Bioconversion of Grape Pomace with Rhizopus oryzae under Solid-State Conditions: Changes in the Chemical Composition and Profile of Phenolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Substrate and Microorganism
2.3. Biological Treatment of Grape Pomace by Rhizopus oryzae
2.4. pH Measurement
2.5. Chemical Composition of Grape Pomace
2.5.1. Reducing Sugars
2.5.2. Biomass Concentration
2.5.3. Analysis of Minerals and Undesirable Substances
2.6. Determination of Antioxidant Activity
2.7. Measurements of Enzyme Activities
2.7.1. Preparation of Crude Enzyme Extracts
2.7.2. Assays for Measuring Enzyme Activity
2.8. Statistical Analysis
3. Results and Discussion
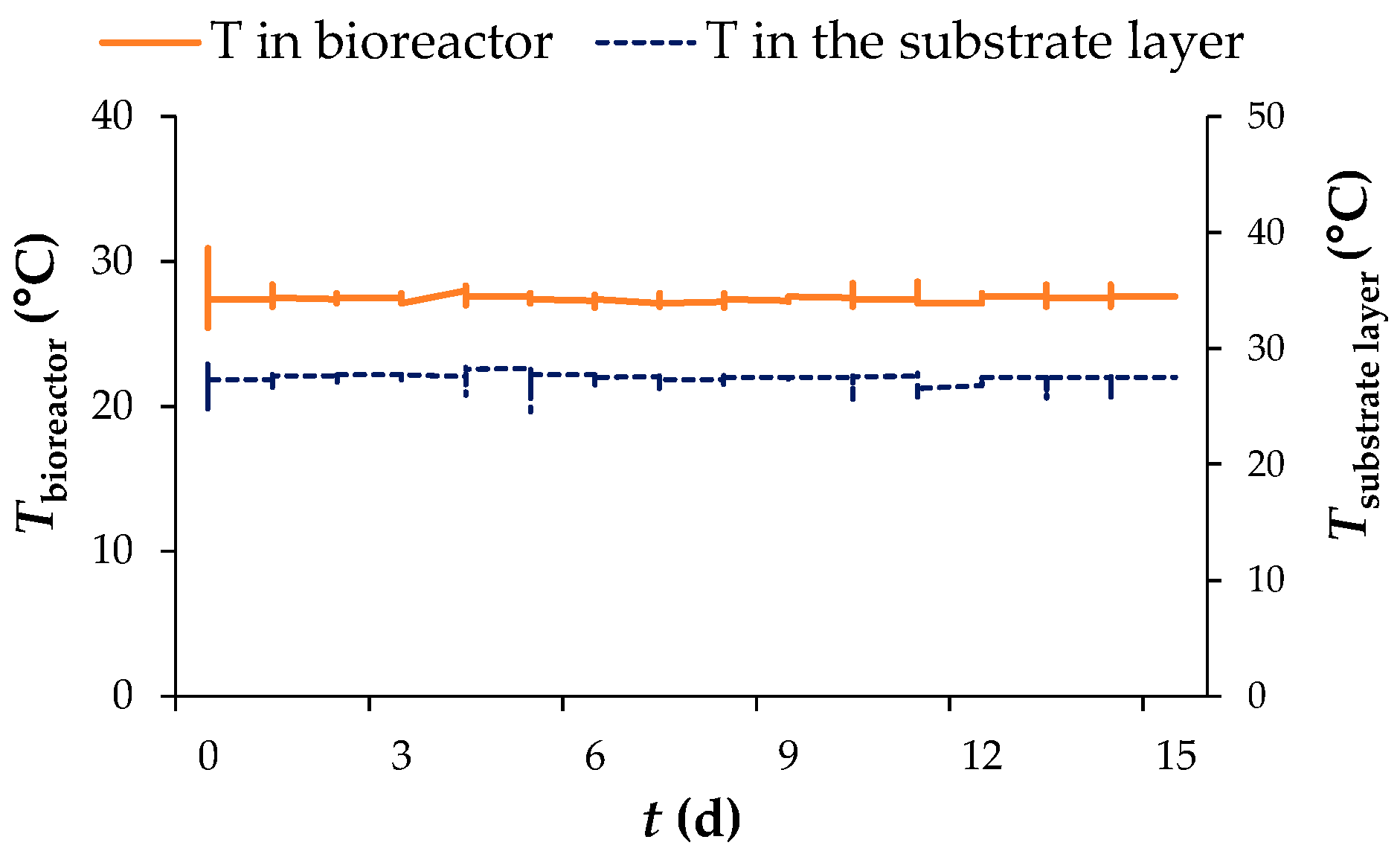
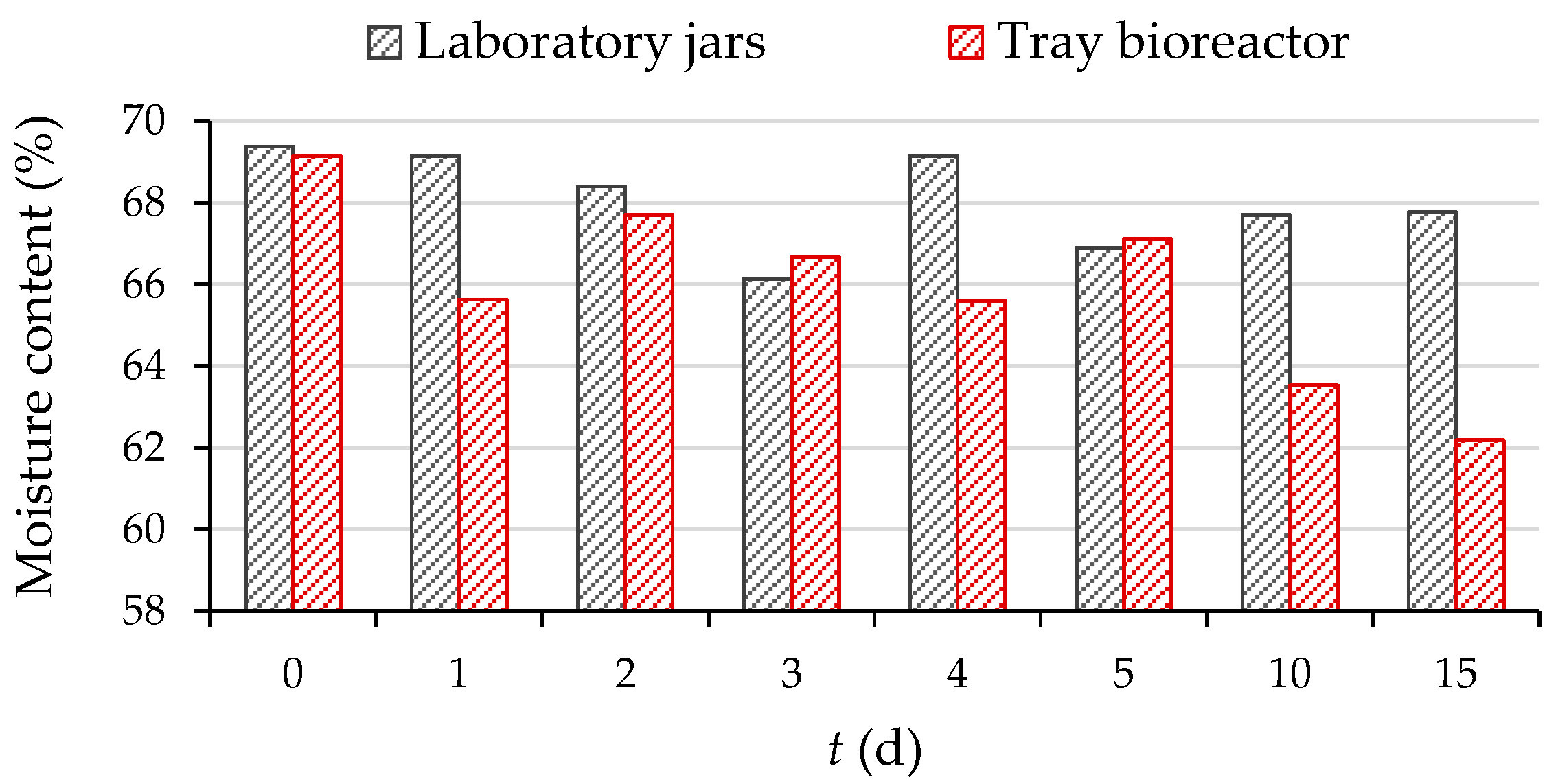
3.1. Biological Treatment of Grape Pomace by R. oryzae in a Tray Bioreactor
3.2. Chemical Composition of Grape Pomace
3.2.1. Lignocellulosic Components
3.2.2. Sugars Content
3.2.3. Ash, Proteins, Total Organic Carbon, Total Nitrogen, and Free Fats Content
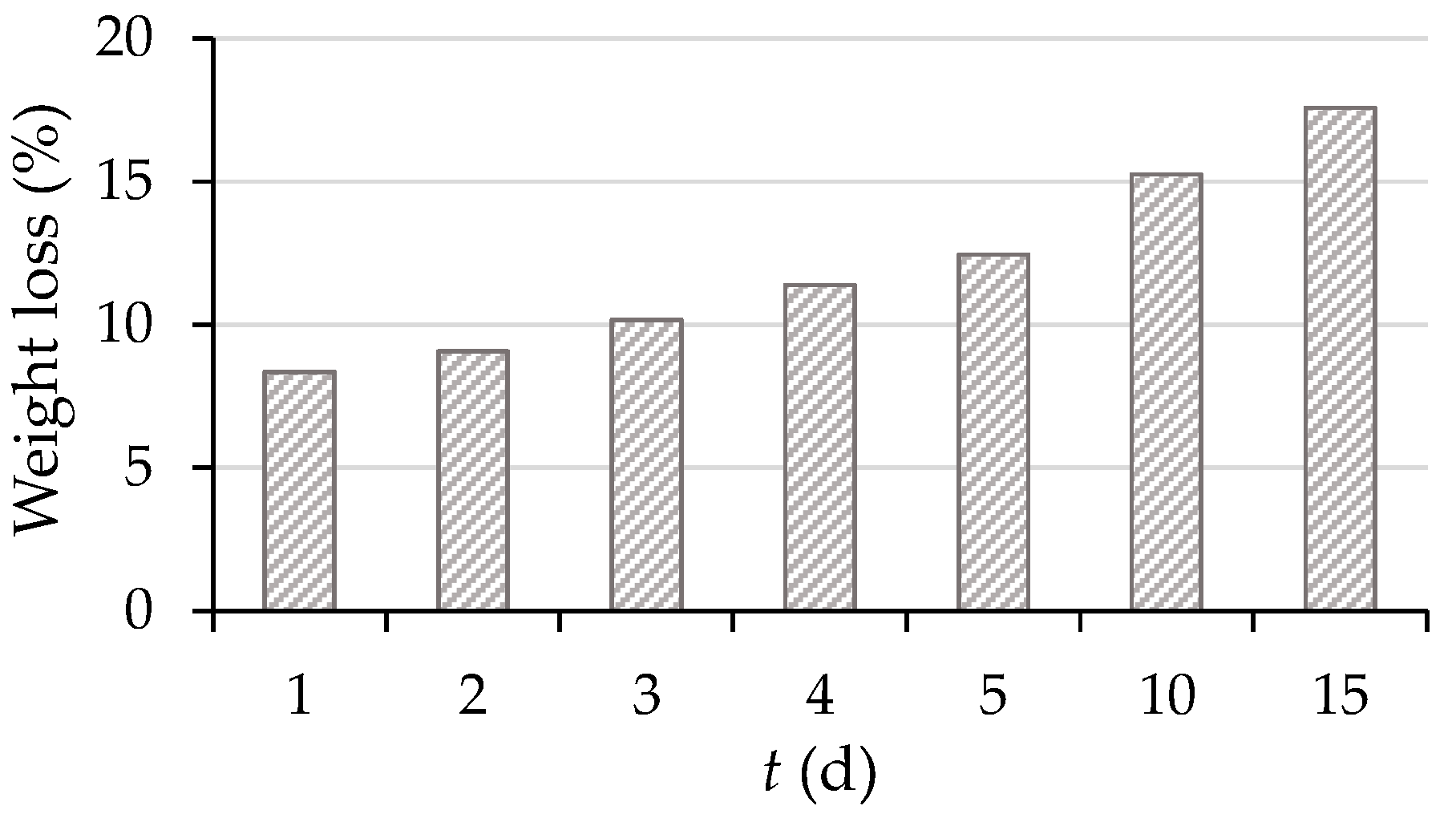
3.2.4. Loss of the Total Substrate Mass
3.2.5. Biomass Concentration
3.2.6. Minerals and Undesirable Substances in Grape Pomace
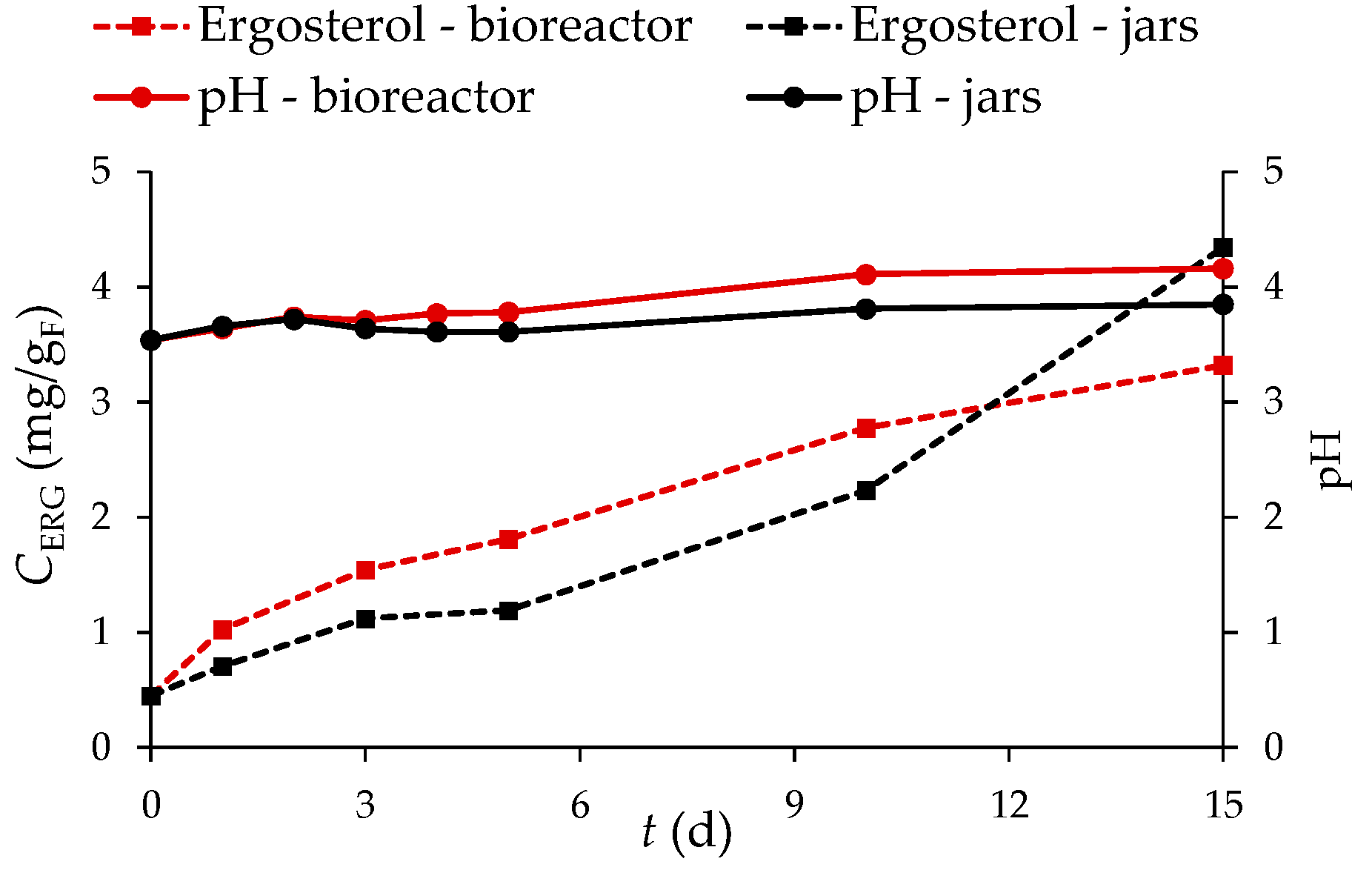
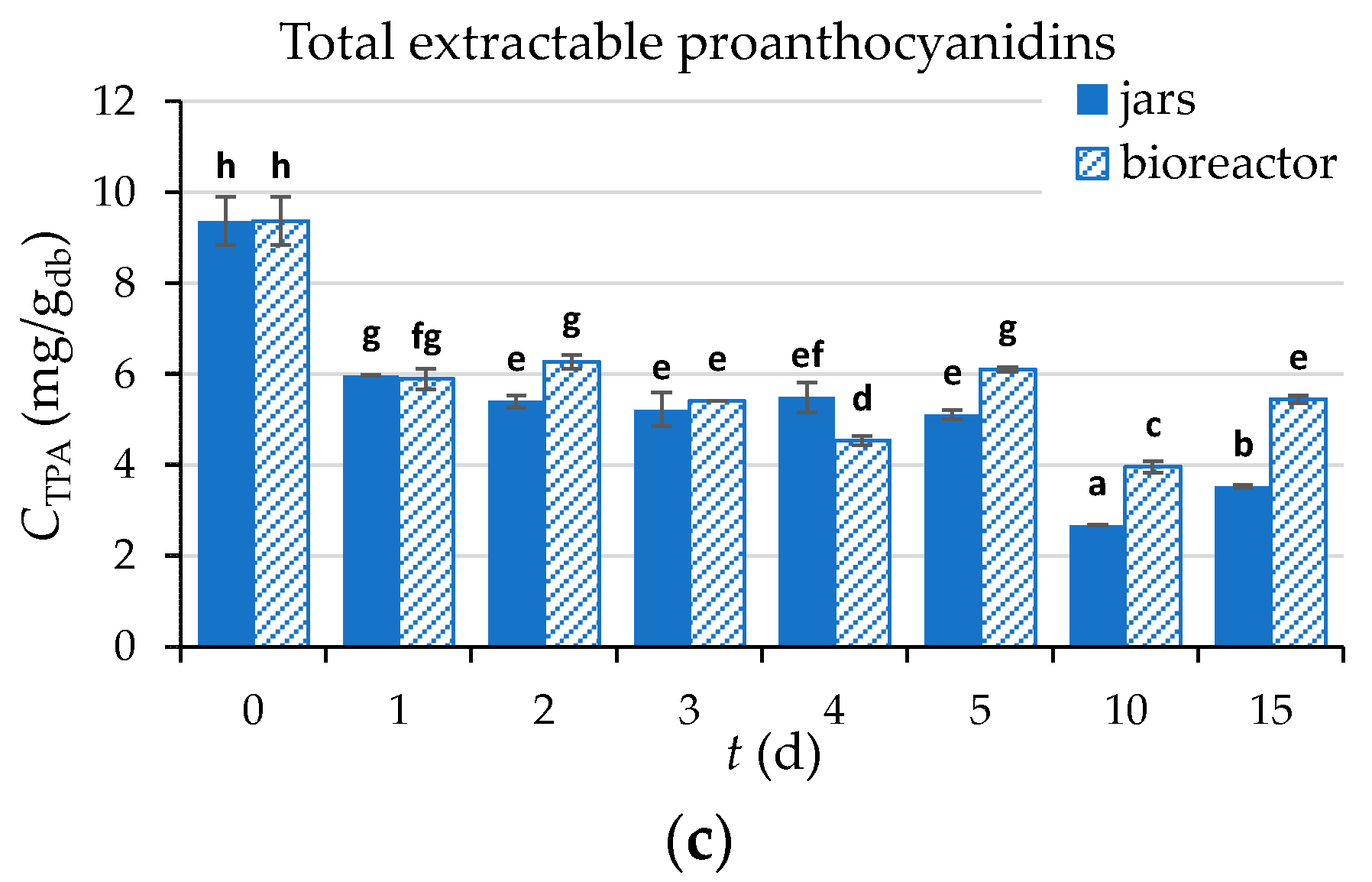
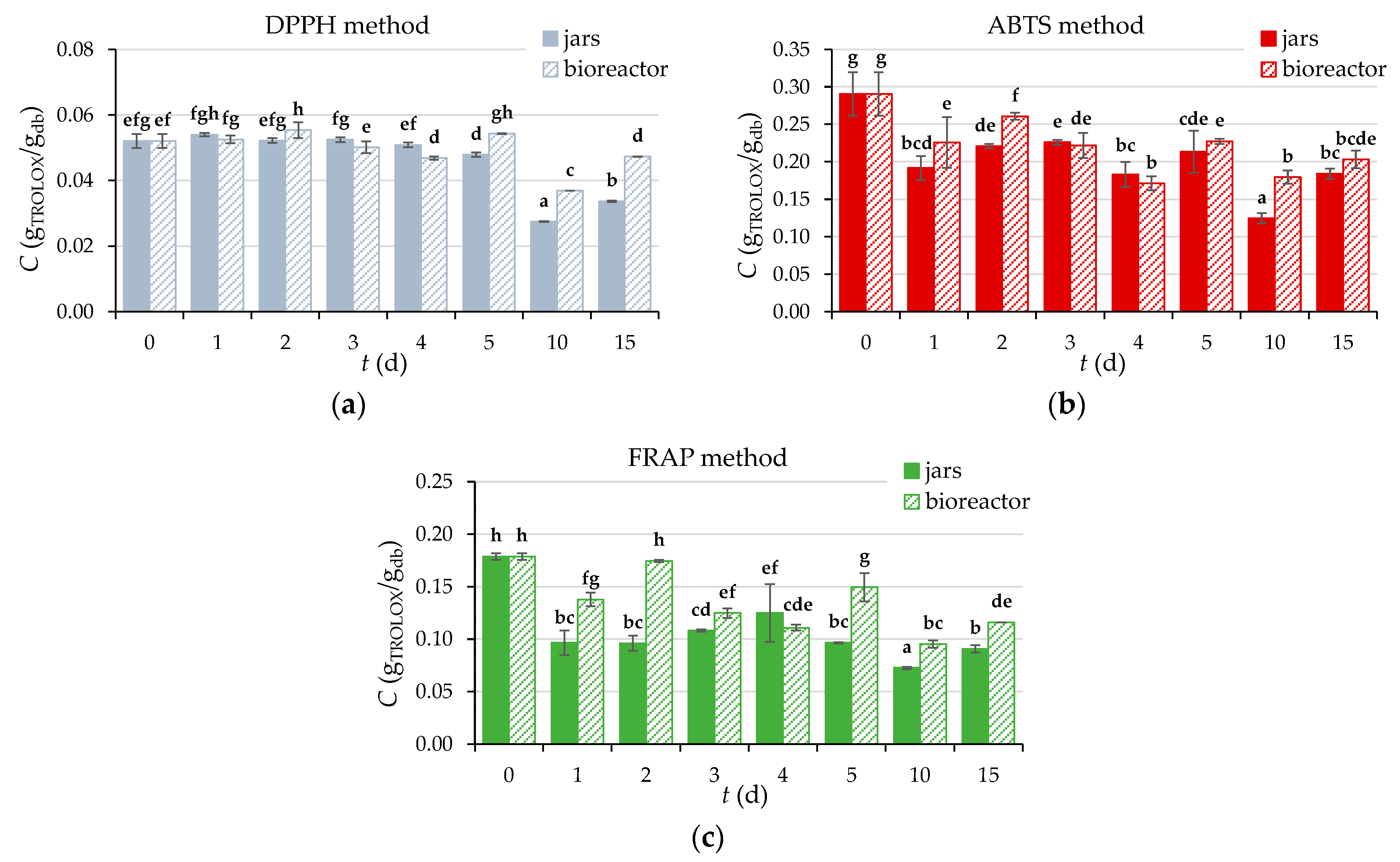
3.2.7. Content of Total Phenolic Compounds (TPC), Total Flavonoids (TF), Total Extractable Proanthocyanidins (TPA), and Antioxidant Activity of GP Extracts
3.2.8. The Effect of Biological Treatment by R. oryzae on the Recovery of Individual Phenolic Compounds from Grape Pomace
3.2.9. Correlation between Hydrolytic and Lignolytic Enzymes and Individual Phenolic Compounds from Grape Pomace
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amaya-Chantaca, D.; Flores-Gallegos, A.C.; Iliná, A.; Aguilar, C.N.; Sepúlveda-Torre, L.; Ascacio-Vadlés, J.A.; Chávez-González, M.L. Comparative Extraction Study of Grape Pomace Bioactive Compounds by Submerged and Solid-State Fermentation. J. Chem. Technol. Biotechnol. 2022, 97, 1494–1505. [Google Scholar] [CrossRef]
