, 259–268. [Google Scholar] [CrossRef]
- Luther, A.; Moehle, K.; Chevalier, E.; Dale, G.; Obrecht, D. Protein epitope mimetic macrocycles as biopharmaceuticals. Curr. Opin. Chem. Biol. 2017, 38, 45–51. [Google Scholar] [CrossRef]
- Kandasamy, S.K.; Larson, R.G. Binding modes of protegrin-1, a beta-strand antimicrobial peptide, in lipid bilayers. Mol. Simul. 2007, 33, 799–807. [Google Scholar] [CrossRef]
- Jang, H.; Ma, B.; Nussinov, R. Conformational study of the protegrin-1 (PG-1) dimer interaction with lipid bilayers and its effect. BMC Struct. Biol. 2007, 7, 21. [Google Scholar] [CrossRef]
- Sader, H.S.; Dale, G.E.; Rhomberg, P.R.; Flamm, R.K. Antimicrobial Activity of Murepavadin Tested against Clinical Isolates of Pseudomonas aeruginosa from the United States, Europe, and China. Antimicrob. Agents Chemother. 2018, 62, e00311-18. [Google Scholar] [CrossRef]
- Li, J.; Nation, R.L.; Kaye, K.S. Polymyxin Antibiotics: From Laboratory Bench to Bedside; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- French, S.; Farha, M.; Ellis, M.J.; Sameer, Z.; Côté, J.-P.; Cotroneo, N.; Lister, T.; Rubio, A.; Brown, E.D. Potentiation of Antibiotics against Gram-Negative Bacteria by Polymyxin B Analogue SPR741 from Unique Perturbation of the Outer Membrane. ACS Infect. Dis. 2020, 6, 1405–1412. [Google Scholar] [CrossRef]
- Corbett, D.; Wise, A.; Langley, T.; Skinner, K.; Trimby, E.; Birchall, S.; Dorali, A.; Sandiford, S.; Williams, J.; Warn, P.; et al. Potentiation of Antibiotic Activity by a Novel Cationic Peptide: Potency and Spectrum of Activity of SPR741. Antimicrob. Agents Chemother. 2017, 61, e00200-17. [Google Scholar] [CrossRef]
- Vaara, M.; Siikanen, O.; Apajalahti, J.; Fox, J.; Frimodt-Møller, N.; He, H.; Poudyal, A.; Li, J.; Nation, R.L.; Vaara, T. A novel polymyxin derivative that lacks the fatty acid tail and carries only three positive charges has strong synergism with agents excluded by the intact outer membrane. Antimicrob. Agents Chemother. 2010, 54, 3341–3346. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Lister, T.; Walpole, S.; Keutzer, T.; Utley, L.; Tomayko, J.; Kopp, E.; Farinola, N.; Coleman, S. Safety, Tolerability, Pharmacokinetics, and Drug Interaction Potential of SPR741, an Intravenous Potentiator, after Single and Multiple Ascending Doses and When Combined with β-Lactam Antibiotics in Healthy Subjects. Antimicrob. Agents Chemother. 2019, 63, e00892-19. [Google Scholar] [CrossRef] [PubMed]
- Lepak, A.J.; Wang, W.; Andes, D.R. Pharmacodynamic Evaluation of MRX-8, a Novel Polymyxin, in the Neutropenic Mouse Thigh and Lung Infection Models against Gram-Negative Pathogens. Antimicrob. Agents Chemother. 2020, 64, e01517-20. [Google Scholar] [CrossRef]
- Smith, J.R.; Rybak, J.M.; Claeys, K.C. Imipenem-Cilastatin-Relebactam: A Novel β-Lactam-β-Lactamase Inhibitor Combination for the Treatment of Multidrug-Resistant Gram-Negative Infections. Pharmacotherapy 2020, 40, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.; Ouweini, A.E.L.; Chahine, E.B.; Karaoui, L.R. Imipenem/cilastatin/relebactam: A new carbapenem β-lactamase inhibitor combination. Am. J. Health Syst. Pharm. 2021, 78, 674–683. [Google Scholar] [CrossRef]
- Viertel, T.M.; Ritter, K.; Horz, H.P. Viruses versus bacteria-novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J. Antimicrob. Chemother. 2014, 69, 2326–2336. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef] [PubMed]
- RC, R. Bacteriophage Effects on Pseudomonas Aeruginosa (MUCOPHAGES). Available online: https://clinicaltrials.gov/ct2/show/NCT01818206 (accessed on 10 March 2023).
- Individual Patient Expanded Access for AB-PA01, an Investigational Anti-Pseudomonas Aeruginosa Bacteriophage Therapeutic. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03395743%20 (accessed on 28 March 2023).
- Tawil, N. Phage Therapy for the Prevention and Treatment of Wound Infections in Burned Patients. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04323475 (accessed on 28 March 2023).
- Armata Pharmaceuticals, Inc. Ph 1/2 Study Evaluating Safety and Tolerability of Inhaled AP-PA02 in Subjects With Chronic Pseudomonas Aeruginosa Lung Infections and Cystic Fibrosis (SWARM-Pa). Available online: https://clinicaltrials.gov/ct2/show/NCT04596319?term=NCT04596319&draw=2 (accessed on 10 March 2023).
- Koff, J. CYstic Fibrosis bacterioPHage Study at Yale (CYPHY). Available online: https://clinicaltrials.gov/ct2/show/NCT04684641?term=NCT04684641&draw=2 (accessed on 10 March 2023).
- Pearson, J.P.; Feldman, M.; Iglewski, B.H.; Prince, A. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 2000, 68, 4331–4334. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Griswold, J.A.; Hamood, A.N. Contribution of the regulatory gene lasR to the pathogenesis of Pseudomonas aeruginosa infection of burned mice. J. Burn Care Rehabil. 1999, 20, 42–49. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Griswold, J.A.; Iglewski, B.H.; Hamood, A.N. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect. Immun. 1999, 67, 5854–5862. [Google Scholar] [CrossRef]
- Wu, H.; Song, Z.; Hentzer, M.; Andersen, J.B.; Heydorn, A.; Mathee, K.; Moser, C.; Eberl, L.; Molin, S.; Hoiby, N.; et al. Detection of N-acylhomoserine lactones in lung tissues of mice infected with Pseudomonas aeruginosa. Microbiology 2000, 146 Pt 10, 2481–2493. [Google Scholar] [CrossRef]
- Tateda, K.; Comte, R.; Pechere, J.C.; Köhler, T.; Yamaguchi, K.; Van Delden, C. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2001, 45, 1930–1933. [Google Scholar] [CrossRef] [PubMed]
- Skindersoe, M.E.; Alhede, M.; Phipps, R.; Yang, L.; Jensen, P.O.; Rasmussen, T.B.; Bjarnsholt, T.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 3648–3663. [Google Scholar] [CrossRef]
- Nalca, Y.; Jänsch, L.; Bredenbruch, F.; Geffers, R.; Buer, J.; Häussler, S. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: A global approach. Antimicrob. Agents Chemother. 2006, 50, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Köhler, T.; Dumas, J.L.; Van Delden, C. Ribosome protection prevents azithromycin-mediated quorum-sensing modulation and stationary-phase killing of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2007, 51, 4243–4248. [Google Scholar] [CrossRef]
- Köhler, T.; Perron, G.G.; Buckling, A.; van Delden, C. Quorum sensing inhibition selects for virulence and cooperation in Pseudomonas aeruginosa. PLoS Pathog. 2010, 6, e1000883. [Google Scholar] [CrossRef]
- Welsh, M.A.; Eibergen, N.R.; Moore, J.D.; Blackwell, H.E. Small molecule disruption of quorum sensing cross-regulation in Pseudomonas aeruginosa causes major and unexpected alterations to virulence phenotypes. J. Am. Chem. Soc. 2015, 137, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Febbraro, S. Safety and Efficacy of Inhaled OligoG CF-5/20 for the Treatment Cystic Fibrosis. Available online: https://clinicaltrials.gov/ct2/show/NCT00970346?term=NCT00970346&draw=2 (accessed on 10 March 2023).
