Antimicrobial Photodynamic Therapy against Escherichia coli and Staphylococcus aureus Using Nanoemulsion-Encapsulated Zinc Phthalocyanine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemical Materials
- Photosensitizer: Zn phthalocyanine was purchased from Sigma Aldrich Co., Ltd. (St. Louis, MI, USA);
- Solvents:
- High-performance liquid chromatography (HPLC) Acetone was purchased from (Loba Chemie laboratory reagents and fine chemicals, Murud, India);
- Tetrahydrofuran (THF) was purchased from (Al-Gomhoria Company for Trade in Medicines, Chemicals, and Medical Supplies, Cairo, Egypt);
- Essential Oil: Miglyol 812N was gifted from Cremer oleo division, Germany;
- Biopolymer surfactants: Poloxamer 188 was purchased from (Sigma Aldrich Co.);
- Lipophilic surfactant: Span 80 was purchased from (Sigma Aldrich Co.);
- Hydrophilic surfactant: Tween 80 was purchased from (Al-Gomhoria Company for Trade in Medicines, Chemicals and Medical Supplies, Cairo, Egypt).
2.1.2. Bacteria Strain and Culture Preparation
2.1.3. Equipment
- The MICROTRAC MRB instrument (Prague, Czechia) at the NAWAH scientific center, Egypt, was used to measure the particle size distribution (mean size), polydispersity index (PdI), zeta potential and viscosity;
- The Transmission Electron Microscope (TEM) (JEM-HR-2100, Tokyo, Japan) at the National Research Centre (NRC), Cairo, Egypt, was used to characterize the structure and morphology of the nanoemulsion (NE) and Zn Phthalocyanine encapsulated in NE (NE + ZnPc). The total magnification was 8.00 kx, and the accelerating voltage was 200 kV;
- A light system composed of a red diode light (Programmable LED, 600–700 nm) at the National Institute of Laser Enhanced Science (NILES), Cairo University, Egypt, was used to irradiate the photosensitizer;
- The water-miscible solvents (acetone and Tetra Hydro Furan) were evaporated in a rotary evaporator device at the National Research Centre, Cairo, Egypt, after complete homogenization of the nanoemulsion and the nanoemulsion with phthalocyanine;
- The Vortex Heidolph reax top (Schwabach, Germany) at the National Research Centre, Cairo, Egypt, was used to mix every mixture thoroughly.
2.2. Methods
2.2.1. Preparation of Nanoemulsion
2.2.2. Preparation of Zn Phthalocyanine Encapsulated in the Nanoemulsion
2.2.3. Physicochemical Characterization of NE) and NE + ZnPc
