The Impact of MOSE (Experimental Electromechanical Module) Flood Barriers on Microphytobenthic Community of the Venice Lagoon
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Physical and Chemical Analyses
2.4. Abundance and Community Structure of Microphytobenthos Using Classical Taxonomy
2.5. Benthic Microeukaryotic Community Composition through Metabarcoding
2.6. Statistical Analyses
3. Results
3.1. Water Column Parameters
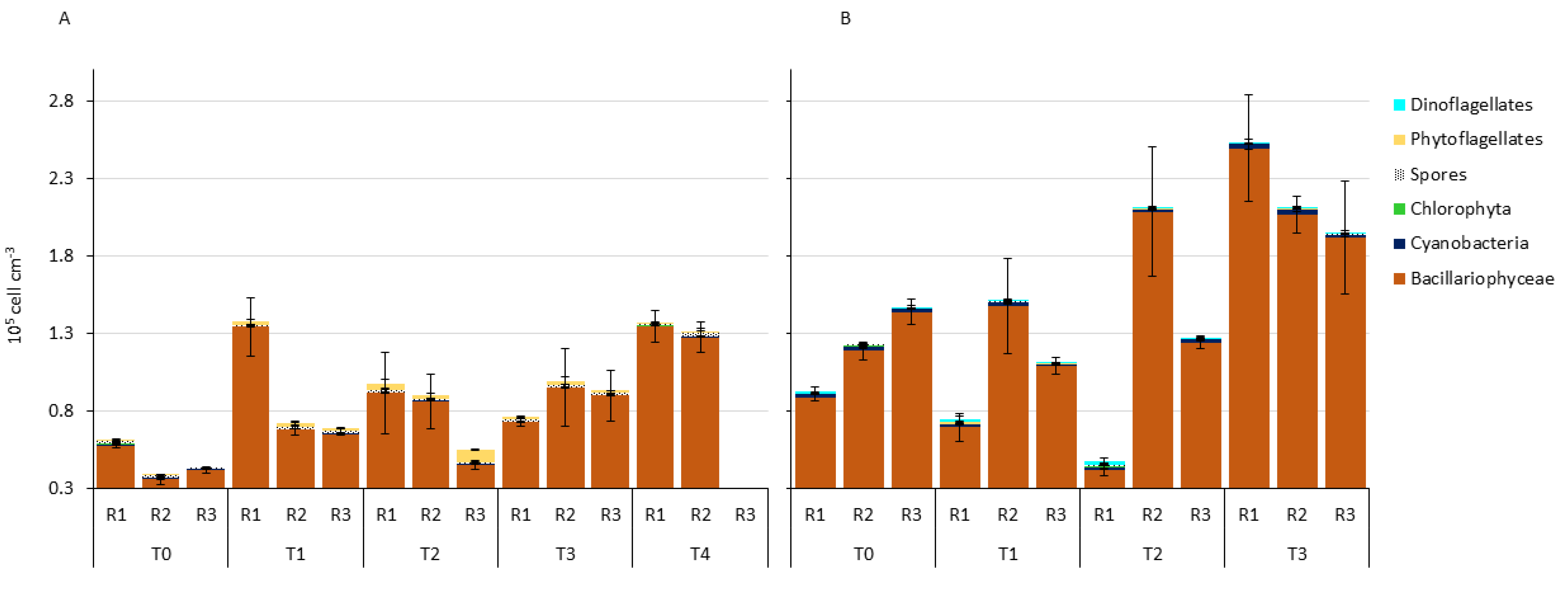
3.2. Microphytobenthic Community Using Classical Taxonomy
3.3. Microphytobenthic Community through Metabarcoding
4. Discussion
4.1. Microphytobenthic Community through Classical Taxonomy
4.2. Microphytobenthic Community through Metabarcoding
4.3. Ecological Aspects of Altered MPB Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change, (IPCC) Climate Change 2022: Mitigation of Climate Change. Available online: https://www.ipcc.ch/report/ar6/wg3/ (accessed on 23 March 2023).