- Cabezudo, I.; Galetto, C.S.; Romanini, D.; Furlán, R.L.E.; Meini, M.R. Production of Gallic Acid and Relevant Enzymes by Aspergillus niger and Aspergillus oryzae in Solid-State Fermentation of Soybean Hull and Grape Pomace. Biomass Convers. Biorefin. 2022, 1–9. [Google Scholar] [CrossRef]
- Filippi, K.; Georgaka, N.; Alexandri, M.; Papapostolou, H.; Koutinas, A. Valorisation of Grape Stalks and Pomace for the Production of Bio-Based Succinic Acid by Actinobacillus succinogenes. Ind. Crops Prod. 2021, 168, 113578. [Google Scholar] [CrossRef]
- Melanouri, E.-M. Cultivating Pleurotus Ostreatus and Pleurotus Eryngii Mushroom Strains on Agro-Industrial Residues in Solid-State Fermentation. Part I: Screening for Growth, Endoglucanase, Laccase and Biomass Production in the Colonization Phase | Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S2588913321000508?token=845D3C3D4D7E170E9F5CFC90D2EF513447FC3791C1ADA17C0DFBE775F1C4B0E0C30D016C2246A28A492F2A0DECD9CB1C&originRegion=eu-west-1&originCreation=20230227211512 (accessed on 27 February 2023).
- Zeko-Pivač, A.; Bošnjaković, A.; Planinić, M.; Parlov Vuković, J.; Novak, P.; Jednačak, T.; Tišma, M. Improvement of the Nutraceutical Profile of Brewer’s Spent Grain after Treatment with Trametes versicolor. Microorganisms 2022, 10, 2295. [Google Scholar] [CrossRef] [PubMed]
- Meini, M.-R.; Cabezudo, I.; Galetto, C.S.; Romanini, D. Production of Grape Pomace Extracts with Enhanced Antioxidant and Prebiotic Activities through Solid-State Fermentation by Aspergillus niger and Aspergillus oryzae. Food Biosci. 2021, 42, 101168. [Google Scholar] [CrossRef]
- Belenioti, M.; Mathioudaki, E.; Spyridaki, E.; Ghanotakis, D.; Chaniotakis, N. Biodegradation of Phenolic Compounds from Grape Pomace of Vitis vinifera Asyrtiko by Chlamydomonas reinhardtii. J. Chem. Technol. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Farru, G.; Cappai, G.; Carucci, A.; De Gioannis, G.; Asunis, F.; Milia, S.; Muntoni, A.; Perra, M.; Serpe, A. A Cascade Biorefinery for Grape Marc: Recovery of Materials and Energy through Thermochemical and Biochemical Processes. Sci. Total Environ. 2022, 846, 157464. [Google Scholar] [CrossRef]