2.2.4. Morphological Characterization of Nanoemulsion (NE) and Zn Phthalocyanine Encapsulated in Nanoemulsion NE + ZnPc
2.2.5. Bacteria Strain and Culture Preparation
2.2.6. The Effect of the Red Diode Light on S. aureus and E. coli
2.2.7. The Safety of Nanoemulsion on S. aureus and E. coli without Zn Phthalocyanine
2.2.8. Safety of Zn Phthalocyanine Encapsulated in Nanoemulsion (NE + ZnPc) Only on S. aureus and E. coli
2.2.9. Photodynamic Therapy against S. aureus and E. coli
2.2.10. The Effect of the Incubation Time before the Photodynamic Therapy for S. aureus and E. coli
3. Results
3.1. Preparation of Nanoemulsion and Zn Phthalocyanine Encapsulated in Nanoemulsion
3.2. The Morphological Characterization of Nanoemulsion (NE) and the Zn Phthalocyanine Encapsulated in Nanoemulsion NE + ZnPc
3.3. Physicochemical Characterization of Nanoemulsion (NE) and Zn Phthalocyanine Encapsulated in (NE + ZnPc)
3.4. The Effect of the Red Diode Light Only on S. aureus and E. coli
3.5. Safety Evaluation of NE and NE + ZnPc on S. aureus and E. coli
3.6. Photodynamic Therapy against S. aureus and E. coli
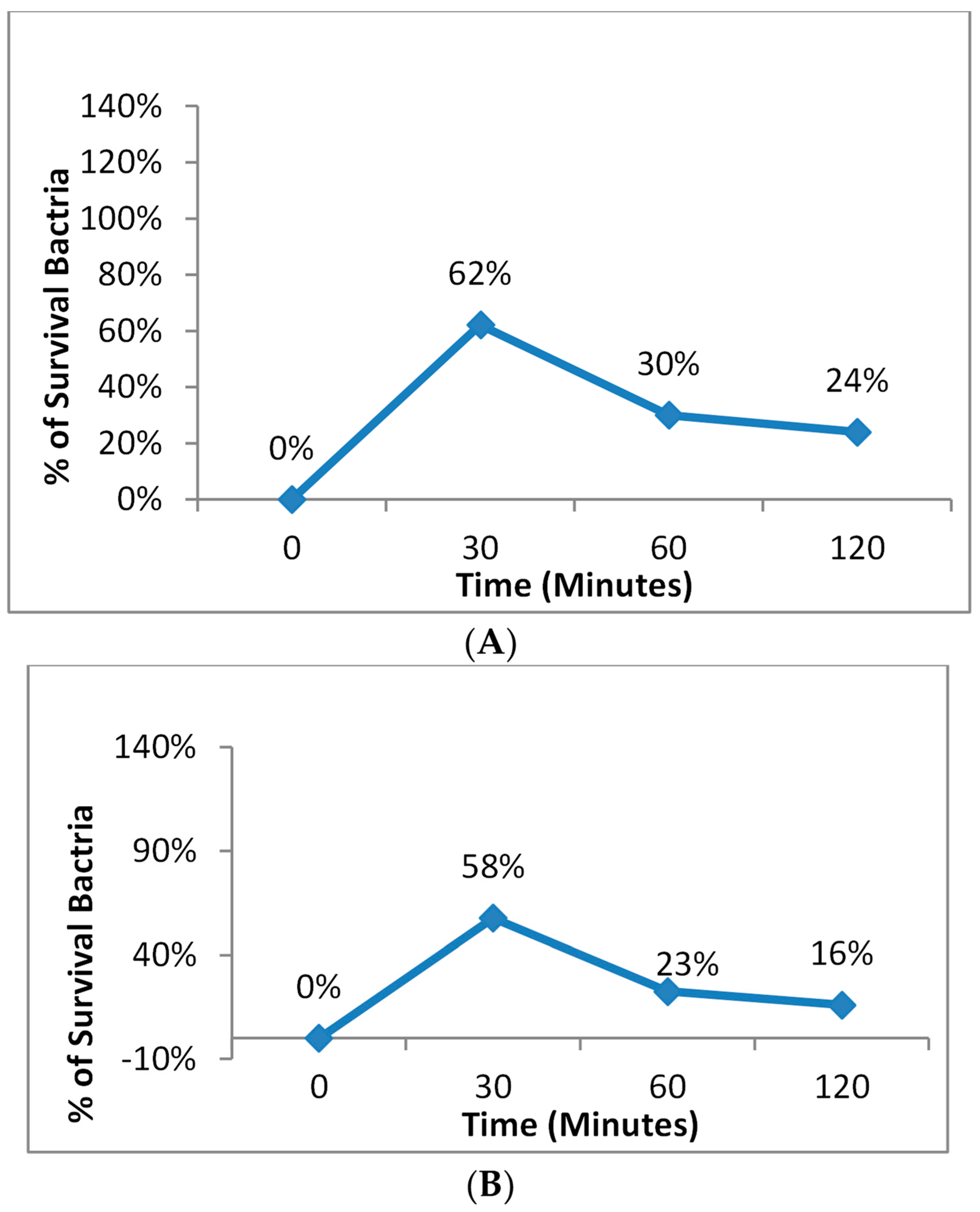
3.7. The Effect of Incubation Time before Photodynamic Therapy for S. aureus and E. coli
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deyno, S.; Fekadu, S.; Astatkie, A. Resistance of Staphylococcus aureus to Antimicrobial Agents in Ethiopia: A Meta-Analysis. Antimicrob. Resist. Infect. Control 2017, 6, 85. [Google Scholar] [CrossRef]
- Alarcon, A.; Omenaca, F. Antimicrobial Resistance in Escherichia coli Sepsis. Pediatr. Infect. Dis. J. 2004, 23, 979–980. [Google Scholar] [CrossRef]
- Chambers, H.F.; DeLeo, F.R. Waves of Resistance: Staphylococcus aureus in the Antibiotic Era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef]
- Klemm, E.J.; Wong, V.K.; Dougan, G. Emergence of Dominant Multidrug-Resistant Bacterial Clades: Lessons from History and Whole-Genome Sequencing. Proc. Natl. Acad. Sci. USA 2018, 115, 12872–12877. [Google Scholar] [CrossRef]
- Nyamu, S.N.; Ombaka, L.; Masika, E.; Ng’ang’a, M. Antimicrobial Photodynamic Activity of Phthalocyanine Derivatives. Adv. Chem. 2018, 2018, 2598062. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Kill Gram-Negative Bacteria. Recent Pat. Anti-Infect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Liu, D.; Li, L.; Chen, J.; Chen, Z.; Jiang, L.; Yuan, C.; Huang, M. Dissociation of Zinc Phthalocyanine Aggregation on Bacterial Surface Is Key for Photodynamic Antimicrobial Effect. J. Porphyr. Phthalocyanines 2018, 22, 925–934. [Google Scholar] [CrossRef]