- Koningsbruggen-Rietschel, S.v. A Dose-finding Study of Inhaled OligoG vs Placebo in Patients With Cystic Fibrosis (SMR3372). Available online: https://clinicaltrials.gov/ct2/show/NCT03698448?term=NCT03698448&draw=2 (accessed on 10 March 2023).
- Peptilogics. Study in Patients Undergoing Debridement, Antibiotics, and Implant Retention (DAIR) for Treatment of a Periprosthetic Joint Infection (PJI) Occurring After Total Knee Arthroplasty (TKA). Available online: https://clinicaltrials.gov/ct2/show/NCT05137314?term=NCT05137314&draw=2 (accessed on 10 March 2023).
- Shunkov, V.; Shvets, A.; Gorelov, D.; Kulagina, L.; Matevosyan, E.; Mozheyko, M.; Sinelnikov, L.; Bushara, M.; Yesayan, A.; Mangushlo, A.; et al. Safety and Efficacy Study of Ftortiazinon in the Treatment of Patients With Complicated Urinary Tract Infections Caused by P. aeruginosa. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03638830 (accessed on 28 March 2023).
- Powell, L.C.; Pritchard, M.F.; Ferguson, E.L.; Powell, K.A.; Patel, S.U.; Rye, P.D.; Sakellakou, S.M.; Buurma, N.J.; Brilliant, C.D.; Copping, J.M.; et al. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. NPJ Biofilms Microbiomes 2018, 4, 13. [Google Scholar] [CrossRef]
- Giorgetti, M.; Klymiuk, N.; Bähr, A.; Hemmerling, M.; Jinton, L.; Tarran, R.; Malmgren, A.; Åstrand, A.; Hansson, G.C.; Ermund, A. New generation ENaC inhibitors detach cystic fibrosis airway mucus bundles via sodium/hydrogen exchanger inhibition. Eur. J. Pharmacol. 2021, 904, 174123. [Google Scholar] [CrossRef]
- Wark, P. A Phase 2b Randomised, Placebo Controlled Study of OligoG in Patients With Cystic Fibrosis. Available online: https://clinicaltrials.gov/ct2/show/NCT03822455 (accessed on 10 March 2023).
- Yasir, M.; Dutta, D.; Hossain, K.R.; Chen, R.; Ho, K.K.K.; Kuppusamy, R.; Clarke, R.J.; Kumar, N.; Willcox, M.D.P. Mechanism of Action of Surface Immobilized Antimicrobial Peptides Against Pseudomonas aeruginosa. Front. Microbiol. 2020, 10, 3053. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. Development of a novel hybrid antimicrobial peptide for targeted killing of Pseudomonas aeruginosa. Eur. J. Med. Chem. 2020, 185, 111814. [Google Scholar] [CrossRef]
- Grishin, S.Y.; Domnin, P.A.; Kravchenko, S.V.; Azev, V.N.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; Surin, A.K.; Makarova, M.A.; Kurpe, S.R.; et al. Is It Possible to Create Antimicrobial Peptides Based on the Amyloidogenic Sequence of Ribosomal S1 Protein of P. aeruginosa? Int. J. Mol. Sci. 2021, 22, 9776. [Google Scholar] [CrossRef] [PubMed]
- Ridyard, K.E.; Elsawy, M.; Mattrasingh, D.; Klein, D.; Strehmel, J.; Beaulieu, C.; Wong, A.; Overhage, J. Synergy between Human Peptide LL-37 and Polymyxin B against Planktonic and Biofilm Cells of Escherichia coli and Pseudomonas aeruginosa. Antibiotics 2023, 12, 389. [Google Scholar] [CrossRef]
- Holder, I.A.; Neely, A.N.; Frank, D.W. Type III secretion/intoxication system important in virulence of Pseudomonas aeruginosa infections in burns. Burns 2001, 27, 129–130. [Google Scholar] [CrossRef]
- Koh, A.Y.; Priebe, G.P.; Pier, G.B. Virulence of Pseudomonas aeruginosa in a murine model of gastrointestinal colonization and dissemination in neutropenia. Infect. Immun. 2005, 73, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Kruczek, C.; Kottapalli, K.R.; Dissanaike, S.; Dzvova, N.; Griswold, J.A.; Colmer-Hamood, J.A.; Hamood, A.N. Major Transcriptome Changes Accompany the Growth of Pseudomonas aeruginosa in Blood from Patients with Severe Thermal Injuries. PLoS ONE 2016, 11, e0149229. [Google Scholar] [CrossRef] [PubMed]
- Plotkowski, M.C.; Estato, V.; Santos, S.A.; da Silva, M.C.; Miranda, A.S.; de Miranda, P.E.; Pinho, V.; Tibirica, E.; Morandi, V.; Teixeira, M.M.; et al. Contribution of the platelet activating factor signaling pathway to cerebral microcirculatory dysfunction during experimental sepsis by ExoU producing Pseudomonas aeruginosa. Pathog. Dis. 2015, 73, ftv046. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Corry, D.B.; Gropper, M.A.; Ohara, M.; Kurahashi, K.; Wiener-Kronish, J.P. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J. Immunol. 1997, 159, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.R. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat. Rev. Microbiol. 2009, 7, 654–665. [Google Scholar] [CrossRef]
- Sheremet, A.B.; Zigangirova, N.A.; Zayakin, E.S.; Luyksaar, S.I.; Kapotina, L.N.; Nesterenko, L.N.; Kobets, N.V.; Gintsburg, A.L. Small Molecule Inhibitor of Type Three Secretion System Belonging to a Class 2,4-disubstituted-4H-[1,3,4]-thiadiazine-5-ones Improves Survival and Decreases Bacterial Loads in an Airway Pseudomonas aeruginosa Infection in Mice. Biomed. Res. Int. 2018, 2018, 5810767. [Google Scholar] [CrossRef] [PubMed]
- Sheremet, A.B.; Nesterenko, L.N.; Zigangirova, N.A. The Type Three Secretion System of Pseudomonas aeruginosa as a Target for Development of Antivirulence Drugs. Mol. Genet. Microbiol. Virol. 2020, 35, 1–13. [Google Scholar] [CrossRef]
- Anantharajah, A.; Mingeot-Leclercq, M.P.; Van Bambeke, F. Targeting the Type Three Secretion System in Pseudomonas aeruginosa. Trends Pharmacol. Sci. 2016, 37, 734–749. [Google Scholar] [CrossRef]
- Pier, G.B.; Small, G.J.; Warren, H.B. Protection against mucoid Pseudomonas aeruginosa in rodent models of endobronchial infections. Science 1990, 249, 537–540. [Google Scholar] [CrossRef]
- Campbell, P.W. Phase II Randomized, Double-Blind, Placebo-Controlled Study of Intravenous Mucoid Exopolysaccharide Pseudomonas Aeruginosa Immune Globulin for Cystic Fibrosis. 2005. Available online: https://clinicaltrials.gov/ct2/show/NCT00004747 (accessed on 28 March 2023).