- Mehvar, S.; Filatova, T.; Dastgheib, A.; De Ruyter van Steveninck, E.; Ranasinghe, R. Quantifying Economic Value of Coastal Ecosystem Services: A Review. J. Mar. Sci. Eng. 2018, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Newton, A.; Brito, A.C.; Icely, J.D.; Derolez, V.; Clara, I.; Angus, S.; Schernewski, G.; Inácio, M.; Lillebø, A.I.; Sousa, A.I.; et al. Assessing, Quantifying and Valuing the Ecosystem Services of Coastal Lagoons. J. Nat. Conserv. 2018, 44, 50–65. [Google Scholar] [CrossRef]
- Molinaroli, E.; Guerzoni, S.; Sarretta, A.; Masiol, M.; Pistolato, M. Thirty-Year Changes (1970 to 2000) in Bathymetry and Sediment Texture Recorded in the Lagoon of Venice Sub-Basins, Italy. Mar. Geol. 2009, 258, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Ridderinkhof, W.; de Swart, H.E.; van der Vegt, M.; Alebregtse, N.C.; Hoekstra, P. Geometry of Tidal Inlet Systems: A Key Factor for the Net Sediment Transport in Tidal Inlets. J. Geophys. Res. Oceans 2014, 119, 6988–7006. [Google Scholar] [CrossRef]
- Toso, C.; Madricardo, F.; Molinaroli, E.; Fogarin, S.; Kruss, A.; Petrizzo, A.; Pizzeghello, N.M.; Sinapi, L.; Trincardi, F. Tidal Inlet Seafloor Changes Induced by Recently Built Hard Structures. PLoS ONE 2019, 14, e0223240. [Google Scholar] [CrossRef] [PubMed]
- Umgiesser, G. The Impact of Operating the Mobile Barriers in Venice (MOSE) under Climate Change. J. Nat. Conserv. 2020, 54, 125783. [Google Scholar] [CrossRef]
- Cavaleri, L.; Bajo, M.; Barbariol, F.; Bastianini, M.; Benetazzo, A.; Bertotti, L.; Chiggiato, J.; Ferrarin, C.; Trincardi, F.; Umgiesser, G. The 2019 Flooding of Venice and Its Implications for Future Predictions. Oceanography 2020, 33, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Ghezzo, M.; Guerzoni, S.; Cucco, A.; Umgiesser, G. Changes in Venice Lagoon Dynamics Due to Construction of Mobile Barriers. Coast. Eng. 2010, 57, 694–708. [Google Scholar] [CrossRef] [Green Version]
- Ferrarin, C.; Cucco, A.; Umgiesser, G.; Bellafiore, D.; Amos, C.L. Modelling Fluxes of Water and Sediment between Venice Lagoon and the Sea. Cont. Shelf Res. 2010, 30, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Ferrarin, C.; Ghezzo, M.; Umgiesser, G.; Tagliapietra, D.; Camatti, E.; Zaggia, L.; Sarretta, A. Assessing Hydrological Effects of Human Interventions on Coastal Systems: Numerical Applications to the Venice Lagoon. Hydrol. Earth Syst. Sci. 2013, 17, 1733–1748. [Google Scholar] [CrossRef] [Green Version]
- Ferrarin, C.; Tomasin, A.; Bajo, M.; Petrizzo, A.; Umgiesser, G. Tidal Changes in a Heavily Modified Coastal Wetland. Cont. Shelf Res. 2015, 101, 22–33. [Google Scholar] [CrossRef]
- Scarpa, G.M.; Braga, F.; Manfè, G.; Lorenzetti, G.; Zaggia, L. Towards an Integrated Observational System to Investigate Sediment Transport in the Tidal Inlets of the Lagoon of Venice. Remote Sens. 2022, 14, 3371. [Google Scholar] [CrossRef]
- Tognin, D.; D’Alpaos, A.; Marani, M.; Carniello, L. Marsh Resilience to Sea-Level Rise Reduced by Storm-Surge Barriers in the Venice Lagoon. Nat. Geosci. 2021, 14, 906–911. [Google Scholar] [CrossRef]
- Brigolin, D.; Rabouille, C.; Demasy, C.; Bombled, B.; Monvoisin, G.; Pastres, R. Early Diagenesis in Sediments of the Venice Lagoon (Italy) and Its Relationship to Hypoxia. Front. Mar. Sci. 2021, 7, 575547. [Google Scholar] [CrossRef]
- Leoni, S.; Dominik, J.; Cassin, D.; Manfè, G.; Tagliapietra, D.; Acri, F.; Zonta, R. Sediment Oxygen Demand Rate in a Flow Regulated Lagoon (Venice, Italy). Front. Environ. Sci. 2022, 10. [Google Scholar] [CrossRef]