- Bekmukhametova, A.; Ruprai, H.; Hook, J.M.; Mawad, D.; Houang, J.; Lauto, A. Photodynamic Therapy with Nanoparticles to Combat Microbial Infection and Resistance. Nanoscale 2020, 12, 21034–21059. [Google Scholar] [CrossRef]
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial Photodynamic Therapy for Inactivation of Biofilms Formed by Oral Key Pathogens. Front. Microbiol. 2014, 5, 405. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Wojtunik-Kulesza, K.A.; Oniszczuk, T.; Kasprzak, K. The Potential of Photodynamic Therapy (PDT)—Experimental Investigations and Clinical Use. Biomed. Pharmacother. 2016, 83, 912–929. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef]
- Dharmaratne, P.; Sapugahawatte, D.N.; Wang, B.; Chan, C.L.; Lau, K.M.; Lau, C.; Fung, K.P.; Ng, D.K.; IP, M. Contemporary Approaches and Future Perspectives of Antibacterial Photodynamic Therapy (APDT) against Methicillin-Resistant Staphylococcus aureus (MRSA): A Systematic Review. Eur. J. Med. Chem. 2020, 200, 112341. [Google Scholar] [CrossRef]
- Almeida, A.; Faustino, M.A.F.; Neves, M.G.P.M.S. Antimicrobial Photodynamic Therapy in the Control of COVID-19. Antibiotics 2020, 9, 320. [Google Scholar] [CrossRef]
- Siqueira-Moura, M.P.; Primo, F.L.; Peti, A.P.F.; Tedesco, A.C. Validated Spectrophotometric and Spectrofluorimetric Methods for Determination of Chloroaluminum Phthalocyanine in Nanocarriers. Pharmazie 2010, 65, 9–14. [Google Scholar] [CrossRef]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic Therapy for Infections: Clinical Applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef]
- Sekkat, N.; Van Den Bergh, H.; Nyokong, T.; Lange, N. Like a Bolt from the Blue: Phthalocyanines in Biomedical Optics. Molecules 2012, 17, 98–144. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Bernegossi, J.; De Freitas, L.M.; Fontana, C.R.; Chorilli, M.; Grumezescu, A.M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Alexeree, S.M.I.; Sliem, M.A.; EL-Balshy, R.M.; Amin, R.M.; Harith, M.A. Exploiting Biosynthetic Gold Nanoparticles for Improving the Aqueous Solubility of Metal-Free Phthalocyanine as Biocompatible PDT Agent. Mater. Sci. Eng. C 2017, 76, 727–734. [Google Scholar] [CrossRef]