- Chastre, J. Pilot Trial of KB001 in Mechanically-Ventilated Patients Colonized With Pseudomonas Aeruginosa. 2009. Available online: https://clinicaltrials.gov/ct2/show/NCT00691587 (accessed on 28 March 2023).
- Milla, C.E.; Chmiel, J.F.; Accurso, F.J.; VanDevanter, D.R.; Konstan, M.W.; Yarranton, G.; Geller, D.E. Anti-PcrV antibody in cystic fibrosis: A novel approach targeting Pseudomonas aeruginosa airway infection. Pediatr. Pulmonol. 2014, 49, 650–658. [Google Scholar] [CrossRef]
- Jain, R.; Beckett, V.V.; Konstan, M.W.; Accurso, F.J.; Burns, J.L.; Mayer-Hamblett, N.; Milla, C.; VanDevanter, D.R.; Chmiel, J.F. KB001-A, a novel anti-inflammatory, found to be safe and well-tolerated in cystic fibrosis patients infected with Pseudomonas aeruginosa. J. Cyst. Fibros. 2018, 17, 484–491. [Google Scholar] [CrossRef]
- Ali, S.O.; Yu, X.Q.; Robbie, G.J.; Wu, Y.; Shoemaker, K.; Yu, L.; DiGiandomenico, A.; Keller, A.E.; Anude, C.; Hernandez-Illas, M.; et al. Phase 1 study of MEDI3902, an investigational anti-Pseudomonas aeruginosa PcrV and Psl bispecific human monoclonal antibody, in healthy adults. Clin. Microbiol. Infect. 2019, 25, 629.e1–629.e6. [Google Scholar] [CrossRef]
- Chastre, J.; François, B.; Bourgeois, M.; Komnos, A.; Ferrer, R.; Rahav, G.; De Schryver, N.; Lepape, A.; Koksal, I.; Luyt, C.E.; et al. 635. Efficacy, Pharmacokinetics (PK), and Safety Profile of MEDI3902, an Anti-Pseudomonas aeruginosa Bispecific Human Monoclonal Antibody in Mechanically Ventilated Intensive Care Unit Patients; Results of the Phase 2 EVADE Study Conducted by the Public-Private COMBACTE-MAGNET Consortium in the Innovative Medicines Initiative (IMI) Program. 2020. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7776862/ (accessed on 28 March 2023).
- Hollsing, A. Anti-pseudomonas IgY to Prevent Infections in Cystic Fibrosis (PseudIgY). 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT00633191 (accessed on 28 March 2023).
- Schuster, A. Efficacy Study of IgY (Antibody Against Pseudomonas) in Cystic Fibrosis Patients (PsAer-IgY). 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT01455675 (accessed on 28 March 2023).
- Lu, Q.; Rouby, J.J.; Laterre, P.F.; Eggimann, P.; Dugard, A.; Giamarellos-Bourboulis, E.J.; Mercier, E.; Garbino, J.; Luyt, C.E.; Chastre, J.; et al. Pharmacokinetics and safety of panobacumab: Specific adjunctive immunotherapy in critical patients with nosocomial Pseudomonas aeruginosa O11 pneumonia. J. Antimicrob. Chemother. 2011, 66, 1110–1116. [Google Scholar] [CrossRef]
- Que, Y.A.; Lazar, H.; Wolff, M.; Francois, B.; Laterre, P.F.; Mercier, E.; Garbino, J.; Pagani, J.L.; Revelly, J.P.; Mus, E.; et al. Assessment of panobacumab as adjunctive immunotherapy for the treatment of nosocomial Pseudomonas aeruginosa pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, V. Safety and Pharmacokinetics of KBPA-101 in Hospital Acquired Pneumonia Caused by O11 Pseudomonas Aeruginosa. 2009. Available online: https://clinicaltrials.gov/ct2/show/NCT00851435 (accessed on 28 March 2023).
- Adjunctive Therapeutic Treatment With Human Monoclonal Antibody AR-105 (Aerucin®) in P. Aeruginosa Pneumonia. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03027609 (accessed on 28 March 2023).
- Ciceri, F. Pentaglobin in CRE and PA Neutropenic Infections (PENTALLO). 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03494959 (accessed on 28 March 2023).
- Bernthal, N.; Conway, J.; Stolarski, E.; Berkowitz, R.; Pulido, L. Study to Evaluate Safety and Activity of TRL1068 in Prosthetic Joint Infections. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04763759?term=TRL1068&draw=2 (accessed on 10 March 2023).
