Finger Millet (Eleusine coracana) Plant–Endophyte Dynamics: Plant Growth, Nutrient Uptake, and Zinc Biofortification
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Endophytic Microbes
2.2. Selection of Effective Zinc Solubilizing Endophytes
2.3. Indole Acetic Acid Synthesis
2.4. HCN Production
2.4.1. Qualitative Assay
2.4.2. Quantitative Assay
2.5. Ammonia Excretion
2.6. Siderophore Production
2.6.1. Qualitative Assay
2.6.2. Quantitative Assay
2.7. Phosphate Solubilization
2.8. Organic Acid Production
2.9. Extra Cellular Enzyme Assay
2.10. Stress Tolerance of Zinc Solubilizers
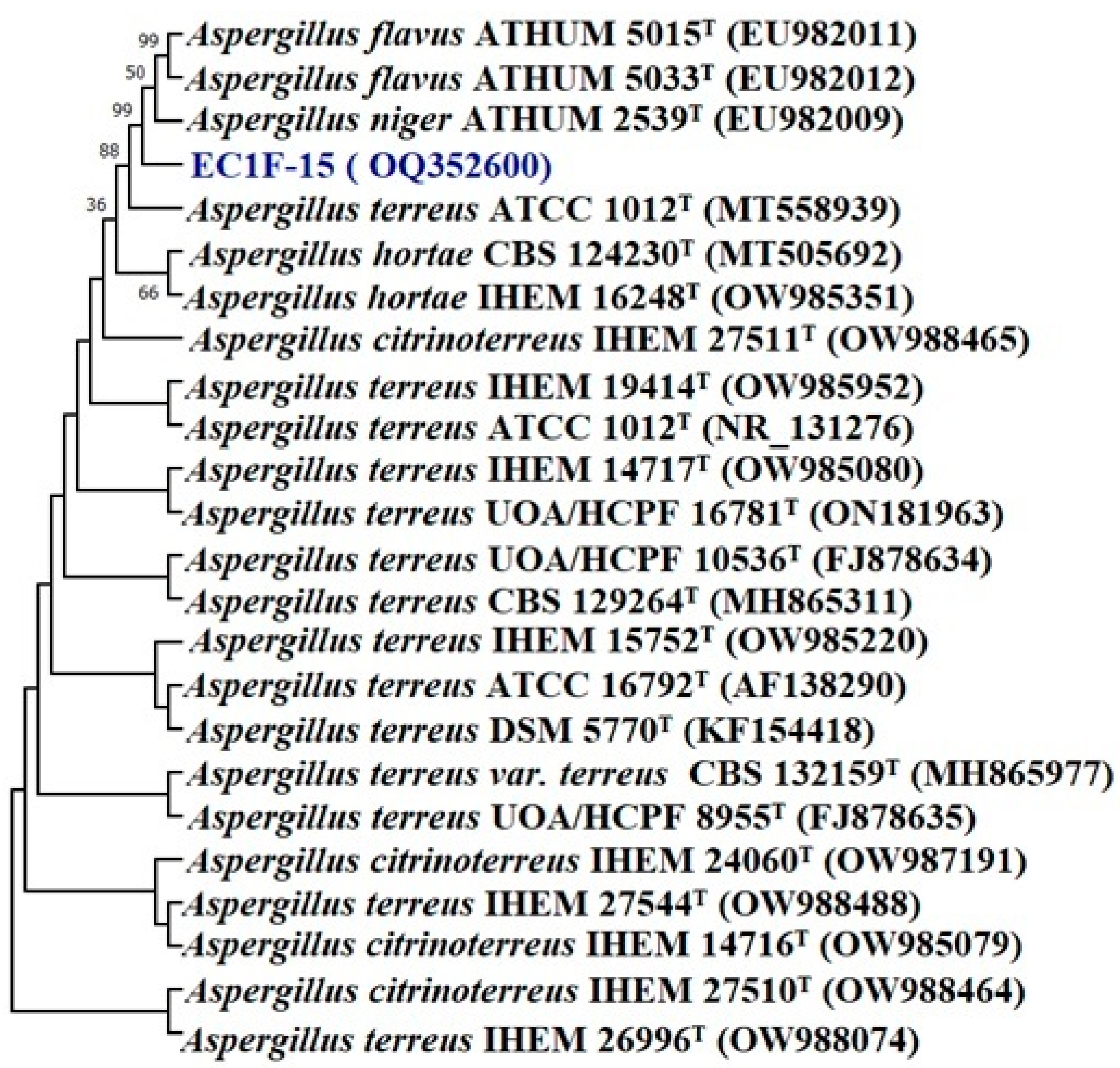
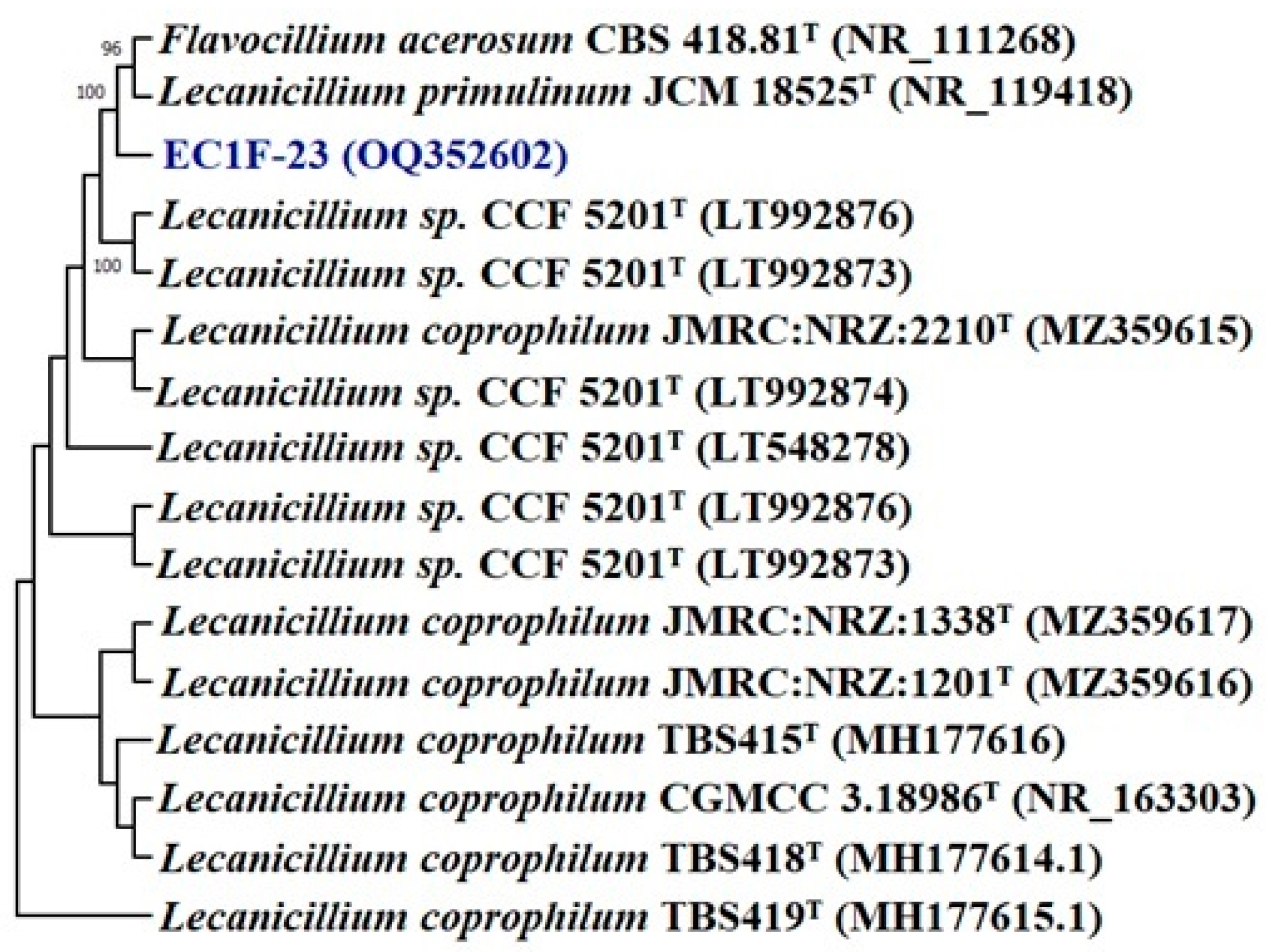
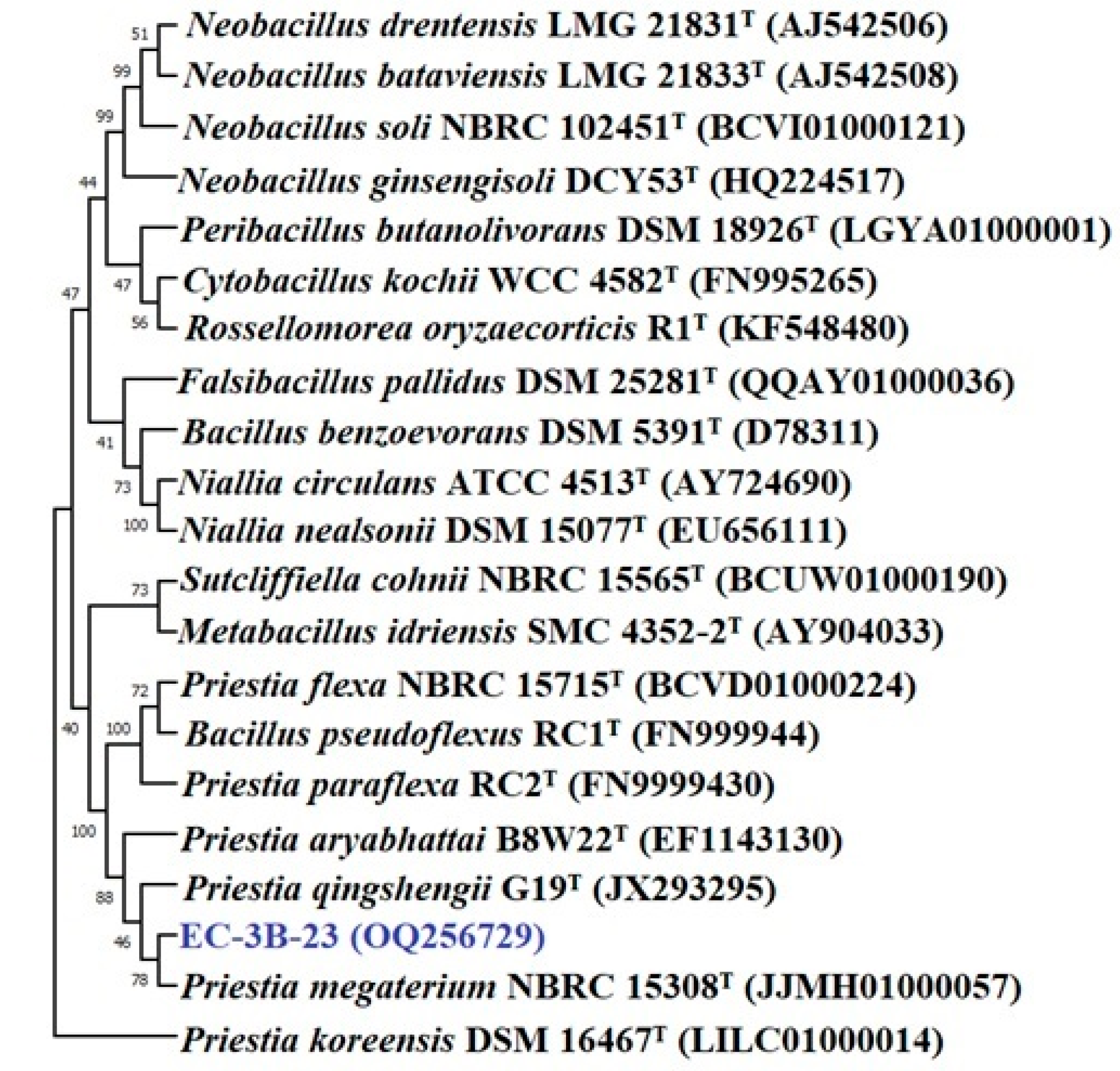
2.11. Identification of Selected Zinc Solubilizers
2.11.1. Morphological and Biochemical Characterization
Substrate Utilization Tests
16S rRNA Sequencing
2.12. Plant Experiment
2.13. Nutrient Analysis
2.14. Statistical Analysis
3. Results
3.1. Isolation of Endophytes
3.2. Endophytes Selection on the Basis of Zinc Solubilization
Phosphate Solubilization
3.3. Plant Growth-Promoting Attributes of Selected Zinc Solubilizers
3.3.1. IAA Production
3.3.2. HCN Production
3.3.3. Ammonia Production
3.3.4. Determination of Siderophore Production
3.3.5. Organic Acid Production
3.3.6. Extra Cellular Enzyme Assay
3.3.7. Stress Tolerance of Zinc Solubilizers
3.3.8. Characterization of Promising Isolates
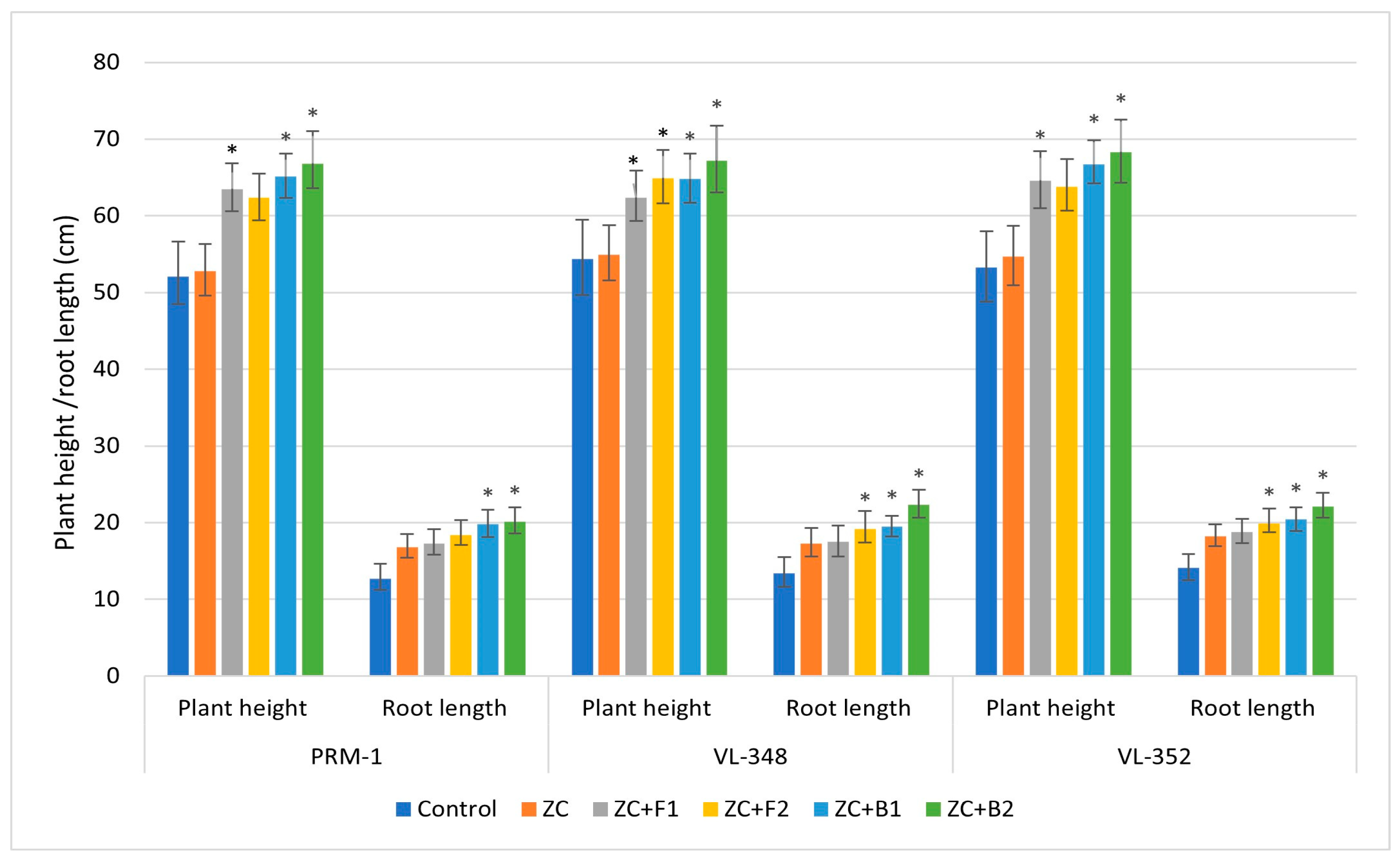
3.3.9. Plant Experiment
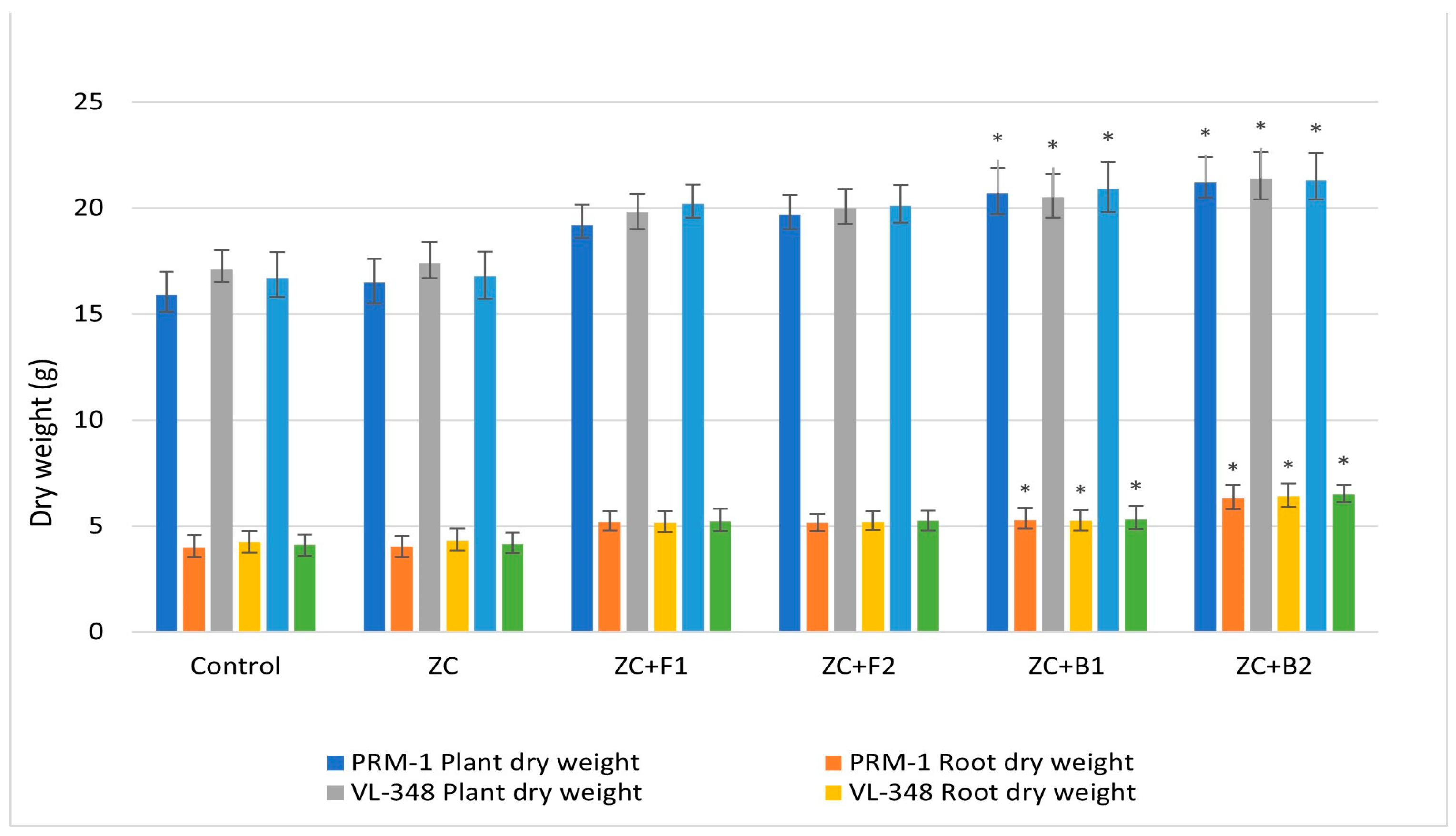
3.3.10. Plant Growth Parameters
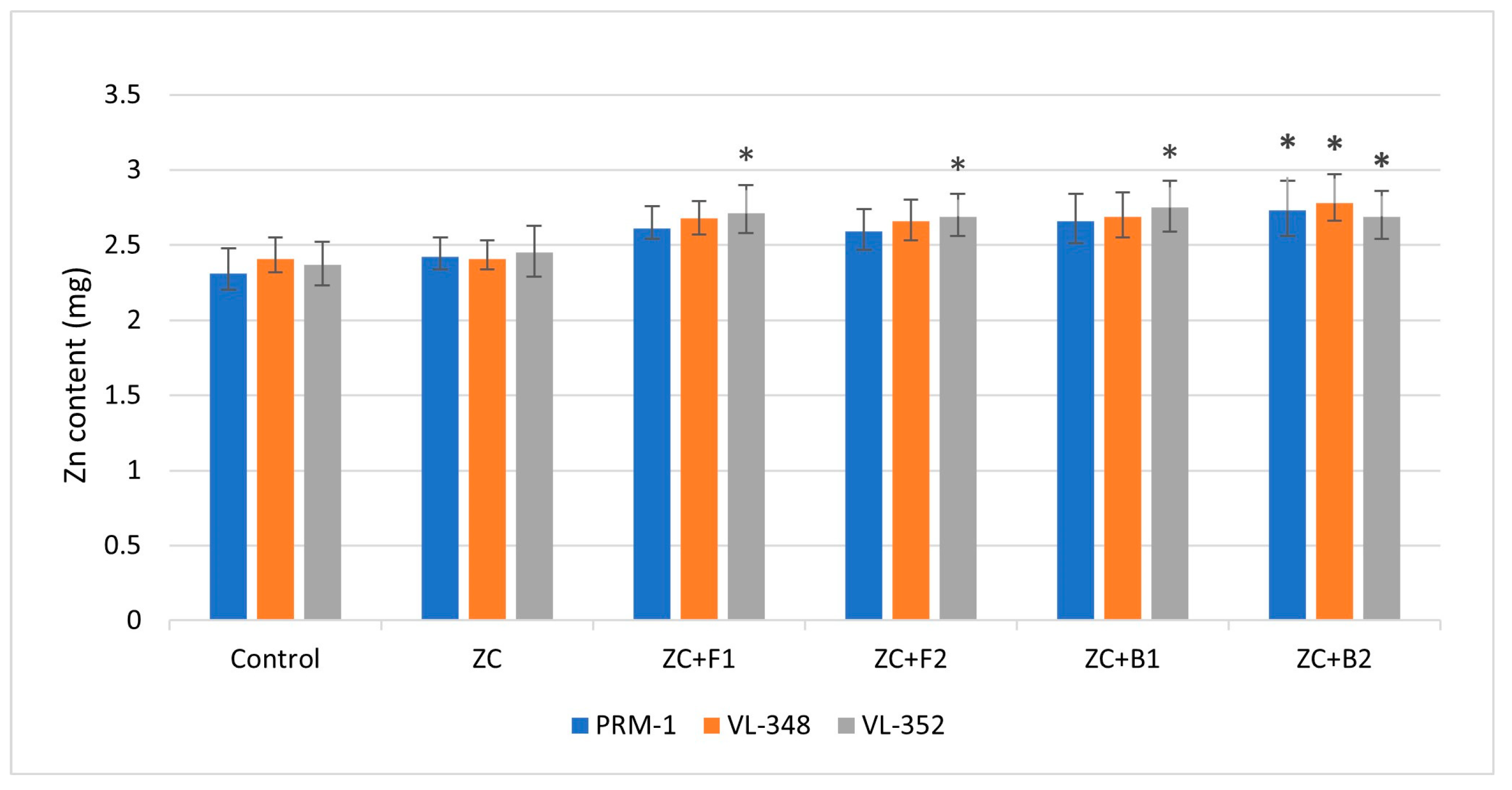
3.3.11. Zinc Content
3.3.12. NPK Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wambi, W.; Otienno, G.; Tumwesigye, W.; Mulumba, J. Genetic and genomic resources for finger millet improvement: Opportunities for advancing climate-smart agriculture. J. Crop Improv. 2020, 35, 204–233. [Google Scholar] [CrossRef]
- Maharajan, T.; Antony Ceasar, S.; Ajeesh Krishna, T.P.; Ignacimuthu, S. Finger millet [Eleusine coracana (L.) Gaertn]: An orphan crop with a potential to alleviate the calcium deficiency in the semi-arid tropics of Asia and Africa. Front. Sustain. Food Syst. 2021, 5, 258. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Anil Kumar, S.; Naravula, J.; Hima Kumari, P.; Kummari, D.; Guddimalli, R.; Edupuganti, S.; Karumanchi, A.R.; Venkatachalam, P.; Suravajhala, P.; et al. Improvement of small seed for big nutritional feed. Physiol. Mol. Biol. Plants 2021, 27, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Gebreyohannes, A.; Shimelis, H.; Laing, M.; Mathew, I.; Odeny, D.A.; Ojulong, H. Finger millet production in Ethiopia: Opportunities, problem diagnosis, key challenges and recommendations for breeding. Sustainability 2021, 13, 13463. [Google Scholar] [CrossRef]
- Rathore, T.; Rakhi Singh, I.; Dinkar Kamble, I.B.; Upadhyay, A.; Thangalakshmi, I.S.; Correspondence Rakhi Singh, I.; Singh, R.; Kamble, D.B.; Thangalakshmi, S. Review on finger millet: Processing and value addition. Pharma Innov. J. 2019, 8, 283–291. [Google Scholar]
- Choudhary, R.; Rawat, G.; Kumar, V.; Kumar, V. Diversity and function of microbes associated with rhizosphere of finger millet (Eleusine coracana). Rhizosphere Microbes Soil Plant Funct. 2020, 23, 431–451. [Google Scholar] [CrossRef]