- Tišma, M.; Bucić-Kojić, A.; Planinić, M. Bio-Based Products from Lignocellulosic Waste Biomass: A State of the Art. Chem. Biochem. Eng. Q. 2021, 35, 139–156. [Google Scholar] [CrossRef]
- Costa-Silva, V.; Anunciação, M.; Andrade, E.; Fernandes, L.; Costa, A.; Fraga, I.; Barros, A.; Marques, G.; Ferreira, L.; Rodrigues, M. Biovalorization of Grape Stalks as Animal Feed by Solid State Fermentation Using White-Rot Fungi. Appl. Sci. 2022, 12, 6800. [Google Scholar] [CrossRef]
- Arancibia-Díaz, A.; Astudillo-Castro, C.; Altamirano, C.; Soto-Maldonado, C.; Vergara-Castro, M.; Córdova, A.; Zúñiga-Hansen, M.E. Development of Solid-State Fermentation Process of Spent Coffee Grounds for the Differentiated Obtaining of Chlorogenic, Quinic, and Caffeic Acids. J. Sci. Food Agric. 2023, 103, 420–427. [Google Scholar] [CrossRef]
- Paz-Arteaga, S.L.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Cadena-Chamorro, E.; Serna-Cock, L.; Aguilar-González, M.A.; Ramírez-Guzmán, N.; Torres-León, C. Bioprocessing of Pineapple Waste for Sustainable Production of Bioactive Compounds Using Solid-State Fermentation. Innov. Food Sci. Emerg. Technol. 2023, 85, 103313. [Google Scholar] [CrossRef]
- Li, Q.; Yi, P.; Zhang, J.; Shan, Y.; Lin, Y.; Wu, M.; Wang, K.; Tian, G.; Li, J.; Zhu, T. Bioconversion of Food Waste to Crayfish Feed Using Solid-State Fermentation with Yeast. Environ. Sci. Pollut. Res. 2023, 30, 15325–15334. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, N.; Oliveira, F.; Pérez-Bibbins, B.; Belo, I.; Torrado Agrasar, A.; Domínguez, J.M. Optimization of Xylanase Production by Filamentous Fungi in Solid-State Fermentation and Scale-up to Horizontal Tube Bioreactor. Appl. Biochem. Biotechnol. 2014, 173, 803–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Londoño-Hernández, L.; Ramírez-Toro, C.; Ruiz, H.A.; Ascacio-Valdés, J.A.; Aguilar-Gonzalez, M.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Rhizopus Oryzae—Ancient Microbial Resource with Importance in Modern Food Industry. Int. J. Food Microbiol. 2017, 257, 110–127. [Google Scholar] [CrossRef]