- Schreiber, J.R.; Pier, G.B.; Grout, M.; Nixon, K.; Patawaran, M. Induction of opsonic antibodies to Pseudomonas aeruginosa mucoid exopolysaccharide by an anti-idiotypic monoclonal antibody. J. Infect. Dis. 1991, 164, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Johansen, H.K.; Hoiby, N.; Pedersen, S.S. Experimental immunization with Pseudomonas aeruginosa alginate induces IgA and IgG antibody responses. APMIS 1991, 99, 1061–1068. [Google Scholar] [CrossRef]
- Holder, I.A.; Neely, A.N.; Frank, D.W. PcrV immunization enhances survival of burned Pseudomonas aeruginosa-infected mice. Infect. Immun. 2001, 69, 5908–5910. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Yahr, T.L.; Ohara, M.; Kurahashi, K.; Gropper, M.A.; Wiener-Kronish, J.P.; Frank, D.W. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 1999, 5, 392–398. [Google Scholar] [CrossRef]
- Frank, D.W.; Vallis, A.; Wiener-Kronish, J.P.; Roy-Burman, A.; Spack, E.G.; Mullaney, B.P.; Megdoud, M.; Marks, J.D.; Fritz, R.; Sawa, T. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J. Infect. Dis. 2002, 186, 64–73. [Google Scholar] [CrossRef]
- Shime, N.; Sawa, T.; Fujimoto, J.; Faure, K.; Allmond, L.R.; Karaca, T.; Swanson, B.L.; Spack, E.G.; Wiener-Kronish, J.P. Therapeutic administration of anti-PcrV F(ab’)(2) in sepsis associated with Pseudomonas aeruginosa. J. Immunol. 2001, 167, 5880–5886. [Google Scholar] [CrossRef]
- Friedman, L.; Kolter, R. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 2004, 51, 675–690. [Google Scholar] [CrossRef]
- DiGiandomenico, A.; Warrener, P.; Hamilton, M.; Guillard, S.; Ravn, P.; Minter, R.; Camara, M.M.; Venkatraman, V.; Macgill, R.S.; Lin, J.; et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J. Exp. Med. 2012, 209, 1273–1287. [Google Scholar] [CrossRef]
- DiGiandomenico, A.; Keller, A.E.; Gao, C.; Rainey, G.J.; Warrener, P.; Camara, M.M.; Bonnell, J.; Fleming, R.; Bezabeh, B.; Dimasi, N.; et al. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl. Med. 2014, 6, 262ra155. [Google Scholar] [CrossRef]
- Cui, J.; Wei, X.; Lv, H.; Li, Y.; Li, P.; Chen, Z.; Liu, G. The clinical efficacy of intravenous IgM-enriched immunoglobulin (pentaglobin) in sepsis or septic shock: A meta-analysis with trial sequential analysis. Ann. Intensive Care 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.N.; Fertmann, J.M.; Vollmar, B.; Laschke, M.W.; Jauch, K.W.; Menger, M.D. Immunoglobulin M-enriched human intravenous immunoglobulins reduce leukocyte-endothelial cell interactions and attenuate microvascular perfusion failure in normotensive endotoxemia. Shock 2008, 29, 133–139. [Google Scholar] [CrossRef]
- Ates, I.; Dogan, N.; Aksoy, M.; Halici, Z.; Gundogdu, C.; Keles, M.S. The protective effects of IgM-enriched immunoglobulin and erythropoietin on the lung and small intestine tissues of rats with induced sepsis: Biochemical and histopathological evaluation. Pharm. Biol. 2015, 53, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Wand, S.; Klages, M.; Kirbach, C.; Warszawska, J.; Meybohm, P.; Zacharowski, K.; Koch, A. IgM-enriched immunoglobulin attenuates systemic endotoxin activity in early severe sepsis: A before-after cohort study. PLoS ONE 2016, 11, e0160907. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Estellés, A.; Woischnig, A.K.; Liu, K.; Stephenson, R.; Lomongsod, E.; Nguyen, D.; Zhang, J.; Heidecker, M.; Yang, Y.; Simon, R.J.; et al. A High-Affinity Native Human Antibody Disrupts Biofilm from Staphylococcus aureus Bacteria and Potentiates Antibiotic Efficacy in a Mouse Implant Infection Model. Antimicrob. Agents Chemother. 2016, 60, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Ryser, S.; Tenorio, E.; Estellés, A.; Kauvar, L.M. Human antibody repertoire frequently includes antibodies to a bacterial biofilm associated protein. PLoS ONE 2019, 14, e0219256. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.B.; Osmon, D.R.; Steckelberg, J.M.; Sierra, R.J.; Walker, R.C.; Tande, A.J.; Berbari, E.F. Pseudomonas Prosthetic Joint Infections: A Review of 102 Episodes. J. Bone Jt. Infect. 2016, 1, 25–30. [Google Scholar] [CrossRef]
- Hsieh, P.H.; Lee, M.S.; Hsu, K.Y.; Chang, Y.H.; Shih, H.N.; Ueng, S.W. Gram-negative prosthetic joint infections: Risk factors and outcome of treatment. Clin. Infect. Dis. 2009, 49, 1036–1043. [Google Scholar] [CrossRef]
- Marculescu, C.E.; Cantey, J.R. Polymicrobial prosthetic joint infections: Risk factors and outcome. Clin. Orthop. Relat. Res. 2008, 466, 1397–1404. [Google Scholar] [CrossRef]
- Zmistowski, B.; Fedorka, C.J.; Sheehan, E.; Deirmengian, G.; Austin, M.S.; Parvizi, J. Prosthetic joint infection caused by gram-negative organisms. J. Arthroplast. 2011, 26, 104–108. [Google Scholar] [CrossRef] [PubMed]
- von Specht, B.U.; Knapp, B.; Muth, G.; Broker, M.; Hungerer, K.D.; Diehl, K.D.; Massarrat, K.; Seemann, A.; Domdey, H. Protection of immunocompromised mice against lethal infection with Pseudomonas aeruginosa by active or passive immunization with recombinant P. aeruginosa outer membrane protein F and outer membrane protein I fusion proteins. Infect. Immun. 1995, 63, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Fito-Boncompte, L.; Chapalain, A.; Bouffartigues, E.; Chaker, H.; Lesouhaitier, O.; Gicquel, G.; Bazire, A.; Madi, A.; Connil, N.; Veron, W.; et al. Full virulence of Pseudomonas aeruginosa requires OprF. Infect. Immun. 2011, 79, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Westritschnig, K.; Hochreiter, R.; Wallner, G.; Firbas, C.; Schwameis, M.; Jilma, B. A randomized, placebo-controlled phase I study assessing the safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein OprF/I vaccine (IC43) in healthy volunteers. Hum. Vaccin Immunother. 2014, 10, 170–183. [Google Scholar] [CrossRef]
- Rello, J.; Krenn, C.G.; Locker, G.; Pilger, E.; Madl, C.; Balica, L.; Dugernier, T.; Laterre, P.F.; Spapen, H.; Depuydt, P.; et al. A randomized placebo-controlled phase II study of a Pseudomonas vaccine in ventilated ICU patients. Crit. Care 2017, 21, 22. [Google Scholar] [CrossRef]
- Adlbrecht, C.; Wurm, R.; Depuydt, P.; Spapen, H.; Lorente, J.A.; Staudinger, T.; Creteur, J.; Zauner, C.; Meier-Hellmann, A.; Eller, P.; et al. Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients-a randomized clinical trial. Crit. Care 2020, 24, 74. [Google Scholar] [CrossRef]
- Goss, C.H. A Pharmacokinetic and Safety Study of IV Gallium Nitrate (Ganite) in Cystic Fibrosis Patients. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT01093521 (accessed on 28 March 2023).
- Pilewski, J. Inhaled Sodium Nitrite as an Antimicrobial for Cystic Fibrosis. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02694393 (accessed on 28 March 2023).
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Shionogi Inc. Clinical Study of Cefiderocol (S-649266) for the Treatment of Nosocomial Pneumonia Caused by Gram-negative Pathogens (APEKS-NP). Available online: https://clinicaltrials.gov/ct2/show/NCT03032380 (accessed on 10 March 2023).