- Govindaraj, M.; Kanatti, A.; Rai, K.N.; Pfeiffer, W.H.; Shivade, H. Association of grain iron and zinc content with other nutrients in pearl millet germplasm, breeding lines, and hybrids. Front. Nutr. 2022, 8, 1357. [Google Scholar] [CrossRef]
- Tahat, M.M.; Alananbeh, K.M.; Othman, Y.A.; Leskovar, D.I. Soil Health and Sustainable Agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Keesstra, S.D.; Bouma, J.; Wallinga, J.; Tittonell, P.; Smith, P.; Cerdà, A.; Montanarella, L.; Quinton, J.N.; Pachepsky, Y.; Van Der Putten, W.H.; et al. The significance of soils and soil science towards realization of the United Nations sustainable development goals. Soil 2016, 2, 111–128. [Google Scholar] [CrossRef]
- Keesstra, S.; Nunes, J.; Novara, A.; Finger, D.; Avelar, D.; Kalantari, Z.; Cerdà, A. The superior effect of nature based solutions in land management for enhancing ecosystem services. Sci. Total Environ. 2017, 610, 997–1009. [Google Scholar] [CrossRef]
- Keesstra, S.; Sannigrahi, S.; López-Vicente, M.; Pulido, M.; Novara, A.; Visser, S.; Kalantari, Z. The role of soils in regulation and provision of blue and green water. Philos. Trans. R. Soc. B 2021, 376, 1834. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.V. Micronutrient Nutritional Problems in Soils of India and Improvement for Human and Animal Health. Indian J. Fertil. 2009, 5, 11–56. [Google Scholar]
- Khan, S.T.; Malik, A.; Alwarthan, A.; Shaik, M.R. The Enormity of the Zinc Deficiency Problem and Available Solutions; an Overview. Arab. J. Chem. 2022, 15, 103668. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.S.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef]
- Hussain, A.; Jiang, W.; Wang, X.; Shahid, S.; Saba, N.; Ahmad, M.; Dar, A.; Masood, S.U.; Imran, M.; Mustafa, A. Mechanistic impact of zinc deficiency in human development. Front. Nutr. 2022, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Praharaj, S.; Skalicky, M.; Maitra, S.; Bhadra, P.; Shankar, T.; Brestic, M.; Hejnak, V.; Vachova, P.; Hossain, A. Zinc biofortification in food crops could alleviate the zinc malnutrition in human health. Molecules 2021, 26, 3509. [Google Scholar] [CrossRef]
- Kamran, S.; Shahid, I.; Baig, D.N.; Rizwan, M.; Malik, K.A.; Mehnaz, S. Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front. Microbiol. 2017, 8, 2593. [Google Scholar] [CrossRef]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front. Plant Sci. 2022, 13, 3970. [Google Scholar] [CrossRef]
- Kumar, K.; Verma, A.; Pal, G.; Anubha; White, J.F.; Verma, S.K. Seed endophytic bacteria of pearl millet (Pennisetum Glaucum L.) promote seedling development and defend against a fungal phytopathogen. Front. Microbiol. 2021, 12, 3660. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dewangan, S.; Lawate, P.; Bahadur, I.; Prajapati, S. Zinc-solubilizing bacteria: A boon for sustainable agriculture. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Singapore, 2019; Volume 12, pp. 139–155. [Google Scholar] [CrossRef]
- Singh, D.; Geat, N.; Rajawat, M.V.S.; Mahajan, M.M.; Prasanna, R.; Singh, S.; Kaushik, R.; Singh, R.N.; Kumar, K.; Saxena, A.K. deciphering the mechanisms of endophyte-mediated biofortification of Fe and Zn in wheat. J. Plant Growth Regul. 2018, 37, 174–182. [Google Scholar] [CrossRef]