- Cibic, T.; Blasutto, O.; Falconi, C.; Fonda Umani, S. Microphytobenthic Biomass, Species Composition and Nutrient Availability in Sublittoral Sediments of the Gulf of Trieste (Northern Adriatic Sea). Estuar. Coast. Shelf Sci. 2007, 75, 50–62. [Google Scholar] [CrossRef]
- Facca, C.; Sfriso, A. Epipelic Diatom Spatial and Temporal Distribution and Relationship with the Main Environmental Parameters in Coastal Waters. Estuar. Coast. Shelf Sci. 2007, 75, 35–49. [Google Scholar] [CrossRef]
- Larson, F.; Sundbäck, K. Role of Microphytobenthos in Recovery of Functions in a Shallow-Water Sediment System after Hypoxic Events. Mar. Ecol. Prog. Ser. 2008, 357, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Dimitriou, P.D.; Papageorgiou, N.; Geropoulos, A.; Kalogeropoulou, V.; Moraitis, M.; Santi, I.; Tsikopoulou, I.; Pitta, P.; Karakassis, I. Benthic Pelagic Coupling in a Mesocosm Experiment: Delayed Sediment Responses and Regime Shifts. Sci. Total Environ. 2017, 605–606, 637–645. [Google Scholar] [CrossRef]
- Lake, S.J.; Brush, M.J. The Contribution of Microphytobenthos to Total Productivity in Upper Narragansett Bay, Rhode Island. Estuar. Coast. Shelf Sci. 2011, 95, 289–297. [Google Scholar] [CrossRef]
- Gambi, C.; Totti, C.; Manini, E. Impact of Organic Loads and Environmental Gradients on Microphytobenthos and Meiofaunal Distribution in a Coastal Lagoon. Chem. Ecol. 2003, 19, 207–223. [Google Scholar] [CrossRef]
- Brito, A.C.; Newton, A.; Fernandes, T.F.; Tett, P. The Role of Microphytobenthos on Shallow Coastal Lagoons: A Modelling Approach. Biogeochemistry 2011, 106, 207–228. [Google Scholar] [CrossRef]
- Cibic, T.; Fazi, S.; Nasi, F.; Pin, L.; Alvisi, F.; Berto, D.; Viganò, L.; Zoppini, A.; Del Negro, P. Natural and Anthropogenic Disturbances Shape Benthic Phototrophic and Heterotrophic Microbial Communities in the Po River Delta System. Estuar. Coast. Shelf Sci. 2019, 222, 168–182. [Google Scholar] [CrossRef]
- Facca, C.; Sfriso, A.; Socal, G. Changes in Abundance and Composition of Phytoplankton and Microphytobenthos Due to Increased Sediment Fluxes in the Venice Lagoon, Italy. Estuar. Coast. Shelf Sci. 2002, 54, 773–792. [Google Scholar] [CrossRef]
- Cibic, T.; Franzo, A.; Celussi, M.; Fabbro, C.; Del Negro, P. Benthic Ecosystem Functioning in Hydrocarbon and Heavy-Metal Contaminated Sediments of an Adriatic Lagoon. Mar. Ecol. Prog. Ser. 2012, 458, 69–87. [Google Scholar] [CrossRef] [Green Version]
- Di Pippo, F.; Magni, P.; Congestri, R. Microphytobenthic Biomass, Diversity and Exopolymeric Substances in a Shallow Dystrophic Coastal Lagoon. J. Mar. Microbiol. 2018, 2, 6–12. [Google Scholar]
- Tolomio, C.; Moro, I.; Moschin, E.; Valandro, A. Resultats Preliminaires Sur Les Diatomees Benthiques De Substrats Meubles Dans La Lagune De Venise, Italie (Mars 1994–Janvier 1995). Diatom Res. 1999, 14, 367–379. [Google Scholar] [CrossRef]
- Cibic, T.; Facca, C. Microphytobenthos. Biol. Mar. Mediterr. 2010, 17 (Suppl. S1), 754–800. [Google Scholar]
- Brito, A.C.; Fernandes, T.F.; Newton, A.; Facca, C.; Tett, P. Does Microphytobenthos Resuspension Influence Phytoplankton in Shallow Systems? A Comparison through a Fourier Series Analysis. Estuar. Coast. Shelf Sci. 2012, 110, 77–84. [Google Scholar] [CrossRef]
- Pivato, M.; Carniello, L.; Moro, I.; D’Odorico, P. On the Feedback between Water Turbidity and Microphytobenthos Growth in Shallow Tidal Environments. Earth Surf. Process. Landf. 2019, 44, 1192–1206. [Google Scholar] [CrossRef]
- Compson, Z.G.; McClenaghan, B.; Singer, G.A.C.; Fahner, N.A.; Hajibabaei, M. Metabarcoding From Microbes to Mammals: Comprehensive Bioassessment on a Global Scale. Front. Ecol. Evol. 2020, 8, 581835. [Google Scholar] [CrossRef]