- Zemke, A.C.; Shiva, S.; Burns, J.L.; Moskowitz, S.M.; Pilewski, J.M.; Gladwin, M.T.; Bomberger, J.M. Nitrite modulates bacterial antibiotic susceptibility and biofilm formation in association with airway epithelial cells. Free Radic. Biol. Med. 2014, 77, 307–316. [Google Scholar] [CrossRef]
- Major, T.A.; Panmanee, W.; Mortensen, J.E.; Gray, L.D.; Hoglen, N.; Hassett, D.J. Sodium nitrite-mediated killing of the major cystic fibrosis pathogens Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia under anaerobic planktonic and biofilm conditions. Antimicrob. Agents Chemother. 2010, 54, 4671–4677. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef]
- Bilton, D.; Fajac, I.; Pressler, T.; Clancy, J.P.; Sands, D.; Minic, P.; Cipolli, M.; Galeva, I.; Solé, A.; Quittner, A.L.; et al. Long-term amikacin liposome inhalation suspension in cystic fibrosis patients with chronic P. aeruginosa infection. J. Cyst. Fibros. 2021, 20, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Ailiyaer, Y.; Wang, X.; Zhang, Y.; Li, C.; Li, T.; Qi, Q.; Li, Y. A Prospective Trial of Nebulized Amikacin in the Treatment of Bronchiectasis Exacerbation. Respiration 2018, 95, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Loebinger, M.R.; Polverino, E.; Blasi, F.; Elborn, S.J.; Chalmers, J.D.; Tiddens, H.A.; Goossens, H.; Tunney, M.; Zhou, W.; Angyalosi, G.; et al. Efficacy and safety of tobramycin inhalation powder in bronchiectasis patients with P. aeruginosa infection: Design of a dose-finding study (iBEST-1). Pulm. Pharmacol. Ther. 2019, 58, 101834. [Google Scholar] [CrossRef]
- Bilton, D.; Pressler, T.; Fajac, I.; Clancy, J.P.; Sands, D.; Minic, P.; Cipolli, M.; Galeva, I.; Solé, A.; Quittner, A.L.; et al. Amikacin liposome inhalation suspension for chronic Pseudomonas aeruginosa infection in cystic fibrosis. J. Cyst. Fibros. 2020, 19, 284–291. [Google Scholar] [CrossRef]
- Nichols, D.P.; Singh, P.K.; Baines, A.; Caverly, L.J.; Chmiel, J.F.; RL, G.I.; Lascano, J.; Morgan, S.J.; Retsch-Bogart, G.; Saiman, L.; et al. Testing the effects of combining azithromycin with inhaled tobramycin for P. aeruginosa in cystic fibrosis: A randomised, controlled clinical trial. Thorax 2022, 77, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Hamblett, N.; Retsch-Bogart, G.; Kloster, M.; Accurso, F.; Rosenfeld, M.; Albers, G.; Black, P.; Brown, P.; Cairns, A.; Davis, S.D.; et al. Azithromycin for Early Pseudomonas Infection in Cystic Fibrosis. The OPTIMIZE Randomized Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Xu, J.F.; Luo, H.; Xu, X.X.; Song, Y.L.; Ma, W.L.; Liang, Z.A.; Liu, X.D.; Zhang, G.J.; Zhang, X.J.; et al. A Double-Blind Randomized Placebo-Controlled Phase 3 Trial of Tobramycin Inhalation Solution in Adults With Bronchiectasis With Pseudomonas aeruginosa Infection. Chest 2023, 163, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.S.; Bilton, D.; Chalmers, J.D.; Davis, A.M.; Froehlich, J.; Gonda, I.; Thompson, B.; Wanner, A.; O’Donnell, A.E. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): Two phase 3, randomised controlled trials. Lancet Respir. Med. 2019, 7, 213–226. [Google Scholar] [CrossRef]
- Preston, R.A.; Mamikonyan, G.; DeGraff, S.; Chiou, J.; Kemper, C.J.; Xu, A.; Mastim, M.; Yeole, R.; Chavan, R.; Patel, A.; et al. Single-Center Evaluation of the Pharmacokinetics of WCK 5222 (Cefepime-Zidebactam Combination) in Subjects with Renal Impairment. Antimicrob. Agents Chemother. 2019, 63, e01484-18. [Google Scholar] [CrossRef]
- Huntington, J.A.; Yu, B.; Li, L.; Jensen, E.; Bruno, C.; Boakye, M.; Zhang, Z.; Gao, W.; Feng, H.P.; Rhee, E. Outcomes in Participants with Renal Impairment from a Phase 3 Clinical Trial for Ceftolozane/Tazobactam Treatment of Nosocomial Pneumonia (ASPECT-NP). Antimicrob. Agents Chemother. 2020, 64, e00731-20. [Google Scholar] [CrossRef]