- Othman, N.M.I.; Othman, R.; Zuan, A.T.K.; Shamsuddin, A.S.; Zaman, N.B.K.; Sari, N.A.; Panhwar, Q.A. isolation, characterization, and identification of zinc-solubilizing bacteria (ZSB) from wetland rice fields in Peninsular Malaysia. Agriculture 2022, 12, 1823. [Google Scholar] [CrossRef]
- Upadhayay, V.K.; Singh, A.V.; Khan, A.; Sharma, A. Contemplating the role of zinc-solubilizing bacteria in crop biofortification: An approach for sustainable bioeconomy. Front. Agron. 2022, 4, 72. [Google Scholar] [CrossRef]
- Upadhayay, V.K.; Singh, A.V.; Khan, A.; Singh, J.; Pareek, N.; Raghav, A. FE-SEM/EDX based zinc mobilization analysis of Burkholderia cepacia and Pantoea Rodasii and their functional annotation in crop productivity, soil quality, and zinc biofortification of paddy. Front. Microbiol. 2022, 13, 1149. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M.; Hussain, M. Improving the productivity, profitability and grain quality of kabuli chickpea with co-application of zinc and endophyte bacteria Enterobacter Sp. MN17. Arch. Agron. Soil Sci. 2019, 66, 897–912. [Google Scholar] [CrossRef]
- Yasmin, R.; Hussain, S.; Rasool, M.H.; Siddique, M.H.; Muzammil, S. Isolation, characterization of Zn solubilizing bacterium (Pseudomonas Protegens RY2) and its contribution in growth of chickpea (Cicer Arietinum L) as deciphered by improved growth parameters and Zn content. Dose-Response 2021, 19, 15593258211036791. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, X.; Zhang, X.; Dong, L.; Zhang, J.; Wei, Y.; Feng, Y.; Lu, L. Improved plant growth and Zn accumulation in grains of rice (Oryza Sativa L.) by inoculation of endophytic microbes isolated from a Zn hyperaccumulator, Sedum Alfredii H. J. Agric. Food Chem. 2014, 62, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Farooq, M.; Naveed, M.; Ozturk, L.; Nawaz, A.; Rehman, A.; Farooq, M.; Naveed, M.; Ozturk, L.; Nawaz, A. Pseudomonas-aided zinc application improves the productivity and biofortification of bread wheat. Crop. Pasture Sci. 2018, 69, 659–672. [Google Scholar] [CrossRef]
- Verma, S.; Chakdar, H.; Kumar, M.; Varma, A.; Saxena, A.K. Microorganisms as a sustainable alternative to traditional biofortification of iron and zinc: Status and prospect to combat hidden hunger. J. Soil Sci. Plant Nutr. 2021, 21, 1700–1717. [Google Scholar] [CrossRef]
- Fasim, F.; Ahmed, N.; Parsons, R.; Gadd, G.M. Solubilization of zinc salts by a bacterium isolated from the air environment of a Tannery. FEMS Microbiol. Lett. 2002, 213, 1–6. [Google Scholar] [CrossRef]
- Sunithakumari, K.; Padma Devi, S.N.; Vasandha, S. Zinc solubilizing bacterial isolates from the agricultural fields of Coimbatore, Tamil Nadu, India. Curr. Sci. 2016, 110, 196–205. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.; Yadav, A.; Giri, D.D.; Singh, P.K.; Pandey, K.D. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech 2016, 6, 60. [Google Scholar] [CrossRef]
- Lorck, H. Production of hydrocyanic acid by bacteria. Physiol. Plant. 1948, 1, 142–146. [Google Scholar] [CrossRef]
- Reetha, A.K.; Pavani, S.L.; Mohan, S. Hydrogen cyanide production ability by bacterial antagonist and their antibiotics inhibition potential on Macrophomina Phaseolina (Tassi.) Goid. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 172–178. [Google Scholar]
- Narula, N.; Gupta, K.G. Ammonia excretion by Azotobacter Chroococcum in liquid culture and soil in the presence of manganese and clay minerals. Plant Soil 1986, 93, 205–209. [Google Scholar] [CrossRef]