- Da Costa, S.; Basri, M.; Shamsudin, N.; Basri, H. Stability of Positively Charged Nanoemulsion Formulation Containing Steroidal Drug for Effective Transdermal Application. J. Chem. 2014, 2014, 748680. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Influence of Surfactant and Processing Conditions in the Stability of Oil-in-Water Nanoemulsions. J. Food Eng. 2015, 167, 89–98. [Google Scholar] [CrossRef]
- Bouchemal, K.; Briançon, S.; Perrier, E.; Fessi, H. Nano-Emulsion Formulation Using Spontaneous Emulsification: Solvent, Oil and Surfactant Optimisation. Int. J. Pharm. 2004, 280, 241–251. [Google Scholar] [CrossRef]
- Rodrigues, G.B.; Primo, F.L.; Tedesco, A.C.; Braga, G.U.L. In Vitro Photodynamic Inactivation of Cryptococcus neoformans Melanized Cells with Chloroaluminum Phthalocyanine Nanoemulsion. Photochem. Photobiol. 2012, 88, 440–447. [Google Scholar] [CrossRef]
- Dong-Hyun Lee, M.D.; Eun-Ha Koh, M.D.; Sae-Rom Choi, M.T.; Sunjoo Kim, M.D. Growth Dynamics of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa as a Function of Time to Detection in BacT/Alert 3D Blood Culture Bottles with Various Preincubation Conditions. Ann. Lab. Med. 2013, 33, 406–409. [Google Scholar]
- Ribeiro, A.P.D.; Andrade, M.C.; Bagnato, V.S.; Vergani, C.E.; Primo, F.L.; Tedesco, A.C.; Pavarina, A.C. Antimicrobial Photodynamic Therapy against Pathogenic Bacterial Suspensions and Biofilms Using Chloro-Aluminum Phthalocyanine Encapsulated in Nanoemulsions. Lasers Med. Sci. 2015, 30, 549–559. [Google Scholar] [CrossRef]
- Gubta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 11, 2826–2841. [Google Scholar] [CrossRef]
- Tan, S.Y.; Tatsumura, Y. Alexander Fleming (1881–1955): Discoverer of Penicillin. Singapore Med. J. 2015, 56, 366–367. [Google Scholar] [CrossRef]
- Davies, J. Where Have All the Raises Gone? Can. J. Infect. Dis. Med. Microbiol. 2006, 17, 287–290. [Google Scholar] [CrossRef]
- Hurley, J.C.; Cosgrove, S.E.; Carmeli, Y. Comparison of Mortality Associated with Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus Bacteremia: An Ecological Analysis (Multiple Letters). Clin. Infect. Dis. 2003, 37, 866–869. [Google Scholar] [CrossRef]
- Weigel, L.M.; Anderson, G.J.; Facklam, R.R.; Tenover, F.C. Genetic Analyses of Mutations Contributing to Fluoroquinolone Resistance in Clinical Isolates of Streptococcus Pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 3517–3523. [Google Scholar] [CrossRef]