- Stone, G.G.; Bradford, P.A.; Tawadrous, M.; Taylor, D.; Cadatal, M.J.; Chen, Z.; Chow, J.W. In Vitro Activity of Ceftazidime-Avibactam against Isolates from Respiratory and Blood Specimens from Patients with Nosocomial Pneumonia, Including Ventilator-Associated Pneumonia, in a Phase 3 Clinical Trial. Antimicrob. Agents Chemother. 2020, 64, e02356-19. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.F.; Huntington, J.A.; Wunderink, R.G.; Shime, N.; Kollef, M.H.; Kivistik, Ü.; Nováček, M.; Réa-Neto, Á.; Martin-Loeches, I.; Yu, B.; et al. Ceftolozane/tazobactam versus meropenem in patients with ventilated hospital-acquired bacterial pneumonia: Subset analysis of the ASPECT-NP randomized, controlled phase 3 trial. Crit. Care 2021, 25, 290. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Zhong, N.; Pachl, J.; Timsit, J.F.; Kollef, M.; Chen, Z.; Song, J.; Taylor, D.; Laud, P.J.; Stone, G.G.; et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): A randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect. Dis. 2018, 18, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.G.; Newell, P.; Gasink, L.B.; Broadhurst, H.; Wardman, A.; Yates, K.; Chen, Z.; Song, J.; Chow, J.W. Clinical activity of ceftazidime/avibactam against MDR Enterobacteriaceae and Pseudomonas aeruginosa: Pooled data from the ceftazidime/avibactam Phase III clinical trial programme. J. Antimicrob. Chemother. 2018, 73, 2519–2523. [Google Scholar] [CrossRef]
- Bruss, J.; Lister, T.; Gupta, V.K.; Stone, E.; Morelli, L.; Lei, Y.; Melnick, D. Single- and Multiple-Ascending-Dose Study of the Safety, Tolerability, and Pharmacokinetics of the Polymyxin Derivative SPR206. Antimicrob. Agents Chemother. 2021, 65, e0073921. [Google Scholar] [CrossRef]
- Palmer, K.L.; Mashburn, L.M.; Singh, P.K.; Whiteley, M. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J. Bacteriol. 2005, 187, 5267–5277. [Google Scholar] [CrossRef]
- Imamura, Y.; Yanagihara, K.; Fukuda, Y.; Kaneko, Y.; Seki, M.; Izumikawa, K.; Miyazaki, Y.; Hirakata, Y.; Sawa, T.; Wiener-Kronish, J.P.; et al. Effect of anti-PcrV antibody in a murine chronic airway Pseudomonas aeruginosa infection model. Eur. Respir. J. 2007, 29, 965–968. [Google Scholar] [CrossRef]
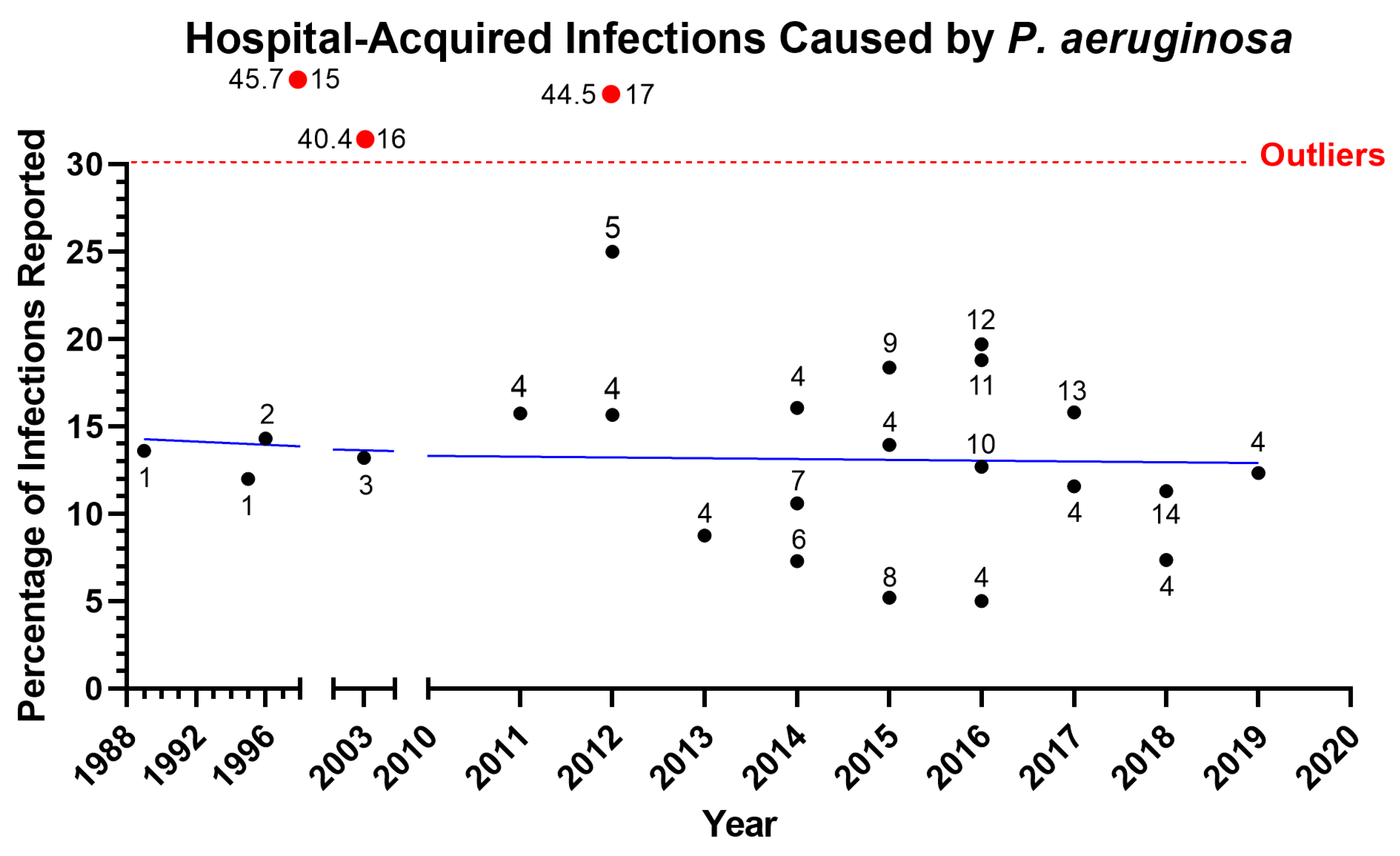
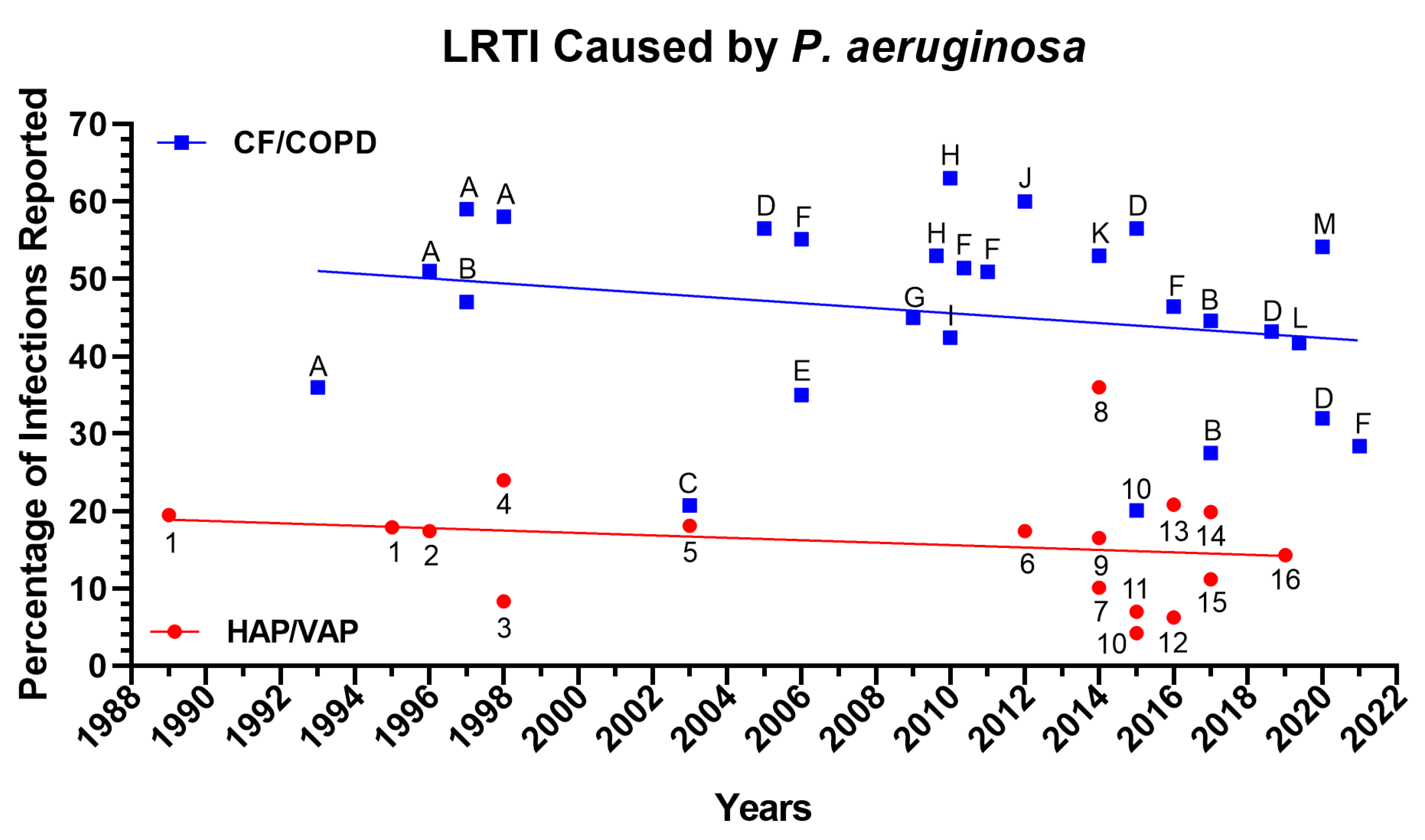

| (A) Novel antibiotics against P. aeruginosa. | ||||||
| Treatment/Study | Mechanism of Action | Population | Purpose | Phase | Number of Patients | Year, Reference |
| Inhaled fosfomycin/tobramycin (FTI) after aztreonam inhalation solution (AZLI) run-in (NCT00794586) | Protein synthesis inhibition | Cystic fibrosis patients with P. aeruginosa respiratory tract infections | Efficacy, safety, and ability to maintain forced expiratory volume (FEV1) in 1 s | II | 120 | 2013, [188] |
| Fosfomycin IV (ZTI-01) and oral fosfomycin (NCT02178254) | Protein synthesis inhibition | Hospital volunteers | Pharmacokinetics, safety | I | 30 | 2016, [190] |
| POL7080 (murepavadin) (NCT02096315) | Outer membrane protein inhibitor | Non–cystic fibrosis patients with bronchiectasis | Safety, pharmacokinetics, pharmacodynamics | II | 20 | 2017, [191] |
| Murepavadin (NCT02110459) | Outer membrane protein inhibitor | Healthy volunteers and renal failure patients | Pharmacokinetics, tolerability, safety | I | 32 | 2019, [192] |
| Murepavadin (PRISM-MDR) (NCT03409679) | Outer membrane protein inhibitor | Ventilated ICU patients | Treating P. aeruginosa VAP | III | 41 | 2019, [193] |
| Murepavadin (PRISM-UDR) (NCT03582007) | Outer membrane protein inhibitor | Hospital patients | Treating P. aeruginosa hospital-associated pneumonia | III | 2 | 2019, [194] |
| RC01 (NCT03832517) | LPS binding | Healthy adult volunteers | Pharmacokinetics, tolerability, safety | I | 8 | 2019, [195] |
| Polymyxin B analogue SPR741 (NCT03376529) | Plasma membrane disruption and/or antibiotic sensitization | Healthy adult volunteers | Pharmacokinetics, tolerability, safety | I | 27 | 2018, [196] |
| Polymyxin B analogue SPR741 (NCT03022175) | Plasma membrane disruption and/or antibiotic sensitization | Healthy adult volunteers | Pharmacokinetics, tolerability, safety | I | 64 | 2017, [197] |
| Polymyxin B analogue SPR206 (NCT03792308) | Plasma membrane disruption and/or antibiotic sensitization | Healthy human volunteers | Pharmacokinetics, tolerability, safety | I | 94 | 2020, [198] |
| Polymyxin B analogue SPR206 (NCT03376529) | Plasma membrane disruption and/or antibiotic sensitization | Healthy human volunteers | Pharmacokinetics, tolerability, safety | I | 27 | 2018, [196] |
| Polymyxin B analogue MRX-8 (NCT04649541) | Plasma membrane disruption and/or antibiotic sensitization | Healthy human volunteers | Pharmacokinetics, tolerability, safety | I | 116 | 2021, [199] |
| (B) Beta-lactamase inhibitors targeting P. aeruginosa. | ||||||
| Treatment/Study | Mechanism of Action | Population | Purpose | Phase | Number of Patients | Year, Reference |