- Hu, Q.-P.; Xu, J.-G. A Simple Double-Layered Chrome Azurol S Agar (SD-CASA) Plate assay to optimize the production of siderophores by a potential biocontrol agent Bacillus. Afr. J. Microbiol. Res. 2011, 5, 4321–4327. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.M. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1993, 1, 66–69. [Google Scholar] [CrossRef]
- Mehta, S.; Nautiyal, C.S. An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr. Microbiol. 2001, 43, 51–56. [Google Scholar] [CrossRef]
- Dezam, A.P.G.; Vasconcellos, V.M.; Lacava, P.T.; Farinas, C.S. Microbial production of organic acids by endophytic fungi. Biocatal. Agric. Biotechnol. 2017, 11, 282–287. [Google Scholar] [CrossRef]
- Sierra, G. A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Van Leeuwenhoek 1957, 23, 15–22. [Google Scholar] [CrossRef]
- Ozturkoglu-Budak, S.; Wiebenga, A.; Bron, P.A.; de Vries, R.P. Protease and lipase activities of fungal and bacterial strains derived from an Artisanal Raw Ewe’s Milk Cheese. Int. J. Food Microbiol. 2016, 237, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Hankin, L.; Anagnostakis, S.L. The Use of Solid Media for Detection of Enzyme Production by Fungi. Mycologia 2018, 67, 597–607. [Google Scholar] [CrossRef]
- Pundir, R.K.; Rana, S.; Kaur, A.; Kashyap, N.; Jain, P. Bioprospecting Potential of Endophytic Bacteria Isolated from Indigenous Plants of Ambala (Haryana, India). Int. J. Pharm. Sci. Res. 2014, 5, 2309–2319. [Google Scholar]
- Manna, S.K.; Maitra, N.; Bandopadhyay, C.; Nandi, L.; Samanta, S. Phytate Mineralizing Bacteria and Their Phytase Activity from Different Matrices of Tropical Floodplain Wetlands. Explor. Anim. Med. Res. 2021, 11, 220–228. [Google Scholar] [CrossRef]
- Balan, S.S.; Nethaji, R.; Sankar, S.; Jayalakshmi, S. Production of Gelatinase Enzyme from Bacillus Spp Isolated from the Sediment Sample of Porto Novo Coastal Sites. Asian Pac. J. Trop. Biomed. 2012, 2, S1811–S1816. [Google Scholar] [CrossRef]
- Girdhari, S.N.; Peshwe, S.A. Isolation and Screening of Tannase Producing Fungi. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 765–774. [Google Scholar]
- Naik, B.; Goyal, S.K.; Tripathi, A.D.; Kumar, V. Exploring the Diversity of Endophytic Fungi and Screening for Their Pullulanase-Producing Capabilities. J. Genet. Eng. Biotechnol. 2021, 19, 110. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, G.; Sardar, B.; Singh, B.; Bharti, A.; Gusain, O.; Singh Bisht, G. An Improved Method for Isolation of Genomic DNA from Filamentous Actinomycetes. Int. J. Eng. Technol. Manag. Appl. Sci. 2010, 2, 2. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic. Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kimura, M. A Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Kumar, V.; Behl, R.K.; Narula, N. Establishment of Phosphate-Solubilizing Strains of Azotobacter Chroococcum in the Rhizosphere and Their Effect on Wheat Cultivars under Green House Conditions. Microbiol. Res. 2001, 156, 87–93. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis, Part 2; Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; IRRI: Los Baños, Philippines, 1971. [Google Scholar]
- Abdu, A.O.; De Groote, H.; Joy, E.J.M.; Kumssa, D.B.; Broadley, M.R.; Gashu, D. Zinc Agronomic Biofortification of Staple Crops May Be a Cost-Effective Strategy to Alleviate Zinc Deficiency in Ethiopia. Front. Nutr. 2022, 9, 2756. [Google Scholar] [CrossRef]