- Yafetto, L.; Odamtten, G.T.; Wiafe-Kwagyan, M. Valorization of Agro-Industrial Wastes into Animal Feed through Microbial Fermentation: A Review of the Global and Ghanaian Case. Heliyon 2023, e14814. [Google Scholar] [CrossRef]
- Ma, X.; Gao, M.; Yin, Z.; Zhu, W.; Liu, S.; Wang, Q. Lactic Acid and Animal Feeds Production from Sophora Flavescens Residues by Rhizopus oryzae Fermentation. Process Biochem. 2020, 92, 401–408. [Google Scholar] [CrossRef]
- Hermansyah, H.; Andikoputro, M.I.; Alatas, A. Production of Lipase Enzyme from Rhizopus oryzae by Solid State Fermentation and Submerged Fermentation Using Wheat Bran as Substrate. In Proceedings of the AIP Conference, Yogyakarta, Indonesia, 7–8 November 2018. [Google Scholar]
- AOAC. Official Method 942.05. In Official Methods of Analysis of AOAC INTERNATIONAL, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- Goering, H.K.; Soest, P.J.V. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1970; pp. 1–24. [Google Scholar]
- AOAC. Official Method 2001.11. In Official Methods of Analysis of AOAC INTERNATIONAL, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Official Method 945.16. In Official Methods of Analysis of AOAC INTERNATIONAL, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Barreira, J.C.M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Development of a Novel Methodology for the Analysis of Ergosterol in Mushrooms. Food Anal. Methods 2014, 7, 217–223. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2001; pp. I1.1.1–I1.1.8. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total Phenolics and Total Flavonoids in Bulgarian Fruits and Vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, Proanthocyanidins, Flavones and Flavonols in Some Plant Materials and Their Antioxidant Activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Šelo, G.; Zelić, B.; Planinić, M.; Tišma, M. Recovery of Phenolic Acid and Enzyme Production from Corn Silage Biologically Treated by Trametes Versicolor. Appl. Biochem. Biotechnol. 