| Imipenem/relebactam/cilastatin (NCT02452047 RESTORE-IMI 1) | Beta-lactamase inhibitor | Patients with HABP, VAPB, urinary tract infections, and intra-abdominal infections | Efficacy at treating HABP, VAPB, urinary tract infections, and intra-abdominal infections | III | 31 | 2019, [200,201] |
| Imipenem/relebactam/cilastatin (NCT02493764-RESTORE-IMI 2) | Beta-lactamase inhibitor | Patients with HABP or VAPB | Efficacy at treating HABP or VAPB | III | 264 | 2021, [202,203] |
| Imipenem/cilastatin/relebactam (NCT05561764) | Beta-lactamase inhibitor | Patients with HABP and VAPB | Efficacy at treating HABP and VAPB | III | 274 | 2022, [204] |
| Imipenem/relebactam/Cilastatin (NCT03583333) | Beta-lactamase inhibitor | Patients with CF pneumonia | Efficacy at treating with CF pneumonia | IV | 16 | 2023, [205] |
| Imipenem/cilastatin/XNW4107 (NCT05204563) | Beta-lactamase inhibitor | Patients with HABP and VAPB | Efficacy at treating patients with HABP and VAPB | III | 450 | 2023, [206] |
| Nacubactam (NCT02134834, NCT02972255, and NCT03182504) | Beta-lactamase inhibitor | Healthy human volunteers | Pharmacokinetics, tolerability, safety | I | 21 | 2018, [207,208,209] |
| Treatment/Study | Mechanism of Action | Population | Purpose | Phase | Number of Patients | Reference/Year |
|---|---|---|---|---|---|---|
| MUCOPHAGES (10 Pseudomonas specific bacteriophages) (NCT01818206) | Binding of bacteriophage to specific Pseudomonas targets to induce lysis | Cystic fibrosis sputa | Clinical study | -- | 59 | 2012, [225] |
| PhagoBurn (PP1131) 12 natural lytic Pseudomonas bacteriophages (NCT02116010) | Binding of bacteriophage to specific Pseudomonas targets to induce lysis | Burn patients | Treat P. aeruginosa burn wound infection | I/II | -- | 2018, [224] |
| B-PAO1 (4 Pseudomonas-specific bacteriophages) (NCT03395743) | Binding of bacteriophage to specific Pseudomonas targets to induce lysis | Cystic fibrosis patients | Prevent/treat P. aeruginosa infection | -- | -- | 2019, [226] |
| Bacteriophage cocktail spray (Phage cocktail-SPK) (NCT04323475) | Binding of bacteriophage to specific Pseudomonas targets to induce lysis | Burn patients | Treatment of P. aeruginosa and infection of burn wounds | I | 12 | 2023, [227] |
| AP-PA02 (NCT04596319) | Binding of bacteriophage to specific Pseudomonas targets to induce lysis | Chronic P. aeruginosa lung infections and CF | Treatment of chronic P. aeruginosa lung infections and CF | I/II | 29 | 2022, [228] |
| YPT-01 (NCT04684641) | Binding of bacteriophage to specific Pseudomonas targets to induce lysis | Chronic P. aeruginosa lung infections and CF | Treatment of chronic P. aeruginosa lung infections and CF | I/II | 8 | 2023, [229] |
| Treatment/Study | Mechanism of Action | Population | Purpose | Phase | Number of Patients | Reference/Year |
|---|---|---|---|---|---|---|
| Azithromycin to inhibit QS (NCT00610623) | Biofilm formation inhibition | Ventilated patients | Reduced P. aeruginosa VAP | II | 92 | 2018, [238] |
| OligoG (NCT00970346, NCT03822455, and NCT03698448) | Biofilm formation inhibition | Healthy volunteers | Pharmacokinetics, tolerability, safety | I | 26 | 2022, [240,241] |
| PLG0206 peptide (NCT05137314) | Biofilm formation inhibition | Patients with prosthetic joints | Treatment for prosthetic joint infection | I | 14 | 2022, [242] |
| Fluorothiazinon (aka Ftortiazinon) (NCT03638830) | Targeting the type III secretion system | Patients with P. aeruginosa complicated urinary tract infection | Adjunct treatment safety, efficacy | II | 777 | 2021, [243] |
| Treatment/Study | Population | Purpose | Phase | Number of Patients | Reference/Year |
|---|---|---|---|---|---|
| MEP IGIV (mucoid exopolysaccharide Ab) (NCT00004747) | Cystic fibrosis patients | Reduce frequency of acute pulmonary exacerbation and mucoid P. aeruginosa colonization | II | 170 | 2005, [261] |
| KB001 (anti-Pa PcrV Fab Ab; humanized, PEGylated recombinant) (NCT00691587) | Ventilated patients | Prevent P. aeruginosa VAP | Pilot | 36 | 2009, [262] |
| KB001 (anti-Pa PcrV Fab Ab; humanized, PEGylated recombinant) (NCT00638365) | Cystic fibrosis patients | Safety, tolerability, pharmacokinetics, pharmacodynamics, and prevent P. aeruginosa respiratory tract infection | I/II | 27 | 2014, [263] |
| KB001 (anti-Pa PcrV Fab Ab; humanized, PEGylated recombinant) (NCT01695343) | Cystic fibrosis patients | Reduce P. aeruginosa infection | II | 169 | 2018, [264] |
| MEDI3902 (anti-PcrV + anti-Psl Mab) (NCT02255760) | Healthy volunteers | Pharmacokinetics, safety | I | 56 | 2019, [265] |