- Chattha, M.U.; Hassan, M.U.; Khan, I.; Chattha, M.B.; Mahmood, A.; Nawaz, M.; Subhani, M.N.; Kharal, M.; Khan, S. Biofortification of Wheat Cultivars to Combat Zinc Deficiency. Front. Plant Sci. 2017, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Nakandalage, N.; Nicolas, M.; Norton, R.M.; Hirotsu, N.; Milham, P.J.; Seneweera, S. Improving Rice Zinc Biofortification Success Rates through Genetic and Crop Management Approaches in a Changing Environment. Front. Plant Sci. 2016, 7, 764. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Zhang, W.; Liu, Y.M.; Chen, X.P.; Zou, C.Q. Soil Application of Zinc Fertilizer Increases Maize Yield by Enhancing the Kernel Number and Kernel Weight of Inferior Grains. Front. Plant Sci. 2020, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Geat, N.; Rajawat, M.V.S.; Prasanna, R.; Kar, A.; Singh, A.M.; Saxena, A.K. Prospecting Endophytes from Different Fe or Zn Accumulating Wheat Genotypes for Their Influence as Inoculants on Plant Growth, Yield, and Micronutrient Content. Ann. Microbiol. 2018, 68, 815–833. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic Microbes: Biodiversity, Plant Growth-Promoting Mechanisms and Potential Applications for Agricultural Sustainability. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2020, 113, 1075–1107. [Google Scholar] [CrossRef]
- Dinesh, R.; Srinivasan, V.; Hamza, S.; Sarathambal, C.; Anke Gowda, S.J.; Ganeshamurthy, A.N.; Gupta, S.B.; Aparna Nair, V.; Subila, K.P.; Lijina, A.; et al. Isolation and Characterization of Potential Zn Solubilizing Bacteria from Soil and Its Effects on Soil Zn Release Rates, Soil Available Zn and Plant Zn Content. Geoderma 2018, 321, 173–186. [Google Scholar] [CrossRef]
- Saravanan, V.S.; Madhaiyan, M.; Thangaraju, M. Solubilization of Zinc Compounds by the Diazotrophic, Plant Growth Promoting Bacterium Gluconacetobacter Diazotrophicus. Chemosphere 2007, 66, 1794–1798. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Inoculation of Zinc Solubilizing Bacillus Aryabhattai Strains for Improved Growth, Mobilization and Biofortification of Zinc in Soybean and Wheat Cultivated in Vertisols of Central India. Appl. Soil Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Bhuvaneswari, G. Effect of Inoculation of Am Fungi and Beneficial Microorganisms on Growth and Nutrient Uptake of Eleusine coracana (L.) Gaertn. (Finger Millet). Int. Lett. Nat. Sci. 2014, 13, 59–69. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An Ecofriendly Technology for Nutrient Recycling and Environmental Sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Marriel, I.E.; De Sousa, S.M.; Lana, U.G.D.P.; Mattos, B.B.; De Oliveira, C.A.; Gomes, E.A. Endophytic Bacillus Strains Enhance Pearl Millet Growth and Nutrient Uptake under Low-P. Braz. J. Microbiol. 2018, 49, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Shahzad, R.; Khan, A.L.; Kang, S.M.; Imran, Q.M.; Al-Harrasi, A.; Yun, B.W.; Lee, I.J. Endophytic Microbial Consortia of Phytohormones-Producing Fungus Paecilomyces Formosus Lhl10 and Bacteria Sphingomonas Sp. Lk11 to Glycine Max l. Regulates Physio-Hormonal Changes to Attenuate Aluminum and Zinc Stresses. Front. Plant Sci. 2018, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Agri, U.; Chaudhary, A.; Kumar, A.; Kumar, G. Endophytes and Their Potential in Biotic Stress Management and Crop Production. Front. Microbiol. 2022, 13, 933017. [Google Scholar] [CrossRef]