2017, 181, 948–960. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Grgić, J.; Perković, G.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comparative Study of the Influence of Various Fungal-Based Pretreatments of Grape Pomace on Phenolic Compounds Recovery. Foods 2022, 11, 1665. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, E.; Hernández, C.; García, G.; Ziarelli, F.; Gutiérrez-Rivera, B.; Musule, R.; Vázquez-Marrufo, G.; Gardner, T.G. Changes in Chemical and Structural Composition of Sugarcane Bagasse Caused by Alkaline Pretreatments [Ca(OH)2 and NaOH] Modify the Amount of Endoglucanase and β-Glucosidase Produced by Aspergillus niger in Solid-State Fermentation. Chem. Eng. Commun. 2021, 209, 594–606. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Croatian Standards Institute. HRN ISO 6491:2001. Available online: http://31.45.242.218/HZN/todb.nsf/wFrameset2?OpenFrameSet&Frame=Down&Src=%2FHZN%2Ftodb.nsf%2FNormaSve%2Fc1256c8f003565d5c1256d29003e1e06%3FOpenDocument%26AutoFramed (accessed on 26 March 2023).
- Croatian Standards Institute. HRN EN ISO/IEC 17025:2017. Available online: http://31.45.242.218/HZN/todb.nsf/wFrameset2?OpenFrameSet&Frame=Down&Src=%2FHZN%2Ftodb.nsf%2FNormaSve%2Fda093fd23afffcb8c12580a4003bbaf4%3FOpenDocument%26AutoFramed (accessed on 26 March 2023).
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of Solvent and Temperature on Extraction of Phenolic Compounds from Grape Seed, Antioxidant Activity and Colour of Extract. Int. J. Food Sci. Technol. 2009, 44, 2394–2401. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory Testing of Methods for Assay of Xylanase Activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of Cellulase Activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Adney, B.; Baker, J. Measurement of Cellulase Activities: Laboratory Analytical Procedure (LAP). Technical Report NREL/TP-510-42628. 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42628.pdf (accessed on 4 April 2023).