- Gielings, R.; Fais, M.; Fontaneto, D.; Creer, S.; Costa, F.O.; Renema, W.; Macher, J.-N. DNA Metabarcoding Methods for the Study of Marine Benthic Meiofauna: A Review. Front. Mar. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, Present, and Future Perspectives of Environmental DNA (EDNA) Metabarcoding: A Systematic Review in Methods, Monitoring, and Applications of Global EDNA. Glob. Ecol. Conserv. 2019, 17, e00547. [Google Scholar] [CrossRef]
- MacIntyre, H.L.; Geider, R.J.; Miller, D.C. Microphytobenthos: The Ecological Role of the “Secret Garden” of Unvegetated, Shallow-Water Marine Habitats. I. Distribution, Abundance and Primary Production. Estuaries 1996, 19, 186–201. [Google Scholar] [CrossRef]
- Molinaroli, E.; Guerzoni, S.; Sarretta, A.; Cucco, A.; Umgiesser, G. Links between Hydrology and Sedimentology in the Lagoon of Venice, Italy. J. Mar. Syst. 2007, 68, 303–317. [Google Scholar] [CrossRef] [Green Version]
- Sarretta, A.; Pillon, S.; Molinaroli, E.; Guerzoni, S.; Fontolan, G. Sediment Budget in the Lagoon of Venice, Italy. Cont. Shelf Res. 2010, 30, 934–949. [Google Scholar] [CrossRef] [Green Version]
- Tagliapietra, D.; UNESCO Office Venice and Regional Bureau for Science and Culture in Europe (Italy); Zonta, R. The Ecological Implications of Climate Change on the Lagoon of Venice; United Nations Educational, Scientific and Cultural Organization (UNESCO): Paris, France, 2011. [Google Scholar]
- Wentworth, C.K. A Scale of Grade and Class Terms for Clastic Sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Nieuwenhuize, J.; Maas, Y.E.M.; Middelburg, J.J. Rapid Analysis of Organic Carbon and Nitrogen in Particulate Materials. Mar. Chem. 1994, 45, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Pella, E.; Colombo, B. Study of Carbon, Hydrogen and Nitrogen Determination by Combustion-Gas Chromatography. Microchim. Acta 1973, 61, 697–719. [Google Scholar] [CrossRef]
- Blasutto, O.; Cibic, T.; Vittor, C.D.; Umani, S.F. Microphytobenthic Primary Production and Sedimentary CarbohydratesAlong Salinity Gradients in the Lagoons of Grado and Marano (Northern Adriatic Sea). Hydrobiologia 2005, 550, 47–55. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Hartree, E.F. Determination of Protein: A Modification of the Lowry Method That Gives a Linear Photometric Response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Fichez, R. Composition and Fate of Organic Matter in Submarine Cave Sediments: Implications for the Biogeochemical Cycle of Organic Carbon. Oceanol. Acta 1991, 14, 369–377. [Google Scholar]
- Fabiano, M.; Danovaro, R.; Fraschetti, S. A Three-Year Time Series of Elemental and Biochemical Composition of Organic Matter in Subtidal Sandy Sediments of the Ligurian Sea (Northwestern Mediterranean). Cont. Shelf Res. 1995, 15, 1453–1469. [Google Scholar] [CrossRef]
- Utermöhl, H. Methods of Collecting Plankton for Various Purposes Are Discussed. SIL Commun. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Rogelja, M.; Cibic, T.; Pennesi, C.; De Vittor, C. Microphytobenthic Community Composition and Primary Production at Gas and Thermal Vents in the Aeolian Islands (Tyrrhenian Sea, Italy). Mar. Environ. Res. 2016, 118, 31–44. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M.; Morrison, L.; Rindi, F.; Miranda, S.V.; Mathieson, A.C.; Parker, B.C.; Langangen, A.; John, D.M.; Bárbara, I.; et al. AlgaeBase: An On-Line Resource for Algae. Cryptogam. Algol. 2014, 35, 105–115. [Google Scholar] [CrossRef]
- Ahyong, S.; Boyko, C.B.; Bailly, N.; Bernot, J.; Bieler, R.; Brandão, S.N.; Daly, M.; De Grave, S.; Gofas, S.; Hernandez, F.; et al. World Register of Marine Species (WoRMS) 2023. Available online: https://www.marinespecies.org/ (accessed on 23 March 2023).