- Maisch, T.; Hackbarth, S.; Regensburger, J.; Felgenträger, A.; Bäumler, W.; Landthaler, M.; Röder, B. Photodynamic Inactivation of Multi-Resistant Bacteria (PIB)—A New Approach to Treat Superficial Infections in the 21st Century. JDDG-J. Ger. Soc. Dermatol. 2011, 9, 360–366. [Google Scholar] [CrossRef]
- Pereira Rosa, L. Antimicrobial Photodynamic Therapy: A New Therapeutic Option to Combat Infections. J. Med. Microbiol. Diagn. 2014, 3, 1. [Google Scholar] [CrossRef]
- Dharmaratne, P.; Wang, B.; Wong, R.C.H.; Chan, B.C.L.; Lau, K.M.; Ke, M.R.; Lau, C.B.S.; Ng, D.K.P.; Fung, K.P.; Ip, M. Monosubstituted Tricationic Zn (II) Phthalocyanine Enhances Antimicrobial Photodynamic Inactivation (APDI) of Methicillin-Resistant Staphylococcus aureus (MRSA) and Cytotoxicity Evaluation for Topical Applications: In Vitro and in Vivo Study. Emerg. Microbes Infect. 2020, 9, 1628–1637. [Google Scholar] [CrossRef]
- Sessler, J.L.; Mody, T.D.; Hemmi, G.W. Phthalocyanine-based molecular materials. Chem. Soc. Rev. 1996, 25, 43–51. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef]
- Mantareva, V.; Angelov, I.P.; Kussovski, V.; Wöhrle, D.; Dimitrov, S. Metallophthalocyanines as photodynamic sensitizers for treatment of pathogenic bacteria. Synthesis and singlet oxygen formation. Comptes Rendus De L’Académie Bulg. Des Sci. Sci. Mathématiques Et Nat. 2009, 62, 1521–1526. [Google Scholar]
- Staicu, A.; Pascu, A.; Nuta, A.; Sorescu, A.; Raditoiu, V.; Pascu, M.L. Studies about Phthalocyanine Photosensitizers to Be Used in Photodynamic Therapy. Rom. Rep. Phys. 2013, 65, 1032–1051. [Google Scholar]
- Tunçel, A.; Öztörk, S.; Ince, M.; Ocakoglu, K.; Hoşgör-Limoncu, M.; Yurt, F. Antimicrobial Photodynamic Therapy against Staphylococcus aureus Using Zinc Phthalocyanine and Zinc Phthalocyanine-Integrated TiO2 Nanoparticles. J. Porphyr. Phthalocyanines 2019, 23, 206–212. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.D.; Brunetti, I.L.; Costa, C.A.D.S.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the Photodynamic Effects of Curcumin against Candida Albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef]
- Setya, S.; Talegaonkar, S.; Razdan, B.K. Nanoemulsions: Formulation Methods and Stability Aspects. World J. Pharm. Pharm. Sci. 2014, 3, 2214–2228. [Google Scholar]