- Kumar, V.; Nautiyal, C.S. Plant Abiotic and Biotic Stress Alleviation: From an Endophytic Microbial Perspective. Curr. Microbiol. 2022, 79, 311. [Google Scholar] [CrossRef]
- Verma, A.; Shameem, N.; Jatav, H.S.; Sathyanarayana, E.; Parray, J.A.; Poczai, P.; Sayyed, R.Z. Fungal Endophytes to Combat Biotic and Abiotic Stresses for Climate-Smart and Sustainable Agriculture. Front. Plant Sci. 2022, 13, 2175. [Google Scholar] [CrossRef]
- Talboys, P.J.; Owen, D.W.; Healey, J.R.; Withers, P.J.A.; Jones, D.L. Auxin Secretion by Bacillus Amyloliquefaciens FZB42 Both Stimulates Root Exudation and Limits Phosphorus Uptake in Triticum Aestivium. BMC Plant Biol. 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Hwang, H.-H.; Chien, P.-R.; Huang, F.-C.; Yeh, P.-H.; Hung, S.-H.W.; Deng, W.-L.; Huang, C.-C.A.; Ramírez-Bahena, H.; Saati-Santamaría, Z.; Strobel, G.A.; et al. A Plant Endophytic Bacterium Priestia megaterium StrainBP-R2 Isolated from the Halophyte Bolboschoenus Planiculmis Enhances Plant Growth under Salt and Drought Stresses. Microorganisms 2022, 10, 2047. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I. Fluorescent Pseudomonas -FAP2 and Bacillus Licheniformis Interact Positively in Biofilm Mode Enhancing Plant Growth and Photosynthetic Attributes. Sci. Rep. 2019, 9, 4547. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Vargas, A.; Rodríguez-Gacha, L.M.; Sánchez-Castro, N.; Garzón-Jaramillo, R.; Pedroza-Camacho, L.D.; Poutou-Piñales, R.A.; Rivera-Hoyos, C.M.; Díaz-Ariza, L.A.; Pedroza-Rodríguez, A.M. Phosphate-Solubilizing Pseudomonas Sp., and Serratia Sp., Co-Culture for Allium cepa L. Growth Promotion. Heliyon 2020, 6, e05218. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.F.; Ashraf, A.; Muzammil, S.; Siddique, M.H.; Ali, T. Assessment of Zinc Solubilization Potential of Zinc-Resistant Pseudomonas Oleovorans Strain ZSB13 Isolated from Contaminated Soil. Braz. J. Biol. 2021, 83, e240015. [Google Scholar] [CrossRef]
- Shahid, M.; Zeyad, M.T.; Syed, A.; Singh, U.B.; Mohamed, A.; Bahkali, A.H.; Elgorban, A.M.; Pichtel, J. Stress-Tolerant Endophytic Isolate Priestia Aryabhattai BPR-9 Modulates Physio-Biochemical Mechanisms in Wheat (Triticum Aestivum L.) for Enhanced Salt Tolerance. Int. J. Environ. Res. Public Heal. 2022, 19, 10883. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Fadiji, A.E.; Ayilara, M.S.; Igiehon, O.N.; Nwachukwu, B.C.; Babalola, O.O. Strategies to Enhance the Use of Endophytes as Bioinoculants in Agriculture. Horticulturae 2022, 8, 498. [Google Scholar] [CrossRef]
- Kumar, N.; Dubey, R.C. Plant Growth Promoting Endophytic Bacteria Bacillus Australimaris BLR41 and Enterobacter Kobei BLR45 Enhance the Growth of Medicinal Plant Barleria Lupulina Lindl. J. Pure Appl. Microbiol. 2022, 16, 2647–2658. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc Solubilizing Bacillus Spp. Potential Candidates for Biofortification in Maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.; Maheshwari, D.K. Zinc Solubilizing Bacteria (Bacillus megaterium) with Multifarious Plant Growth Promoting Activities Alleviates Growth in Capsicum annuum L. 3 Biotech 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.; Srivastava, R.; Pandiyan, K.; Singh, A.; Chakdar, H.; Kashyap, P.L.; Bhardwaj, A.K.; Murugan, K.; Karthikeyan, N.; Bagul, S.Y.; et al. Enhancement in Plant Growth and Zinc Biofortification of Chickpea (Cicer arietinum L.) by Bacillus Altitudinis. J. Soil Sci. Plant Nutr. 2021, 21, 922–935. [Google Scholar] [CrossRef]