- Karpe, A.V.; Dhamale, V.V.; Morrison, P.D.; Beale, D.J.; Harding, I.H.; Palombo, E.A. Winery Biomass Waste Degradation by Sequential Sonication and Mixed Fungal Enzyme Treatments. Fungal Genet. Biol. 2017, 102, 22–30. [Google Scholar] [CrossRef]
- Margetić, A.; Vujčić, Z. Comparative Study of Stability of Soluble and Cell Wall Invertase from Saccharomyces cerevisiae. Prep. Biochem. Biotechnol. 2017, 47, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Lueangjaroenkit, P.; Kunitake, E.; Sakka, M.; Kimura, T.; Teerapatsakul, C.; Sakka, K.; Chitradon, L. Light Regulation of Two New Manganese Peroxidase-Encoding Genes in Trametes polyzona KU-RNW027. Microorganisms 2020, 8, 852. [Google Scholar] [CrossRef] [PubMed]
- Field, J.A.; Vledder, R.H.; van Zelst, J.G.; Rulkens, W.H. The Tolerance of Lignin Peroxidase and Manganese-Dependent Peroxidase to Miscible Solvents and the in Vitro Oxidation of Anthracene in Solvent: Water Mixtures. Enzyme Microb. Technol. 1996, 18, 300–308. [Google Scholar] [CrossRef]
- Linko, S.; Haapala, R. A Critical Study of Lignin Peroxidase Activity Assay by Veratryl Alcohol Oxidation. Biotechnol. Tech. 1993, 7, 75–80. [Google Scholar] [CrossRef]
- Van Kuijk, S.J.A.; Sonnenberg, A.S.M.; Baars, J.J.P.; Hendriks, W.H.; Cone, J.W. Fungal Treatment of Lignocellulosic Biomass: Importance of Fungal Species, Colonization and Time on Chemical Composition and in Vitro Rumen Degradability. Anim. Feed. Sci. Technol. 2015, 209, 40–50. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Oliveira, R.A.; Bon, E.P.S.; Terzi, S.C.; Souza, E.F.; Gottschalk, L.M.F.; Tonon, R.V. Use of Grape Pomace for the Production of Hydrolytic Enzymes by Solid-State Fermentation and Recovery of Its Bioactive Compounds. Food Res. Int. 2019, 120, 441–448. [Google Scholar] [CrossRef]
- Niu, D.; Zuo, S.; Jiang, D.; Tian, P.; Zheng, M.; Xu, C. Treatment Using White Rot Fungi Changed the Chemical Composition of Wheat Straw and Enhanced Digestion by Rumen Microbiota In Vitro. Anim. Feed Sci. Technol. 2018, 237, 46–54. [Google Scholar] [CrossRef]
- Leite, P.; Silva, C.; Salgado, J.M.; Belo, I. Simultaneous Production of Lignocellulolytic Enzymes and Extraction of Antioxidant Compounds by Solid-State Fermentation of Agro-Industrial Wastes. Ind. Crops Prod. 2019, 137, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Mushollaeni, W.; Tantalu, L. Anthocyanin and Nutritional Contents of Fermented Lebui Bean (Cajanus sp.) through SSF Method and Induced by Rhizopus sp. and Saccharomyces sp. IOP Conf. Ser. Earth Environ. Sci 2020, 465, 012037. [Google Scholar] [CrossRef]
- Singh, S.; Khajuria, R. Regulation by Metal Ions. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Secondary Metabolites Biochemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–166. ISBN 978-0-444-63504-4. [Google Scholar]
- Bastos, R.G.; Ribeiro, H.C. Citric Acid Production by the Solid-State Cultivation Consortium of and from Sugarcane Bagasse. Open Biotechnol. J. 2020, 14, 32–41. [Google Scholar] [CrossRef]
- Yu, H.; Xie, B.; Khan, R.; Dong, J.; Shen, G. The Changes in Macronutrients and Microbial Community Structure during the Co-Composting of White Wine Distillers’ Grains and Potassium Silicate. J. Clean. Prod. 2021, 319, 128681. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Toşa, M.I.; Dulf, E.-H. Simultaneous Enrichment of Grape Pomace with γ-Linolenic Acid and Carotenoids by Solid-State Fermentation with Zygomycetes fungi and Antioxidant Potential of the Bioprocessed Substrates. Food Chem. 2020, 310, 125927. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, W.; El-Sheikh, H.; Abu-Elreesh, G.; Hashem, A. Isolation and Screening of Promising Oleaginous Rhizopus sp. and Designing of Taguchi Method for Increasing Lipid Production. J. Innov. Pharm. Biol. Sci. 2018, 5, 8–15. [Google Scholar]
- KKupski, L.; Cipolatti, E.; Rocha, M.D.; Oliveira, M.D.S.; Souza-Soares, L.D.A.; Badiale-Furlong, E. Solid-State Fermentation for the Enrichment and Extraction of Proteins and Antioxidant Compounds in Rice Bran by Rhizopus oryzae. Braz. Arch. Biol. Technol. 2012, 55, 937–942. [Google Scholar] [CrossRef] [Green Version]
- Larios-Cruz, R.; Buenrostro-Figueroa, J.; Prado-Barragán, A.; Rodríguez-Jasso, R.M.; Rodríguez-Herrera, R.; Montañez, J.C.; Aguilar, C.N. Valorization of Grapefruit By-Products as Solid Support for Solid-State Fermentation to Produce Antioxidant Bioactive Extracts. Waste Biomass Valoriz. 2019, 10, 763–769. [Google Scholar] [CrossRef]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent Developments on Solid-State Fermentation for Production of Microbial Secondary Metabolites: Challenges and Solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef]
- Botella, C.; Diaz, A.; de Ory, I.; Webb, C.; Blandino, A. Xylanase and Pectinase Production by Aspergillus awamori on Grape Pomace in Solid State Fermentation. Process Biochem. 2007, 42, 98–101. [Google Scholar] [CrossRef]
- Lopes, V.R.O.; Farias, M.A.; Belo, I.M.P.; Coelho, M.A.Z. Nitrogen Sources on TPOMW Valorization through Solid State Fermentation Performed by Yarrowia lipolytica. Braz. J. Chem. Eng. 2016, 33, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Eur-LEX. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed. Available online: https://eur-lex.europa.eu/eli/dir/2002/32/2019-11-28 (accessed on 21 February 2023).