- De Paula, C.S.; Tedesco, A.C.; Primo, F.L.; Vilela, J.M.C.; Andrade, M.S.; Mosqueira, V.C.F. Chloroaluminium Phthalocyanine Polymeric Nanoparticles as Photosensitisers: Photophysical and Physicochemical Characterisation, Release and Phototoxicity in Vitro. Eur. J. Pharm. Sci. 2013, 49, 371–381. [Google Scholar] [CrossRef]
- Gurpreet, K.; Singh, S.K. Review of Nanoemulsion Formulation and Characterization Techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Primo, F.L.; Rodrigues, M.M.A.; Simioni, A.R.; Bentley, M.V.L.B.; Morais, P.C.; Tedesco, A.C. In Vitro Studies of Cutaneous Retention of Magnetic Nanoemulsion Loaded with Zinc Phthalocyanine for Synergic Use in Skin Cancer Treatment. J. Magn. Magn. Mater. 2008, 320, e211–e214. [Google Scholar] [CrossRef]
- Baboota, S.; Shakeel, F.; Ahuja, A.; Ali, J.; Shafiq, S. Design, Development and Evaluation of Novel Nanoemulsion Formulations for Transdermal Potential of Celecoxib. Acta Pharm. 2007, 57, 315–332. [Google Scholar] [CrossRef]
- García Vior, M.C.; Monteagudo, E.; Dicelio, L.E.; Awruch, J. A Comparative Study of a Novel Lipophilic Phthalocyanine Incorporated into Nanoemulsion Formulations: Photophysics, Size, Solubility and Thermodynamic Stability. Dye. Pigment. 2011, 91, 208–214. [Google Scholar] [CrossRef]
- Schuenck-Rodrigues, R.A.; de Oliveira de Siqueira, L.B.; dos Santos Matos, A.P.; da Costa, S.P.; da Silva Cardoso, V.; Vermelho, A.B.; Colombo, A.P.V.; Oliveira, C.A.; Santos-Oliveira, R.; Ricci-Júnior, E. Development, Characterization and Photobiological Activity of Nanoemulsion Containing Zinc Phthalocyanine for Oral Infections Treatment. J. Photochem. Photobiol. B Biol. 2020, 211, 112010. [Google Scholar] [CrossRef]
- Primo, F.L.; Da Costa Reis, M.B.; Porcionatto, M.A.; Tedesco, A.C. In Vitro Evaluation of Chloroaluminum Phthalocyanine Nanoemulsion and Low-Level Laser Therapy on Human Skin Dermal Equivalents and Bone Marrow Mesen-Chymal Stem Cells. Curr. Med. Chem. 2011, 18, 3376–3381. [Google Scholar] [CrossRef]
- Kamel, F.H.; Saeed, C.H.; Hassan, N.I. Comparative Effect of Different Visible Light Energy on Bacterial Growth. Int. J. Adv. Res. 2016, 4, 263–270. [Google Scholar]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic Therapy in the Treatment of Microbial Infections: Basic Principles and Perspective Applications. Lasers Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef]