- Peragallo, H.; Peragallo, M. Diatomées Marines de France et des Districts Maritimes Voisins; Micrographe-Editeur: Grez-sur-Loing, France, 1897–1908. [Google Scholar]
- Van Heurck, H. Traité des Diatomées; Édité aux Frais de L’Auteur: Anvers, Belgium, 1899. [Google Scholar]
- Hustedt, F. Bacillariophyta (Diatomeae) Zweite Auflage. In Die Süsswasser-Flora Mitteleuropas. Heft 10; Pascher, A., Ed.; Verlag von Gustav Fischer: Jena, Germany, 1930. [Google Scholar]
- Germain, H. Flore Des Diatomées, Diatomophycées: Eaux Douces et Saumâtres Du Massif Armoricain et Des Contrées Voisines d’Europe Occidentale; Collection Faunes et flores actuelles; Société Nouvelle des Éditions Boubée: Paris, France, 1981; ISBN 978-2-85004-023-8. [Google Scholar]
- Ricard, M. Atlas du Phytoplancton Marin; du Centre National du la Richerche Scientifique: Paris, France, 1987; ISBN 978-2-222-03987-7. [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; ISBN 978-0-521-36318-1. [Google Scholar]
- Tomas, C.R. Identifying Marine Phytoplankton; Elsevier Science: Amsterdam, The Netherlands, 1997; ISBN 978-0-08-053442-8. [Google Scholar]
- Jin, D.; Cheng, Z.; Lin, J.; Liu, S. The Marine Benthic Diatoms in China. Volume 1.313 Pp. Beijing: China Ocean Press, and Berlin: Springer-Verlag, 1985. J. Mar. Biol. Assoc. U. K. 1986, 66, 763. [Google Scholar] [CrossRef]
- Great Britain. Ministry of Agriculture, Fisheries and Food. In An Introductory Account of the Smaller Algae of British Coastal Waters; H.M. Stationery Office: Richmond, UK, 1959. [Google Scholar]
- Lange-Bertalot, H.; Witkowski, A.; Metzeltin, D. Iconographia Diatomologica Volume 7. In Annotated Diatom Micrographs; Lange-Bertalot, H., Ed.; ARG Gantner Verlag KG: Königstein, Germany, 2000; ISBN 978-3-904144-10-0. [Google Scholar]
- Canter-Lund, H.; Lund, J.W.G. Freshwater Algae: Their Microscopic World Explored; Biopress Limited: Bristol, UK, 1995; ISBN 978-0-948737-25-1. [Google Scholar]
- Amaral-Zettler, L.A.; McCliment, E.A.; Ducklow, H.W.; Huse, S.M. A Method for Studying Protistan Diversity Using Massively Parallel Sequencing of V9 Hypervariable Regions of Small-Subunit Ribosomal RNA Genes. PLoS ONE 2009, 4, e6372. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- R Core Team R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 23 March 2023).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; de Vargas, C.; Decelle, J.; et al. The Protist Ribosomal Reference Database (PR2): A Catalog of Unicellular Eukaryote Small Sub-Unit RRNA Sequences with Curated Taxonomy. Nucleic Acids Res. 2013, 41, D597–D604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 23 March 2023).
- Available online: https://www.primer-e.com/PRIMER-e (accessed on 23 March 2023).
- Margalef, R. Ecología; Biología Y Ciencias De La Vida-Ecologia; Omega: Barcellona, Spain, 1977; ISBN 978-84-282-0405-7. [Google Scholar]
- Pielou, E.C. Shannon’s Formula as a Measure of Specific Diversity: Its Use and Misuse. Am. Nat. 1966, 100, 463–465. [Google Scholar] [CrossRef]
- Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949; p. 117. [Google Scholar]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Kruskal, J.; Wish, M. Multidimensional Scaling; Sage: Thousand Oaks, CA, USA, 1978. [Google Scholar]
- Clarke, K.; Ainsworth, M. A Method of Linking Multivariate Community Structure to Environmental Variables. Mar. Ecol. Prog. Ser. 1993, 92, 205–219. [Google Scholar] [CrossRef]
- Lambshead, P.J.D.; Platt, H.M.; Shaw, K.M. The Detection of Differences among Assemblages of Marine Benthic Species Based on an Assessment of Dominance and Diversity. J. Nat. Hist. 1983, 17, 859–874. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Reimann, L.; Vafeidis, A.T.; Brown, S.; Hinkel, J.; Tol, R.S.J. Mediterranean UNESCO World Heritage at Risk from Coastal Flooding and Erosion Due to Sea-Level Rise. Nat. Commun. 2018, 9, 4161. [Google Scholar] [CrossRef] [Green Version]