| MEDI3902 (anti-PcrV + anti-Psl Mab) (NCT02696902) | Ventilated patients | Prevent P. aeruginosa VAP | II | 168 | 2020, [266] |
| PseudIgY (avian Ab to Pa) (NCT00633191) | Cystic fibrosis patients’ safety | Prevent P. aeruginosa respiratory tract colonization; preserve pulmonary function | I/II | 14 | 2012, [267] |
| PseudIgY (avian Ab to Pa) (NCT01455675) | Cystic fibrosis patients | Reduce P. aeruginosa reinfection | III | 164 | 2017, [268] |
| KBPA-101 (panobacumab) human IgM anti-Pa O11 LPS Mab obtained from a volunteer immunized with LPS-toxin A conjugate vaccine (NCT00851435) | Patients with P. aeruginosa hospital-acquired pneumonia | Safety, Pharmacokinetics, adjunct treatment of P. aeruginosa hospital-acquired pneumonia | I/II | 14 | 2014, [269,270,271] |
| AR-105 (Aerucin, anti-alginate Mab) (NCT03027609) | Ventilated patients | Adjunct treatment of P. aeruginosa VAP | II | 158 | 2019, [272] |
| Pentaglobin (PENTALLO) (NCT03494959) | Neutropenic acute leukemia or transplant patients with carbapenem-resistant Enterobacteriaceae | Decrease P. aeruginosa mortality | II | 120 | 2023, [273] |
| TRL1068 monoclonal antibody (NCT04763759) | Prosthetic joint infection patients secondary to P. aeruginosa | Safety and pharmacokinetics. Decrease C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), IL-6, and IL-10 | I | 18 | 2023, [274] |
| Treatment/Study | Mechanism of Action | Population | Purpose | Phase | Number of Patients | Reference/Year |
|---|---|---|---|---|---|---|
| IC43 vaccine (recombinant P. aeruginosa outer membrane protein OprF/I) (NCT00778388) | Induce immune response against P. aeruginosa outer membrane protein (OprF/I) | Healthy volunteers | Safety pharmacokinetics immunogenicity | I | 163 | 2013, [297] |
| IC43 vaccine (recombinant P. aeruginosa outer membrane protein OprF/I) (NCT00876252) | Induce immune response against P. aeruginosa outer membrane protein (OprF/I) | Ventilated patients | Safety immunogenicity | II | 400 | 2017, [298] |
| IC43 vaccine (recombinant P. aeruginosa outer membrane protein OprF/I) (NCT01563263) | Induce immune response against P. aeruginosa outer membrane protein (OprF/I) | Ventilated ICU patients | Efficacy in preventing P. aeruginosa infection immunogenicity safety | II/III | 800 | 2020, [299] |
| Treatment/Study | Mechanism of Action | Population | Purpose | Phase | Number of Patients | Reference/Year |
|---|---|---|---|---|---|---|
| Gallium (GANITE), inhaled (NCT01093521) | Decreasing bacterial Fe uptake and interfering with Fe signaling | Cystic fibrosis patients with chronic P. aeruginosa respiratory tract infection | Pharmacokinetics, safety, and reduce P. aeruginosa burden | I | 20 | 2013, [157] |
| Gallium (IGNITE) (NCT02354859) | Decreasing bacterial Fe uptake and interfering with Fe signaling | Cystic fibrosis patients | Improve pulmonary function | II | 119 | 2018, [300] |
| Inhaled sodium nitrite (NCT02694393) | Decreasing bacterial Fe uptake and interfering with Fe signaling | Cystic fibrosis patients with chronic P. aeruginosa infection | Safety and efficacy reduced P. aeruginosa bioburden | I/II | 35 | 2023, [301] |
| Cefiderocol (NCT03032380) | Decreasing bacterial Fe uptake and interfering with Fe signaling | Nosocomial pneumonia secondary to P. aeruginosa | Efficacy against nosocomial pneumonia secondary to P. aeruginosa | III | 300 | 2020, [303] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmassry, M.M.; Colmer-Hamood, J.A.; Kopel, J.; San Francisco, M.J.; Hamood, A.N. Anti-Pseudomonas aeruginosa Vaccines and Therapies: An Assessment of Clinical Trials. Microorganisms 2023, 11, 916. https://doi.org/10.3390/microorganisms11040916
Elmassry MM, Colmer-Hamood JA, Kopel J, San Francisco MJ, Hamood AN. Anti-Pseudomonas aeruginosa Vaccines and Therapies: An Assessment of Clinical Trials. Microorganisms. 2023; 11(4):916. https://doi.org/10.3390/microorganisms11040916
Chicago/Turabian StyleElmassry, Moamen M., Jane A. Colmer-Hamood, Jonathan Kopel, Michael J. San Francisco, and Abdul N. Hamood. 2023. "Anti-Pseudomonas aeruginosa Vaccines and Therapies: An Assessment of Clinical Trials" Microorganisms 11, no. 4: 916. https://doi.org/10.3390/microorganisms11040916
APA StyleElmassry, M. M., Colmer-Hamood, J. A., Kopel, J., San Francisco, M. J., & Hamood, A. N. (2023). Anti-Pseudomonas aeruginosa Vaccines and Therapies: An Assessment of Clinical Trials. Microorganisms, 11(4), 916. https://doi.org/10.3390/microorganisms11040916