- Seven, O.; Dindar, B.; Aydemir, S.; Cilli, F. Synthesis, Properties and Photodynamic Activities of Some Zinc (II) Phthalocyanines against Escherichia coli and Staphylococcus aureus. J. Porphyr. Phthalocyanines 2008, 12, 953–963. [Google Scholar] [CrossRef]
- Ryskova, L.; Buchta, V.; Karaskova, M.; Rakusan, J.; Cerny, J.; Slezak, R. In Vitro Antimicrobial Activity of Light-Activated Phthalocyanines. Cent. Eur. J. Biol. 2012, 8, 168–177. [Google Scholar] [CrossRef]
- Dimaano, M.L.; Rozario, C.; Nerandzic, M.M.; Donskey, C.J.; Lam, M.; Baron, E.D. The Photodynamic Antibacterial Effects of Silicon Phthalocyanine (Pc) 4. Int. J. Mol. Sci. 2015, 16, 7851–7860. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Wang, D.; Li, L.; Zhou, S.; Huang, J.H.; Chen, J.; Hu, P.; Huang, M. Photodynamic Antimicrobial Chemotherapy Using Zinc Phthalocyanine Derivatives in Treatment of Bacterial Skin Infection. J. Biomed. Opt. 2016, 21, 018001. [Google Scholar] [CrossRef]
- Cabral, Á.S.; Leonel, E.C.R.; Candido, N.M.; Piva, H.L.; de Melo, M.T.; Taboga, S.R.; Rahal, P.; Tedesco, A.C.; Calmon, M.F. Combined Photodynamic Therapy with Chloroaluminum Phthalocyanine and Doxorubicin Nanoemulsions in Breast Cancer Model. J. Photochem. Photobiol. B Biol. 2021, 218, 112181. [Google Scholar] [CrossRef]
- da Costa, V.G.; Moreli, M.L.; Saivish, M.V. The Emergence of SARS, MERS and Novel SARS-2 Coronaviruses in the 21st Century. Arch. Virol. 2020, 165, 1517–1526. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef]
- Svyatchenko, V.A.; Nikonov, S.D.; Mayorov, A.P.; Gelfond, M.L.; Loktev, V.B. Since January 2020 Elsevier Has Created a COVID-19 Resource Centre with Free Information in English and Mandarin on the Novel Coronavirus COVID-19. The COVID-19 Resource Centre Is Hosted on Elsevier Connect, the Company’s Public News and Information. 2020. Available online: https://cir.nii.ac.jp/crid/1370576118741734285 (accessed on 1 January 2020).
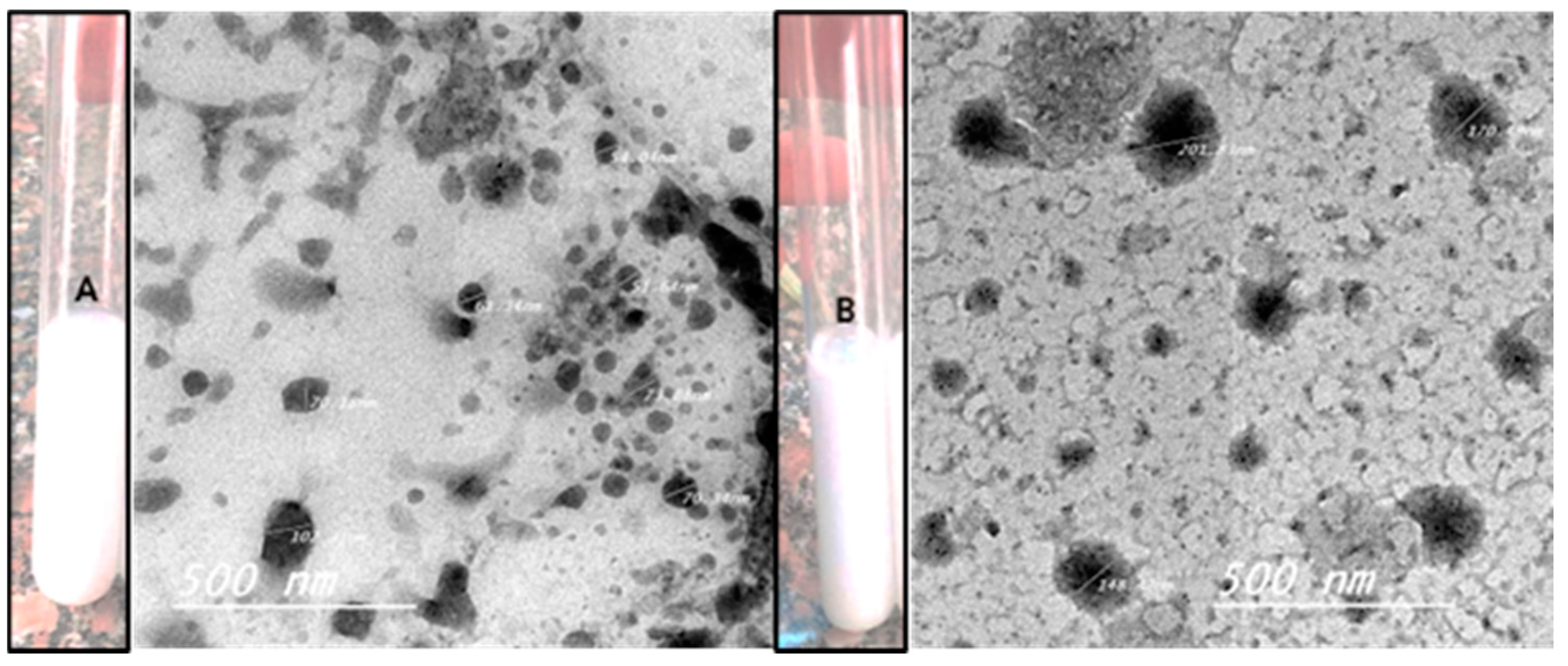
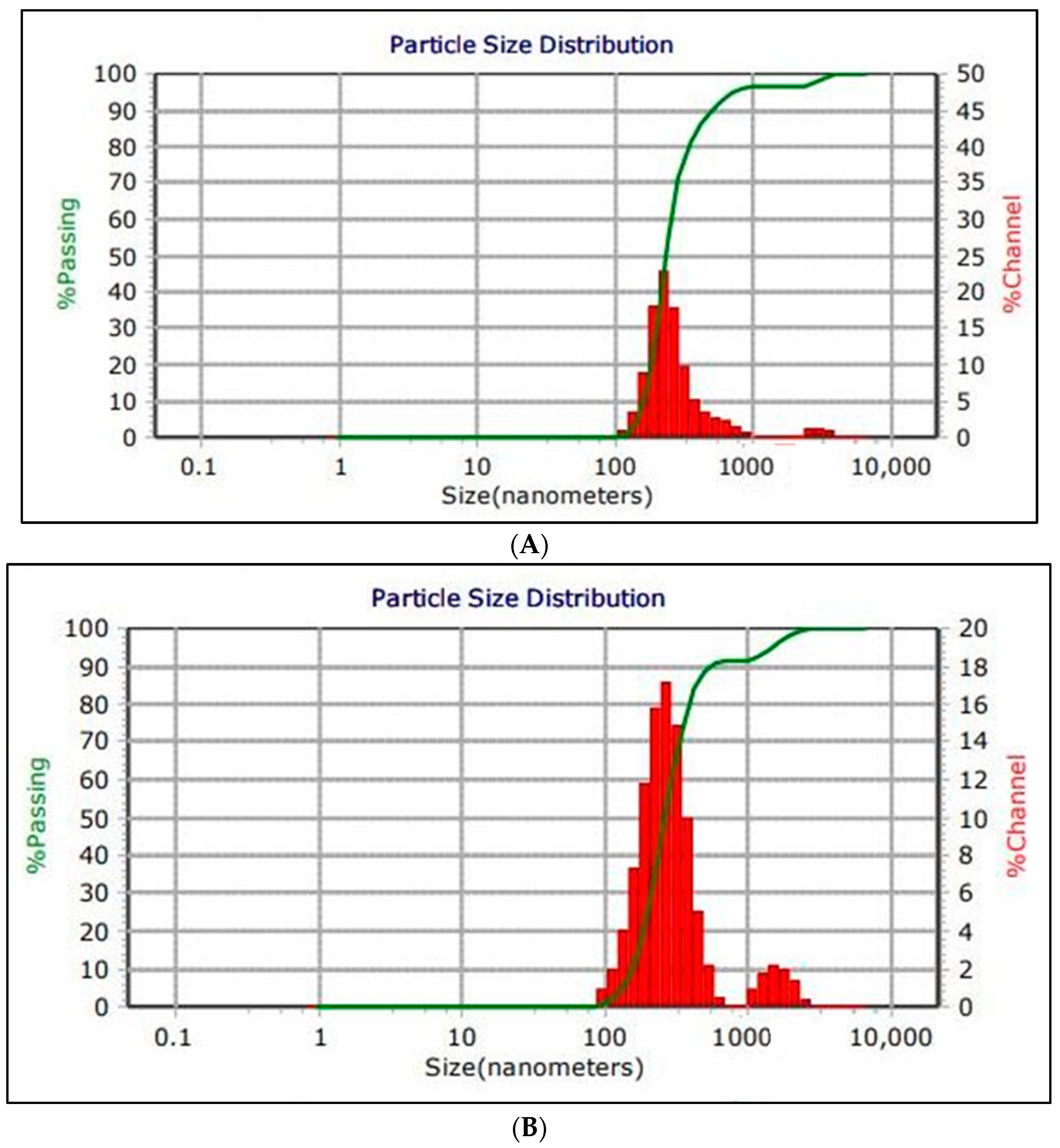
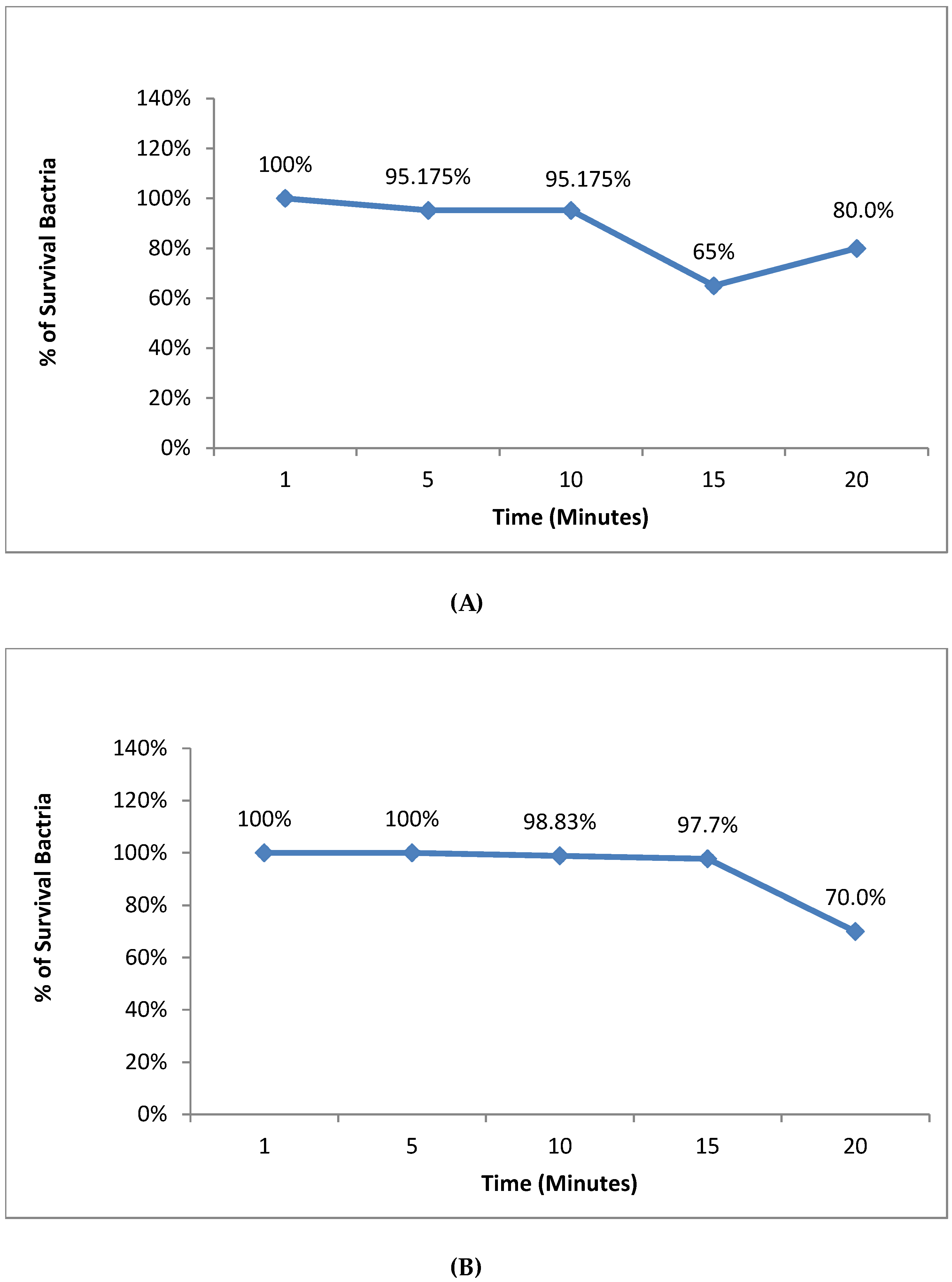


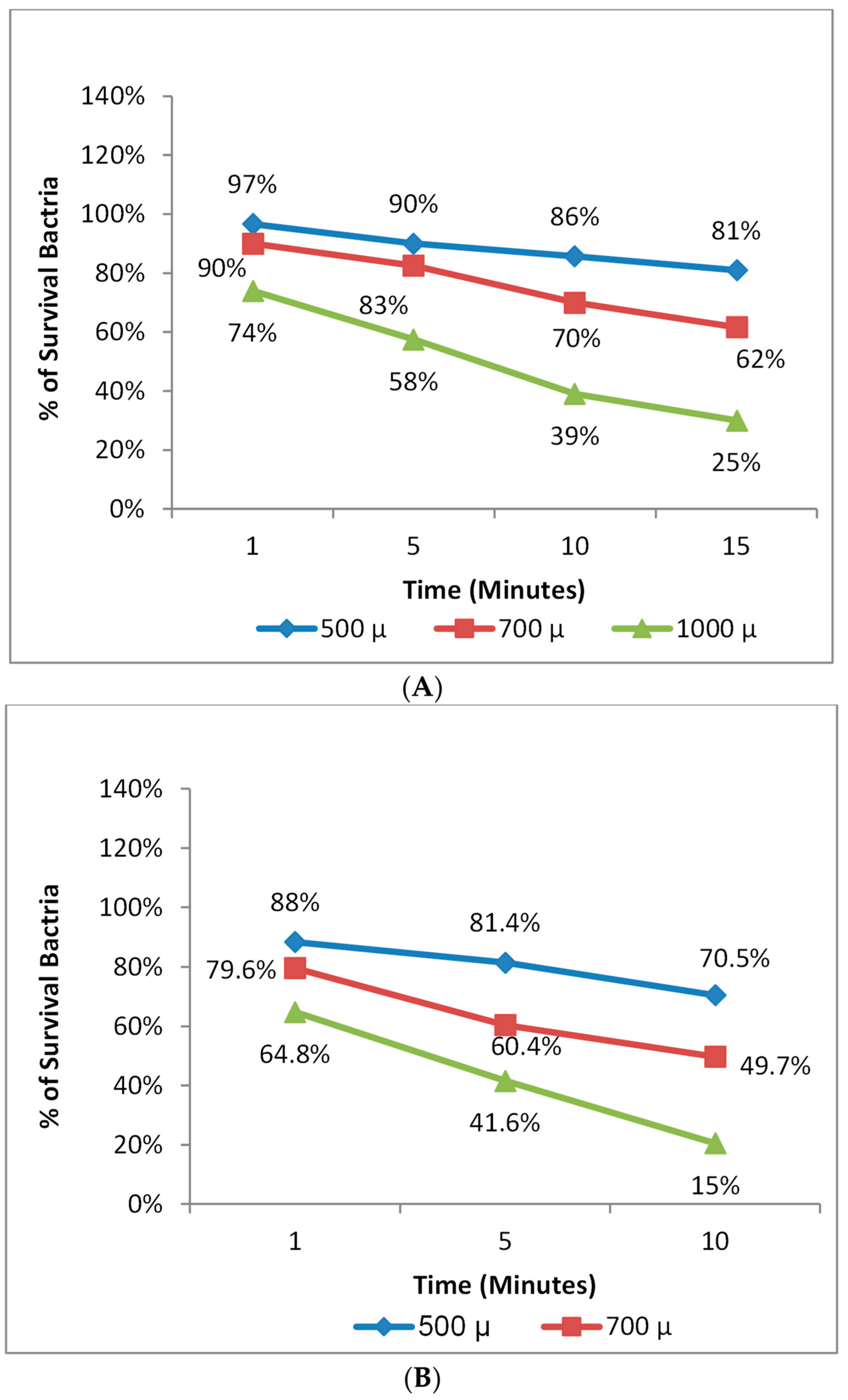
| Treatments | Control | 1 | 2 | 3 |
|---|---|---|---|---|
| S. aureus or E. coli (µL) | 50 | 50 | 50 | 50 |
| Concentration of NE (µL) | 0 | 500 | 1000 | 1500 |
| Distilled water (µL) | 1950 | 1450 | 950 | 450 |