- Mallin, M.A.; Cahoon, L.B.; Toothman, B.R.; Parsons, D.C.; McIver, M.R.; Ortwine, M.L.; Harrington, R.N. Impacts of a Raw Sewage Spill on Water and Sediment Quality in an Urbanized Estuary. Mar. Pollut. Bull. 2007, 54, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Cibic, T.; Acquavita, A.; Aleffi, F.; Bettoso, N.; Blasutto, O.; De Vittor, C.; Falconi, C.; Falomo, J.; Faresi, L.; Predonzani, S.; et al. Integrated Approach to Sediment Pollution: A Case Study in the Gulf of Trieste. Mar. Pollut. Bull. 2008, 56, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Franzo, A.; Cibic, T.; Del Negro, P.; Solidoro, C. Microphytobenthic Response to Mussel Farm Biodeposition in Coastal Sediments of the Northern Adriatic Sea. Mar. Pollut. Bull. 2014, 79, 379–388. [Google Scholar] [CrossRef]
- Rogelja, M.; Cibic, T.; Rubino, F.; Belmonte, M.; Del Negro, P. Active and Resting Microbenthos in Differently Contaminated Marine Coastal Areas: Insights from the Gulf of Trieste (Northern Adriatic, Mediterranean Sea). Hydrobiologia 2018, 806, 283–301. [Google Scholar] [CrossRef]
- Facca, C.; Sfriso, A.; Socal, G. Temporal and Spatial Distribution of Diatoms in the Surface Sediments of the Venice Lagoon. Bot. Mar. 2002, 45, 170–183. [Google Scholar] [CrossRef]
- Cibic, T.; Baldassarre, L.; Cerino, F.; Comici, C.; Fornasaro, D.; Kralj, M.; Giani, M. Benthic and Pelagic Contributions to Primary Production: Experimental Insights from the Gulf of Trieste (Northern Adriatic Sea). Front. Mar. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Agatz, M.; Asmus, R.M.; Deventer, B. Structural Changes in the Benthic Diatom Community along a Eutrophication Gradient on a Tidal Flat. Helgol. Mar. Res. 1999, 53, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Cibic, T.; Blasutto, O. Living Marine Benthic Diatoms as Indicators of Nutrient Enrichment: A Case Study in the Gulf of Trieste. Diatoms Ecol. Life Cycle 2011, 169–184. [Google Scholar]
- Round, F.E. The Ecology of Algae; Cambridge University Press: Cambridge, UK, 1981; ISBN 978-0-521-26906-3. [Google Scholar]
- Hunter, J. Diatoms As Environmental Indicators: A Case Study In The Bioluminescent Bays Of Vieques, Puerto Rico. 2007. Available online: http://keck.wooster.edu/publications (accessed on 23 March 2023).
- SABANCI, F. An Illustrated Survey on the Morphological Characters in Three Species of the Diatom Genus Mastogloia (Bacillariophyceae). Turk. J. Bot. 2012, 36, 727–737. [Google Scholar] [CrossRef]
- Giovagnetti, V.; Cataldo, M.L.; Conversano, F.; Brunet, C. Growth and Photophysiological Responses of Two Picoplanktonic Minutocellus Species, Strains RCC967 and RCC703 (Bacillariophyceae). Eur. J. Phycol. 2012, 47, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Nanjappa, D.; Dorrell, R.G.; Vieira, F.R.J.; Kazamia, E.; Tirichine, L.; Veluchamy, A.; Heilig, R.; Aury, J.-M.; Jaillon, O.; et al. Genome-Enabled Phylogenetic and Functional Reconstruction of an Araphid Pennate Diatom Plagiostriata Sp. CCMP470, Previously Assigned as a Radial Centric Diatom, and Its Bacterial Commensal. Sci. Rep. 2020, 10, 9449. [Google Scholar] [CrossRef] [PubMed]
- Underwood, G.J.C.; Paterson, D.M. The Importance of Extracellular Carbohydrate Productionby Marine Epipelic Diatoms. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2003; Volume 40, pp. 183–240. [Google Scholar]