- Şahin, T.; Dalğa, S.; Ölmez, M.; Şahin, T.; Dalğa, S.; Ölmez, M. Polycyclic Aromatic Hydrocarbons (PAHs) and Their Importance in Animal Nutrition; IntechOpen: London, UK, 2022; ISBN 978-1-80355-127-2. [Google Scholar]
- Zambrano, C.; Kotogán, A.; Bencsik, O.; Papp, T.; Vágvölgyi, C.; Mondal, K.C.; Krisch, J.; Takó, M. Mobilization of Phenolic Antioxidants from Grape, Apple and Pitahaya Residues via Solid State Fungal Fermentation and Carbohydrase Treatment. LWT 2018, 89, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Papadaki, A.; Kachrimanidou, V.; Papanikolaou, S.; Philippoussis, A.; Diamantopoulou, P. Upgrading Grape Pomace through Pleurotus spp. Cultivation for the Production of Enzymes and Fruiting Bodies. Microorganisms 2019, 7, 207. [Google Scholar] [CrossRef] [Green Version]
- Troncozo, M.I.; Figoli, C.B.; Franco, M.E.E.; Mirífico, M.V.; Bosch, A.; Rajchenberg, M.; Balatti, P.A.; Saparrat, M.C.N. Biotransformation of Grape Pomace from Vitis labrusca by Peniophora albobadia LPSC # 285 (Basidiomycota). An. Acad. Bras. Ciênc. 2020, 92, e20181174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, R.K.; Brar, S.K.; Verma, M. A Fermentative Approach towards Optimizing Directed Biosynthesis of Fumaric Acid by Rhizopus oryzae 1526 Utilizing Apple Industry Waste Biomass. Fungal Biol. 2015, 119, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of Total Content of Phenolic Compounds and Their Antioxidant Activity in VegetablesEvaluation of Spectrophotometric Methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Lennartsson, P.R.; Edebo, L.; Taherzadeh, M.J. Zygomycetes-Based Biorefinery: Present Status and Future Prospects. Bioresour. Technol. 2013, 135, 523–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipe, D.; Fernandes, H.; Castro, C.; Peres, H.; Oliva-Teles, A.; Belo, I.; Salgado, J.M. Improved Lignocellulolytic Enzyme Production and Antioxidant Extraction Using Solid-state Fermentation of Olive Pomace Mixed with Winery Waste. Biofuels Bioprod. Bioref. 2020, 14, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Díaz, A.B.; Alvarado, O.; de Ory, I.; Caro, I.; Blandino, A. Valorization of Grape Pomace and Orange Peels: Improved Production of Hydrolytic Enzymes for the Clarification of Orange Juice. Food Bioprod. Process. 2013, 91, 580–586. [Google Scholar] [CrossRef]
- Ratner, B. The Correlation Coefficient: Its Values Range between +1/−1, or Do They? J. Target Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef] [Green Version]

| Enzyme | Buffer | pH |
|---|---|---|
| Xylanase | 50 mM sodium citrate buffer | 5.3 |
| Cellulase | 50 mM sodium citrate buffer | 4.8 |
| β-glucosidase | 100 mM sodium acetate buffer | 5.0 |
| Invertase | 100 mM sodium acetate buffer | 4.5 |
| Laccase | 50 mM sodium malonate buffer | 4.5 |
| Manganese peroxidase (MnP) | 50 mM sodium malonate buffer | 4.5 |
| Lignin peroxidase (LiP) | 50 mM sodium tartrate buffer | 3.0 |
| Compound | GP | SSF in Laboratory Jars | SSF in a Tray Bioreactor | ||||
|---|---|---|---|---|---|---|---|
| Day “0” | Day 5 | Day 10 | Day 15 | Day 5 | Day 10 | Day 15 | |
| Ash (%db) | 8.65 ± 0.00 c | 8.19 ± 0.11 bc | 8.30 ± 0.18 bc | 7.59 ± 0.73 a | 7.84 ± 0.14 ab | 8.13 ± 0.09 abc | 7.95 ± 0.08 ab |
| Proteins (%db) | 11.35 ± 0.09 e | 9.09 ± 0.18 b | 8.41 ± 0.10 a | 9.91 ± 0.10 d | 9.41 ± 0.00 c | 9.56 ± 0.10 c | 11.31 ± 0.10 e |
| Free fats (%db) | 7.50 ± 0.20 a | 9.01 ± 0.29 b | 10.66 ± 0.29 d | 10.77 ± 0.10 d | 11.13 ± 0.47 d | 11.00 ± 0.03 d | 9.73 ± 0.04 c |
| TOCGP (%db) | 72.18 ± 0.56 a | 73.03 ± 0.20 ab | 73.77 ± 1.17 bc | 74.75 ± 0.21 cd | 74.20 ± 0.56 c | 75.33 ± 0.12 d | 80.14 ± 0.47 e |
| NDF (%db) | 52.81 ± 1.10 a | 57.60 ± 0.64 b | 58.64 ± 1.12 b | 56.69 ± 0.20 b | 53.16 ± 1.13 a | 58.78 ± 1.44 b | 61.72 ± 1.84 c |
| ADF (%db) | 44.26 ± 2.41 a | 52.18 ± 0.77 bc | 52.84 ± 0.66 c | 50.17 ± 0.55 b | 44.84 ± 1.77 a | 49.66 ± 1.40 b | 50.60 ± 0.94 bc |
| ADL/lignin (%db) | 31.35 ± 1.49 a | 36.59 ± 0.72 cd | 37.97 ± 0.33 d | 34.28 ± 0.57 c | 30.81 ± 0.26 a | 35.45 ± 0.14 bc | 37.11 ± 1.01 d |
| Hemicellulose (%db) | 8.55 ± 1.68 c | 5.42 ± 0.30 a | 5.80 ± 1.72 a | 6.52 ± 0.76 ab | 8.32 ± 0.64 bc | 9.12 ± 1.11 c | 11.64 ± 0.20 d |
| Cellulose (%db) | 12.90 ± 1.70 a | 15.58 ± 0.05 bc | 14.87 ± 0.27 bc | 15.89 ± 0.01 c | 14.03 ± 1.58 ab | 14.97 ± 0.57 bc | 13.96 ± 0.55 ab |
| Sucrose (mg/gdb) | 12.04 ± 0.27 e | 6.21 ± 0.03 d | 4.77 ± 0.03 e | 4.28 ± 0.02 b | 2.88 ± 0.09 a | 4.40 ± 0.03 b | 4.88 ± 0.14 c |
| Glucose (mg/gdb) | 7.01 ± 0.29 e | 1.63 ± 0.04 b | 2.13 ± 0.07 c | 0.78 ± 0.49 a | 1.98 ± 0.04 bc | 2.64 ± 0.11 d | 2.14 ± 0.16 c |
| Fructose (mg/gdb) | 1.50 ± 0.12 d | 0.21 ± 0.02 bc | 0.07 ± 0.01 a | 0.15 ± 0.01 ab | 0.30 ± 0.01 c | 0.18 ± 0.01 b | 0.19 ± 0.07 b |
| RS (mgGLUCOSE/gdb) | 30.00 ± 0.76 g | 21.93 ± 0.20 f | 18.77 ± 0.19 d | 13.96 ± 0.01 c | 19.78 ± 0.41 e | 13.08 ± 0.37 b | 9.19 ± 0.41 a |
| TOCE (mg/gdb) | 79.25 ± 7.15 d | 49.65 ± 1.30 b | 45.27 ± 1.13 b | 30.54 ± 1.18 a | 63.78 ± 1.40 c | 44.90 ± 0.47 b | 26.50 ± 0.20 a |
| TN (mg/gdb) | 1.45 ± 0.00 e | 0.85 ± 0.02 d | 0.72 ± 0.02 c | 0.41 ± 0.04 a | 0.90 ± 0.02 d | 0.66 ± 0.03 b | 0.36 ± 0.04 a |
| Element | Unit | Content | |
|---|---|---|---|
| Day “0” | Day 15 | ||
| Phosphorus (P) | % | 0.30 | 0.28 |
| Calcium (Ca) | mg/kg | 4967 | 5030 |
| Potassium (K) | mg/kg | 22,130 | 11,219 |
| Iron (Fe) | mg/kg | 103 | 87 |
| Lead (Pb) | mg/kg | 0.064 | 0.060 |
| Cadmium (Cd) | mg/kg | 0.025 | 0.027 |