| Treatments | Control | 1 | 2 | 3 |
|---|---|---|---|---|
| S. aureus or E. coli (µL) | 50 | 50 | 50 | 50 |
| Concentration of NE + ZnPc (µL) | 0 | 500 | 1000 | 1500 |
| Distilled water (µL) | 1950 | 1450 | 950 | 450 |
| Photodynamic Therapy Duration (min) | 0 (Control) | 1 | 5 | 10 |
|---|---|---|---|---|
| Concentration of S. aureus (µL) | 50 | 50 | 50 | 50 |
| Concentration of NE + ZnPc (µL) | 0 | 500 | 700 | 1000 |
| Distilled water (µL) | 1950 | 1450 | 1250 | 950 |
| Photodynamic Therapy Duration (min) | 0 (Control) | 1 | 5 | 10 | 15 |
|---|---|---|---|---|---|
| The concentration of E. coli (µL) | 50 | 50 | 50 | 50 | 50 |
| Concentration of NE + ZnPc (µL) | 0 | 500 | 700 | 1000 | 1000 |
| Distilled water (µL) | 1950 | 1450 | 1250 | 950 | 950 |
| Incubation Time (min) | 0 (Control) | 30 min | 60 | 120 |
|---|---|---|---|---|
| S. aureus or E. coli (µL) | 50 | 50 | 50 | 50 |
| Concentration of NE + ZnPc (µL) | 0 | 1000 | 1000 | 1000 |
| Distilled water (µL) | 1950 | 950 | 950 | 950 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felifel, N.T.; Sliem, M.A.; Kamel, Z.; Bojarska, J.; Seadawy, M.G.; Amin, R.M.; Elnagdy, S.M. Antimicrobial Photodynamic Therapy against Escherichia coli and Staphylococcus aureus Using Nanoemulsion-Encapsulated Zinc Phthalocyanine. Microorganisms 2023, 11, 1143. https://doi.org/10.3390/microorganisms11051143
Felifel NT, Sliem MA, Kamel Z, Bojarska J, Seadawy MG, Amin RM, Elnagdy SM. Antimicrobial Photodynamic Therapy against Escherichia coli and Staphylococcus aureus Using Nanoemulsion-Encapsulated Zinc Phthalocyanine. Microorganisms. 2023; 11(5):1143. https://doi.org/10.3390/microorganisms11051143
Chicago/Turabian StyleFelifel, Nada T., Mahmoud A. Sliem, Zienat Kamel, Joanna Bojarska, Mohamed G. Seadawy, Rehab M. Amin, and Sherif M. Elnagdy. 2023. "Antimicrobial Photodynamic Therapy against Escherichia coli and Staphylococcus aureus Using Nanoemulsion-Encapsulated Zinc Phthalocyanine" Microorganisms 11, no. 5: 1143. https://doi.org/10.3390/microorganisms11051143
APA StyleFelifel, N. T., Sliem, M. A., Kamel, Z., Bojarska, J., Seadawy, M. G., Amin, R. M., & Elnagdy, S. M. (2023). Antimicrobial Photodynamic Therapy against Escherichia coli and Staphylococcus aureus Using Nanoemulsion-Encapsulated Zinc Phthalocyanine. Microorganisms, 11(5), 1143. https://doi.org/10.3390/microorganisms11051143