- Moreno-Lora, A.; Recena, R.; Delgado, A. Bacillus Subtilis QST713 and Cellulose Amendment Enhance Phosphorus Uptake While Improving Zinc Biofortification in Wheat. Appl. Soil Ecol. 2019, 142, 81–89. [Google Scholar] [CrossRef]
- Daniel, A.I.; Fadaka, A.O.; Gokul, A.; Bakare, O.O.; Aina, O.; Fisher, S.; Burt, A.F.; Mavumengwana, V.; Keyster, M.; Klein, A. Biofertilizer: The Future of Food Security and Food Safety. Microorganisms 2022, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.K.; Schwan, A.; Davidson, J.; Strange, P.; Liu, H.; Zhou, T.; Auzanneau, F.I.; Raizada, M.N. An Endophytic Fungus Isolated from Finger Millet (Eleusine coracana) Produces Anti-Fungal Natural Products. Front. Microbiol. 2015, 6, 1157. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.K.; Schwan, A.L.; Raizada, M.N. Characterization of Antifungal Natural Products Isolated from Endophytic Fungi of Finger Millet (Eleusine coracana). Molecules 2016, 21, 1171. [Google Scholar] [CrossRef] [PubMed]
- Prasannakumar, M.K.; Mahesh, H.B.; Desai, R.U.; Kunduru, B.; Narayan, K.S.; Teli, K.; Puneeth, M.E.; Rajadurai, R.C.; Parivallal, B.; Babu, G.V. Metagenome Sequencing of Fingermillet-Associated Microbial Consortia Provides Insights into Structural and Functional Diversity of Endophytes. 3 Biotech 2020, 10, 15. [Google Scholar] [CrossRef]


| Isolates | Zinc Solubilization | Phosphate Solubilization | ||
|---|---|---|---|---|
| Zinc Carbonate | Zinc Oxide | Zinc Phosphate | ||
| SI (mm) | SI (mm) | SI (mm) | SI (mm) | |
| Fungal isolates | ||||
| EC1F-15 | 3.0 ± 0.63 | 2.9 ± 0.52 | 2.6 ± 0.49 | 4.3 ± 0.74 |
| EC1F-23 | 3.3 ± 0.65 | 3.6 ± 0.61 | 3.1 ± 0.53 | 3.6 ± 0.68 |
| Bacterial isolates | ||||
| EC3B-22 | 4.3 ± 0.58 | 3.4 ± 0.71 | 2.1 ± 0.35 | 2.4 ± 0.63 |
| EC3B-23 | 4.8 ± 0.61 | 5.6 ± 0.82 | 2.3 ± 0.49 | 2.5 ± 0.56 |
| Endophytic Isolates | Siderophore Production | IAA Production (µg/mL) | HCN Production | Ammonia Production | Organic Acid Production | pH | ||
|---|---|---|---|---|---|---|---|---|
| Qualitative | Quantitative Percent Siderophore Unit (psu) | Qualitative | Quantitative (OD) | |||||
| Fungal isolates | ||||||||
| EC1F-15 | + | 63.7 ± 0.54 | 35.4 ± 0.46 | ++ | 0.031 ± 0.002 | − | + | 4.76 ± 0.13 |
| EC1F-23 | + | 60.6 ± 0.48 | 82.4 ± 0.74 | − | 0.009 ± 0.001 | − | + | 4.54 ± 0.18 |
| Bacterial isolates | ||||||||
| EC3B-22 | + | 23.74 ± 0.60 | 117.2 ± 0.67 | +++ | 0.043 ± 0.002 | − | + | 5.16 ± 0.12 |
| EC3B-23 | + | 71.8 ± 0.63 | 105.5 ± 0.59 | ++ | 0.029 ± 0.001 | + | + | 5.04 ± 0.16 |
| Isolates | Enzymes | ||||||
|---|---|---|---|---|---|---|---|
| Amylase | Lipase | Urease | Tannase | Gelatinase | Protease | Phytase | |
| Fungal isolate | |||||||
| EC1F-15 | + | + | + | − | − | − | − |
| EC1F-23 | + | + | − | + | + | + | + |
| Bacterial isolate | |||||||
| EC3B-22 | − | − | − | − | + | + | + |
| EC3B-23 | + | + | − | − | − | − | + |
| Endophytic Isolates | Fungal Isolates | Bacterial Isolates | ||
|---|---|---|---|---|
| EC1F-15 | EC1F-23 | EC3B-22 | EC3B-23 | |
| Growth at different pHs | ||||