- de Brouwer, J.F.C.; Wolfstein, K.; Ruddy, G.K.; Jones, T.E.R.; Stal, L.J. Biogenic Stabilization of Intertidal Sediments: The Importance of Extracellular Polymeric Substances Produced by Benthic Diatoms. Microb. Ecol. 2005, 49, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Underwood, G.J.C.; Smith, D.J. Predicting Epipelic Diatom Exopolymer Concentrations in Intertidal Sediments from Sediment Chlorophyll a. Microb. Ecol. 1998, 35, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, C.G.; Rehm, C.; Grossart, H.-P.; Kroth, P.G. Growth and Release of Extracellular Organic Compounds by Benthic Diatoms Depend on Interactions with Bacteria. Environ. Microbiol. 2011, 13, 1052–1063. [Google Scholar] [CrossRef]
- Kim, B.; Lee, J.; Noh, J.; Bae, H.; Lee, C.; Ha, H.J.; Hwang, K.; Kim, D.-U.; Kwon, B.-O.; Ha, H.K.; et al. Spatiotemporal Variation of Extracellular Polymeric Substances (EPS) Associated with the Microphytobenthos of Tidal Flats in the Yellow Sea. Mar. Pollut. Bull. 2021, 171, 112780. [Google Scholar] [CrossRef]





| n. Variables | Correlation | Variables | |
|---|---|---|---|
| SUMMER 2019 | 1 | 0.500 | C-LIP |
| 2 | 0.375 | TOC+C-LIP | |
| 3 | 0.375 | Sand+TOC+C-LIP | |
| 4 | 0.500 | Sand+Clay+TOC+C-LIP | |
| 5 | 0.375 | Sand+Silt+Clay+TN+C-LIP | |
| AUTUMN 2020 | 1 | 0.625 | C-LIP |
| 2 | 0.625 | TN+C-PRT | |
| 3 | 0.500 | TN+C-PRT+C-LIP | |
| 4 | 0.625 | TN+TOC+C-PRT+C-LIP | |
| 5 | 0.375 | TN+Sand+Silt+Clay+C-PRT |
| A | SUMMER 2019 | B | AUTUMN 2020 | ||
|---|---|---|---|---|---|
| T0 | LDA | T0 | LDA | ||
| Bacillariophyta.Araphidpennate.Fragilariales | 3.35 | Bacillariophyta.Staurosiraceae | 3.57 | ||
| Chlorophyta.Tetraselmis convolutae | 3.12 | Bacillariophyta.Araphidpennate.Fragilariales endosymbiont | 3.47 | ||
| Bacillariophyta.Araphidpennate.Fragilariales | 2.98 | Bacillariophyta.Raphidpennate.Naviculales | 3.43 | ||
| Bacillariophyta.Araphidpennate.Fragilariales | 2.98 | Bacillariophyta.Araphidpennate.Fragilariales endosymbiont | 3.41 | ||
| Bacillariophyta.Araphidpennate.Fragilariales | 2.85 | Dinoflagellata.Gymnodinium smaydae | 3.30 | ||
| Bacillariophyta.Raphidpennate.Amphora | 2.85 | Bacillariophyta.Araphidpennate.Fragilariales | 3.23 | ||
| Bacillariophyta.Araphidpennate.Dimeregramma | 2.83 | Bacillariophyta.Araphidpennate.Fragilariales | 3.14 | ||
| Dinoflagellata.Gymnodinium | 2.82 | Chlorophyta.Nannochloris.Picochlorum | 3.06 | ||
| Dinoflagellata.Gymnodinium smaydae | 2.82 | Bacillariophyta.Araphidpennate.Fragilariales endosymbiont | 2.98 | ||
| Bacillariophyta.Raphidpennate.Sellaphora | 2.75 | Bacillariophyta.Araphidpennate.Nanofrustulum shiloi | 2.85 | ||
| Dinoflagellata.Luciella sp. | 2.75 | Bacillariophyta.Raphidpennate.Naviculales | 2.84 | ||
| Bacillariophyta.Naviculales | 2.69 | Chlorophyta.Picochlorum.Nannochloris sp. MI37 | 2.82 | ||
| Bacillariophyta.Araphidpennate.Fragilariales-Saurosira | 2.66 | Bacillariophyta.Araphidpennate.Fragilariales endosymbiont | 2.82 | ||
| Bacillariophyta.Araphidpennate.Fragilariales | 2.66 | Chlorophyta.Picochlorum.Nannochloris sp. MI37 | 2.71 | ||
| Bacillariophyta.Polarcentric Mediophyceae.Cyclotella striata | 2.61 | Bacillariophyta.Araphidpennate.Fragilariales endosymbiont | 2.66 | ||
| Bacillariophyta.Araphidpennate.Plagiostriata goreensis | 2.52 | Chlorophyta.Picochlorum eukaryotum | 2.65 | ||
| Chlorophyta.Desmodesmus communis | 2.51 | Bacillariophyta.Araphidpennate.Fragilariales endosymbiont | 2.65 | ||
| Bacillariophyta.Polarcentric Mediophyceae.Skeletonema | 2.51 | Chlorophyta.Chlorellales.Nannochloris sp. MBIC10053 | 2.64 | ||
| Cryptophyta.Urgorri complanatus | 2.39 | Chlorophyta.Chlorellales.Trebouxiophyceae | 2.61 | ||
| Dinoflagellata.Clade4Xsp90 | 2.36 | Dinoflagellata.Blixaea quinquecornis | 2.57 | ||
| Bacillariophyta.Raphidpennate.Naviculales | 2.27 | Dinoflagellata.Luciella sp. | 2.57 | ||
| T4 | LDA | Bacillariophyta.Pleurosigma sp. mgcode 4 | 2.55 | ||
| Bacillariophyta.Raphidpennate.Tryblionella | 3.40 | Bacillariophyta.Naviculales | 2.52 | ||