| Arsenic (As) | mg/kg | 0.024 | 0.018 |
| Component | Unit | Content | |
|---|---|---|---|
| Day “0” | Day 15 | ||
| Harmful Substances and Pollutants | |||
| PAHs | mg/kg | <0.020 | <0.020 |
| PCBs | mg/kg | <0.035 | <0.035 |
| Mycotoxins * | |||
| Aflatoxins | mg/kg | <0.003 | <0.003 |
| Fumonisins | mg/kg | <0.030 | <0.030 |
| Zearalenone | mg/kg | <0.030 | <0.030 |
| T-2/HT-2 toxin | µg/kg | <20 | <20 |
| Deoxynivalenol | mg/kg | <0.020 | <0.020 |
| Ochratoxin A | mg/kg | <0.001 | <0.001 |
| Phenolic Compounds | Day “0” | SSF in Laboratory Jars | SSF in Tray Bioreactor | p *** | ||||
|---|---|---|---|---|---|---|---|---|
| Co (µg/gdb) * | Ci,max. (µg/gdb) * | p ** | tSSF (d) | Ci,max. (µg/gdb) * | p ** | tSSF (d) | ||
| Phenolic acid (hydroxybenzoic acids) | ||||||||
| GA | 307.20 ± 3.10 | 447.88 ± 33.15 | 0.0149 | 4. | 490.77 ± 15.31 | 0.0033 | 4. | 0.1117 |
| EA | 208.44 ± 4.42 | 235.50 ± 6.27 | 0.0483 | 3. | 233.49 ± 1.62 | 0.0188 | 10. | 0.6183 |
| p-HBA | 6.07 ± 0.44 | 14.30 ± 0.79 | 0.0006 | 4. | 11.79 ± 0.17 | 0.0008 | 3. | 0.0057 |
| SA | 236.63 ± 1.28 | 424.04 ± 4.96 | 0.0004 | 5. | 282.00 ± 18.34 | 0.0441 | 1. | 0.0002 |
| VA | 80.81 ± 1.31 | 97.49 ± 0.79 | 0.0053 | 2. | 93.68 ± 6.57 | 0.0514 | 1. | 0.3749 |
| 3,4-DHBA | 111.51 ± 2.17 | 234.14 ± 9.27 | 0.0029 | 10. | 194.24 ± 3.78 | 0.0017 | 4. | 0.0023 |
| Phenolic acid (hydroxycinnamic acids) | ||||||||
| p-CoA | 8.76 ± 0.70 | 9.65 ± 0.55 | 0.0098 | 4. | 10.23 ± 0.17 | 0.1000 | 4. | 0.1544 |
| CAF | 13.12 ± 0.46 | 15.07 ± 0.17 | 0.0070 | 1. | 17.14 ± 0.99 | 0.0058 | 1. | 0.0235 |
| Flavan-3-ol | ||||||||
| EPG | 319.26 ± 3.39 | 366.73 ± 5.08 | 0.0004 | 1. | 453.49 ± 15.62 | 0.0028 | 1. | 0.0008 |
| Flavonol | ||||||||
| QU | 403.10 ± 9.04 | 657.79 ± 13.33 | 0.0001 | 15. | 714.91 ± 15.92 | 0.0021 | 10. | 0.0089 |
| Stilbene | ||||||||
| RES | 30.40 ± 0.69 | 30.63 ± 0.37 | 0.7370 | 3. | 76.19 ± 0.41 | 0.0000 | 15. | 0.0000 |
| tSSF (d) | Hydrolytic Enzymes | Lignolytic Enzymes * | |||||
|---|---|---|---|---|---|---|---|
| β-Glucosidase (U/gdb) | Xylanase (U/gdb) | Cellulase (U/gdb) | Invertase (U/gdb) | Laccase (U/gdb) | MnP (U/gdb) | LiP (U/gdb) | |
| Laboratory jars | |||||||
| 1. | 0.421 | 25.517 | 0.234 | n.d. | 0.005 | n.d. | 0.013 |
| 5. | 0.208 | 62.964 | 0.675 | n.d. | 0.001 | 0.001 | 0.006 |
| 10. | 0.315 | 40.054 | n.d. | 8.198 | 0.001 | 0.005 | 0.039 |
| 15. | 0.347 | 33.181 | 1.020 | 6.316 | 0.002 | n.d. | 0.040 |
| Tray bioreactor | |||||||
| 1. | 0.290 | 9.271 | 0.293 | 6.135 | 0.005 | n.d. | 0.036 |
| 5. | 0.533 | 17.684 | 0.685 | 2.700 | 0.009 | n.d. | 0.026 |
| 10. | 0.440 | 46.133 | 0.666 | 10.706 | 0.071 | 0.018 | 0.069 |
| 15. | 0.430 | 47.673 | 0.523 | 7.344 | 0.072 | 0.018 | 0.074 |
| Phenolic Compounds * | Hidrolytic Enzymes | Lignolytic Enzymes ** | |||||
|---|---|---|---|---|---|---|---|
| β-Glucosidase | Xylanase | Cellulase | Invertase | Laccase | MnP | LiP | |
| HBA | −0.433 | 0.555 | −0.565 | −0.552 | −0.233 | 0.188 | −0.739 |
| HCA | 0.149 | 0.014 | −0.579 | −0.755 | 0.404 | −0.181 | −0.790 |
| F3L | 0.116 | 0.051 | −0.095 | −0.970 | 0.474 | −0.643 | −0.919 |
| FL | 0.192 | −0.323 | 0.717 | 0.533 | 0.014 | −0.205 | 0.687 |
| PCD | −0.476 | 0.618 | −0.225 | −0.810 | −0.163 | −0.201 | −0.937 |
| ST | −0.105 | 0.273 | −0.274 | −0.923 | 0.236 | −0.398 | −0.962 |
| TP | −0.331 | 0.487 | −0.154 | −0.913 | 0.019 | −0.379 | −0.987 |
| Phenolic Compounds * | Hidrolytic Enzymes | Lignolytic Enzymes ** | |||||
|---|---|---|---|---|---|---|---|
| β-Glucosidase | Xylanase | Cellulase | Invertase | Laccase | MnP | LiP | |
| HBA | −0.494 | −0.854 | −0.472 | −0.257 | −0.801 | −0.790 | −0.735 |
| HCA | −0.349 | −0.966 | −0.431 | −0.534 | −0.943 | −0.937 | −0.897 |
| F3L | −0.235 | −0.994 | −0.387 | −0.700 | −0.992 | −0.989 | −0.961 |
| FL | 0.162 | 0.240 | 0.477 | 0.570 | 0.253 | 0.238 | 0.161 |
| PCD | 0.638 | −0.532 | 0.472 | −0.781 | −0.635 | −0.669 | −0.790 |
| ST | 0.203 | 0.917 | 0.253 | 0.508 | 0.908 | 0.910 | 0.903 |
| TP | 0.217 | −0.792 | 0.133 | −0.643 | −0.838 | −0.857 | −0.922 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šelo, G.; Planinić, M.; Tišma, M.; Martinović, J.; Perković, G.; Bucić-Kojić, A. Bioconversion of Grape Pomace with Rhizopus oryzae under Solid-State Conditions: Changes in the Chemical Composition and Profile of Phenolic Compounds. Microorganisms 2023, 11, 956. https://doi.org/10.3390/microorganisms11040956
Šelo G, Planinić M, Tišma M, Martinović J, Perković G, Bucić-Kojić A. Bioconversion of Grape Pomace with Rhizopus oryzae under Solid-State Conditions: Changes in the Chemical Composition and Profile of Phenolic Compounds. Microorganisms. 2023; 11(4):956. https://doi.org/10.3390/microorganisms11040956
Chicago/Turabian StyleŠelo, Gordana, Mirela Planinić, Marina Tišma, Josipa Martinović, Gabriela Perković, and Ana Bucić-Kojić. 2023. "Bioconversion of Grape Pomace with Rhizopus oryzae under Solid-State Conditions: Changes in the Chemical Composition and Profile of Phenolic Compounds" Microorganisms 11, no. 4: 956. https://doi.org/10.3390/microorganisms11040956
APA StyleŠelo, G., Planinić, M., Tišma, M., Martinović, J., Perković, G., & Bucić-Kojić, A. (2023). Bioconversion of Grape Pomace with Rhizopus oryzae under Solid-State Conditions: Changes in the Chemical Composition and Profile of Phenolic Compounds. Microorganisms, 11(4), 956. https://doi.org/10.3390/microorganisms11040956