| pH 4.0 | + | +++ | ++ | ++ |
| pH 5.0 | ++ | +++ | ++ | ++ |
| pH 6.0 | ++ | +++ | +++ | +++ |
| pH 7.0 | ++ | +++ | +++ | +++ |
| pH 8.0 | ++ | +++ | +++ | ++ |
| pH 9.0 | + | +++ | + | + |
| pH 10.0 | + | +++ | − | − |
| Growth at different temperatures | ||||
| 5 °C | − | − | − | − |
| 15 °C | ++ | ++ | +++ | +++ |
| 25 °C | +++ | +++ | +++ | +++ |
| 30 °C | +++ | +++ | +++ | +++ |
| 35 °C | ++ | +++ | ++ | +++ |
| 40 °C | + | + | + | ++ |
| 50 °C | − | + | − | ++ |
| Growth at different NaCl concentrations | ||||
| 0.5% | +++ | +++ | +++ | +++ |
| 1% | +++ | +++ | +++ | +++ |
| 2% | +++ | +++ | +++ | +++ |
| 3% | ++ | +++ | +++ | +++ |
| 4% | ++ | ++ | − | − |
| 5% | − | ++ | − | − |
| 6% | − | + | − | − |
| 7% | − | − | − | − |
| 8% | − | − | − | − |
| 9% | − | − | − | − |
| 10% | − | − | − | − |
| 11% | − | − | − | − |
| 12% | − | − | − | − |
| Isolates | Fungal Isolates | Bacterial Isolates | ||
|---|---|---|---|---|
| EC1F-15 | EC1F-23 | EC3B-22 | EC3B-23 | |
| Nitrogen source | ||||
| Peptone | +++ | +++ | +++ | +++ |
| Yeast extract | +++ | +++ | +++ | ++ |
| Beef extract | +++ | +++ | +++ | +++ |
| Ammonium bromide | +++ | +++ | +++ | +++ |
| Ammonium thiocyanate | +++ | +++ | − | − |
| Ammonium persulphate | +++ | +++ | +++ | +++ |
| Ammonium vanadate | +++ | +++ | − | − |
| Ammonium bicarbonate | +++ | +++ | +++ | +++ |
| Ammonium acetate | ++ | +++ | ++ | +++ |
| Ammonium molybdate | +++ | +++ | +++ | +++ |
| Ammonium dichromate | +++ | ++ | +++ | +++ |
| Ammonium ferrous sulphate | +++ | +++ | +++ | +++ |
| Carbon source | ||||
| Glucose | +++ | ++ | +++ | +++ |
| Fructose | ++ | ++ | +++ | +++ |
| Lactose | ++ | + | + | +++ |
| Inositol | + | + | +++ | +++ |
| Maltose | ++ | ++ | + | +++ |
| Malt extract | +++ | +++ | +++ | +++ |
| Sorbitol | + | ++ | +++ | +++ |
| Mannitol | ++ | − | +++ | +++ |
| Galactose | +++ | + | +++ | − |
| Arabinose | + | +++ | +++ | − |
| Rhamnose | ++ | ++ | + | − |
| Ribose | ++ | ++ | − | − |
| Trehalose | ++ | − | − | − |
| Sucrose | +++ | +++ | +++ | +++ |
| Mannose | ++ | − | +++ | − |
| Dextrin | +++ | +++ | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, R.; Kumar, V.; Gupta, S.; Naik, B.; Prasad, R.; Mishra, S.; Saris, P.E.J.; Kumar, V. Finger Millet (Eleusine coracana) Plant–Endophyte Dynamics: Plant Growth, Nutrient Uptake, and Zinc Biofortification. Microorganisms 2023, 11, 973. https://doi.org/10.3390/microorganisms11040973
Chaudhary R, Kumar V, Gupta S, Naik B, Prasad R, Mishra S, Saris PEJ, Kumar V. Finger Millet (Eleusine coracana) Plant–Endophyte Dynamics: Plant Growth, Nutrient Uptake, and Zinc Biofortification. Microorganisms. 2023; 11(4):973. https://doi.org/10.3390/microorganisms11040973
Chicago/Turabian StyleChaudhary, Renu, Vijay Kumar, Sanjay Gupta, Bindu Naik, Ram Prasad, Sadhna Mishra, Per Erik Joakim Saris, and Vivek Kumar. 2023. "Finger Millet (Eleusine coracana) Plant–Endophyte Dynamics: Plant Growth, Nutrient Uptake, and Zinc Biofortification" Microorganisms 11, no. 4: 973. https://doi.org/10.3390/microorganisms11040973
APA StyleChaudhary, R., Kumar, V., Gupta, S., Naik, B., Prasad, R., Mishra, S., Saris, P. E. J., & Kumar, V. (2023). Finger Millet (Eleusine coracana) Plant–Endophyte Dynamics: Plant Growth, Nutrient Uptake, and Zinc Biofortification. Microorganisms, 11(4), 973. https://doi.org/10.3390/microorganisms11040973