| Bacillariophyta.Raphidpennate.Fragilariopsis sublineata | 2.93 | Bacillariophyta.Araphidpennate.Plagiostriata goreensis | 2.50 | ||
| Dinoflagellata.Gymnodinium aureolum | 2.79 | Bacillariophyta.Coscinodiscophyceae | 2.48 | ||
| Chlorophyta.Mantoniella antarctica | 2.70 | Bacillariophyta.Polarcentric Mediophyceae.Thalassiosira.Thalassiosira | 2.47 | ||
| Dinoflagellata.Scrippsiella acuminata | 2.69 | Chlorophyta.Pseudoscourfieldia marina | 2.45 | ||
| Dinoflagellata.Prorocentrum micans | 2.62 | Chlorophyta.Ostreococcus mediterraneus | 2.44 | ||
| Chlorophyta.Chlamydomonas | 2.59 | Chlorophyta.Nannochloris sp. | 2.44 | ||
| Bacillariophyta.Raphidpennate.Nitzschia amphibia | 2.47 | Bacillariophyta.Polarcentric Mediophyceae.Cyclotella meneghiniana | 2.44 | ||
| Dinoflagellata.Gymnodiniaceae | 2.43 | ||||
| Dinoflagellata.Symbiodiniaceae.Symbiodinium | 2.41 | ||||
| Bacillariophyta.Araphidpennate.Fragilariales endosymbiont | 2.37 | ||||
| Dinoflagellata.Thoracosphaeraceae | 2.36 | ||||
| Cryptophyta.Hemiselmis.Hemiselmis cryptochromatica | 2.28 | ||||
| Bacillariophyta.Polarcentric Mediophyceae.Odontellaceae | 2.28 | ||||
| Bacillariophyta.Papiliocellulus elegans | 2.28 | ||||
| Bacillariophyta.Polarcentric Mediophyceae.Chaetoceros.Chaetoceros | 2.27 | ||||
| Dinoflagellata.DinoGroupIClade1Xsp | 2.25 | ||||
| Dinoflagellata.Heterocapsa niei | 2.23 | ||||
| Bacillariophyta.Raphidpennate.Craticula importuna | 2.22 | ||||
| BacillariophytaRaphidpennate.Naviculales | 2.20 | ||||
| BacillariophytaRaphidpennate.Pleurosigma sp. mgcode 4 | 2.16 | ||||
| Bacillariophyta.Araphidpennate.Plagiostriata goreensis | 2.07 | ||||
| T3 | LDA | ||||
| BacillariophytaRaphidpennate.Navicula | 4.10 | ||||
| BacillariophytaRaphidpennate.Navicula.Navicula | 3.53 | ||||
| BacillariophytaRaphidpennate.Navicula.Navicula | 3.26 | ||||
| BacillariophytaRaphidpennate.Pleurosigma | 3.23 | ||||
| BacillariophytaRaphidpennate.Navicula | 3.06 | ||||
| BacillariophytaRaphidpennate.Navicula.Navicula | 2.83 | ||||
| Bacillariophyta.Raphidpennate.Navicula cryptotenella | 2.75 | ||||
| Bacillariophyta.Raphidpennate.Navicula cryptotenella | 2.66 | ||||
| Bacillariophyta.Raphidpennate.Naviculaceae | 2.53 | ||||
| Bacillariophyta.Raphidpennate.Naviculales | 2.50 | ||||
| Cryptophyta.Hemiselmis tepida | 2.47 | ||||
| BacillariophytaRaphidpennate.Naviculaceae | 2.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldassarre, L.; Natali, V.; De Pascale, F.; Vezzi, A.; Banchi, E.; Bazzaro, M.; Relitti, F.; Tagliapietra, D.; Cibic, T. The Impact of MOSE (Experimental Electromechanical Module) Flood Barriers on Microphytobenthic Community of the Venice Lagoon. Microorganisms 2023, 11, 936. https://doi.org/10.3390/microorganisms11040936
Baldassarre L, Natali V, De Pascale F, Vezzi A, Banchi E, Bazzaro M, Relitti F, Tagliapietra D, Cibic T. The Impact of MOSE (Experimental Electromechanical Module) Flood Barriers on Microphytobenthic Community of the Venice Lagoon. Microorganisms. 2023; 11(4):936. https://doi.org/10.3390/microorganisms11040936
Chicago/Turabian StyleBaldassarre, Laura, Vanessa Natali, Fabio De Pascale, Alessandro Vezzi, Elisa Banchi, Matteo Bazzaro, Federica Relitti, Davide Tagliapietra, and Tamara Cibic. 2023. "The Impact of MOSE (Experimental Electromechanical Module) Flood Barriers on Microphytobenthic Community of the Venice Lagoon" Microorganisms 11, no. 4: 936. https://doi.org/10.3390/microorganisms11040936
APA StyleBaldassarre, L., Natali, V., De Pascale, F., Vezzi, A., Banchi, E., Bazzaro, M., Relitti, F., Tagliapietra, D., & Cibic, T. (2023). The Impact of MOSE (Experimental Electromechanical Module) Flood Barriers on Microphytobenthic Community of the Venice Lagoon. Microorganisms, 11(4), 936. https://doi.org/10.3390/microorganisms11040936







