C-Terminal Lysine Residue of Pneumococcal Triosephosphate Isomerase Contributes to Its Binding to Host Plasminogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Media
2.2. Construction of the Brevibacillus Strains Producing rTpiA
2.3. Purification of rTpiA
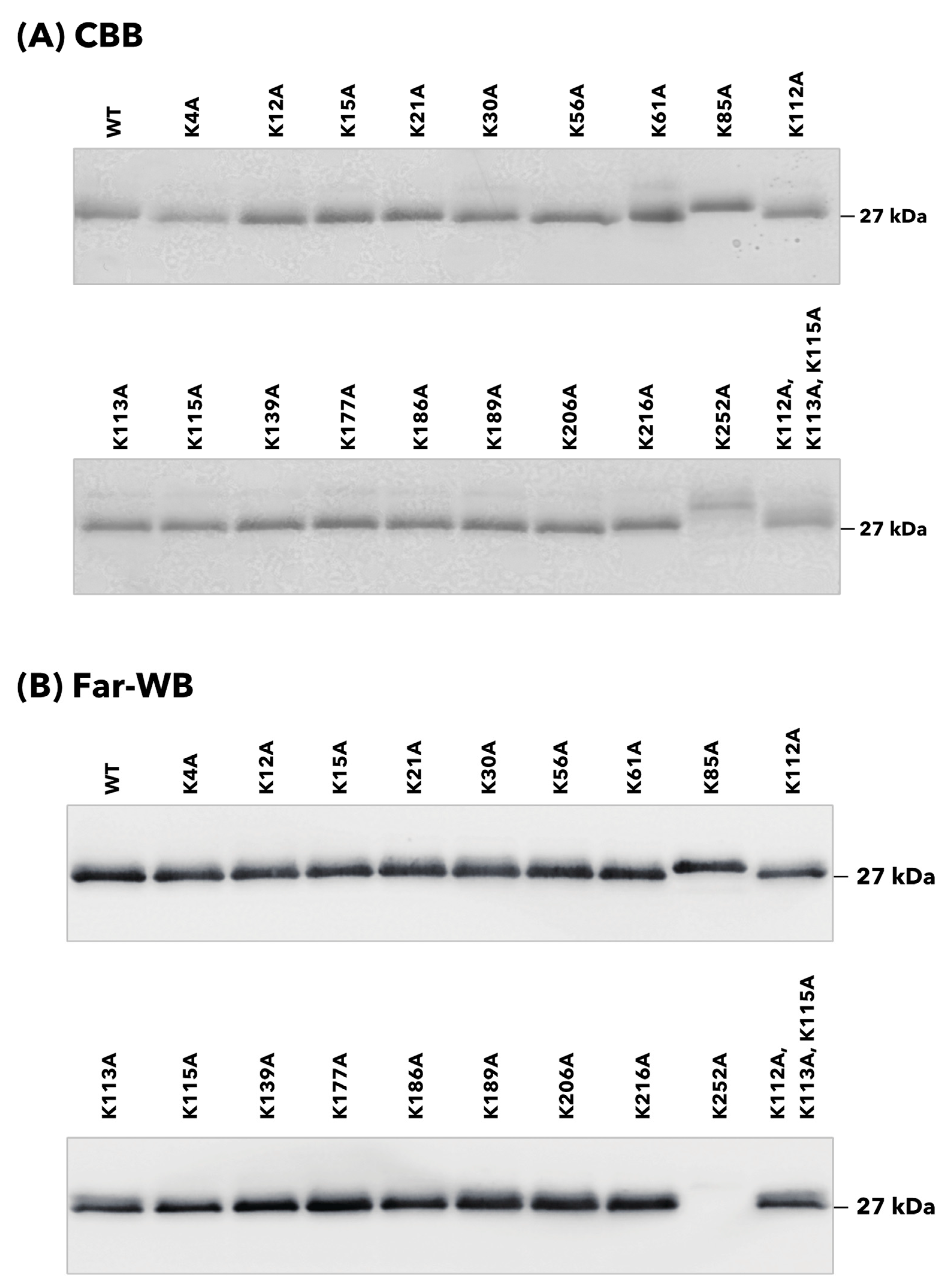
2.4. SDS-PAGE and Far-Western Blotting
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Evaluation of Protein Binding Activity by Surface Plasmon Resonance
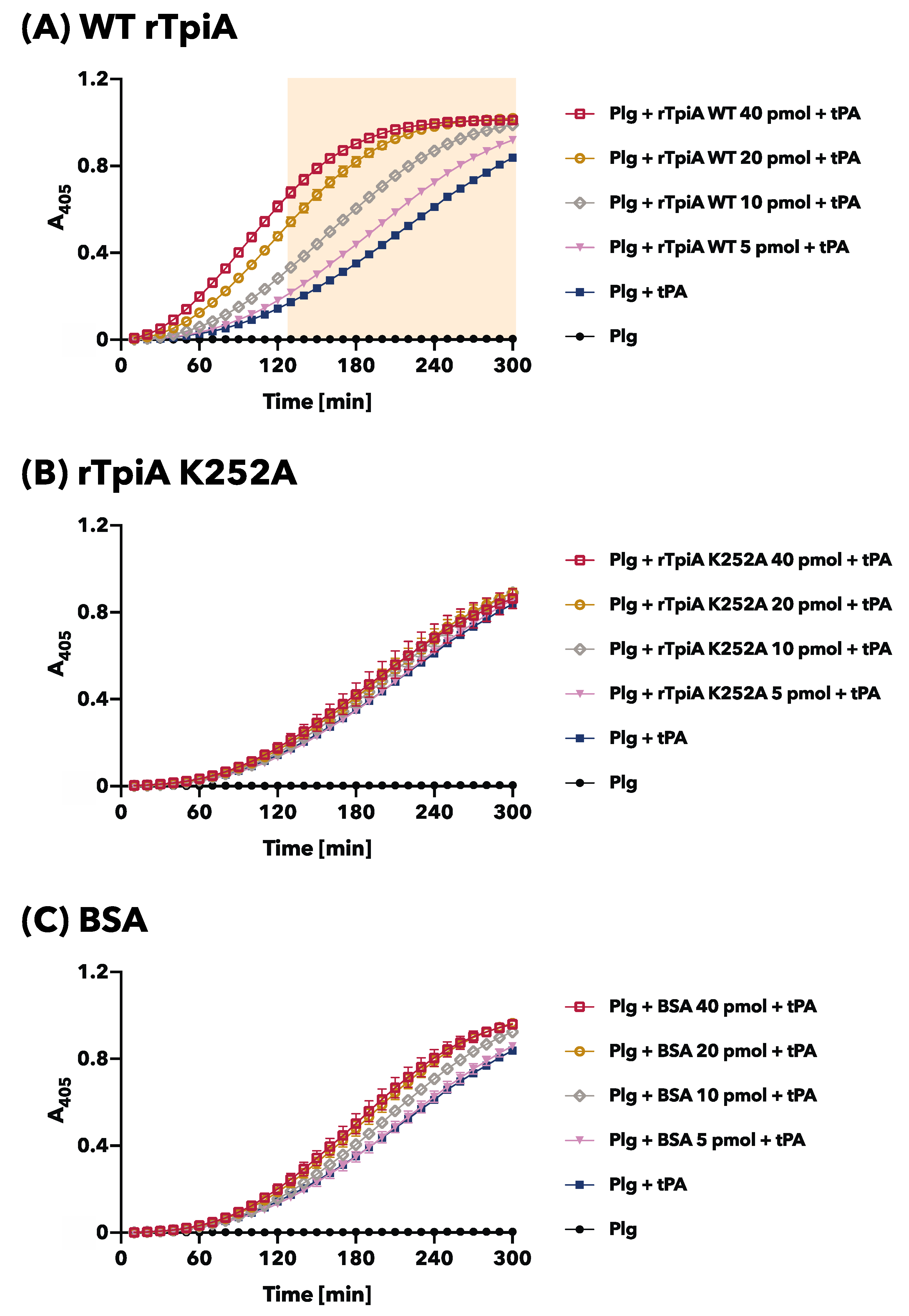
2.7. Plasminogen Activation
2.8. Statistical Analysis
3. Results
3.1. rTpiA Proteins with Site-Specific Substitution of Lysine Residues with Alanine Residues
3.2. Binding of rTpiA Proteins with Site-Specific Substitutions to Human Plasminogen
3.3. The Site-Specific Substituted rTpiA, Which Does Not Bind to Plasminogen, Does Not Promote Plasminogen Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scelfo, C.; Menzella, F.; Fontana, M.; Ghidoni, G.; Galeone, C.; Facciolongo, N.C. Pneumonia and Invasive Pneumococcal Diseases: The Role of Pneumococcal Conjugate Vaccine in the Era of Multi-Drug Resistance. Vaccines 2021, 9, 420. [Google Scholar] [CrossRef]
- Tan, T.Q. Pediatric invasive pneumococcal disease in the United States in the era of pneumococcal conjugate vaccines. Clin. Microbiol. Rev. 2012, 25, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Amblar, M.; Zaballos, Á.; de la Campa, A.G. Role of PatAB Transporter in Efflux of Levofloxacin in Streptococcus pneumoniae. Antibiotics 2022, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T.; et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Duan, N.; Chen, W.; Zhao, X.; Wang, L.; Du, P.; Guo, J. Genomic epidemiology of Streptococcus pneumoniae isolated in a tertiary hospital in Beijing, China, from 2018 to 2022. Pathogens 2023, 12, 284. [Google Scholar] [CrossRef]
- Eberhard, T.; Kronvall, G.; Ullberg, M. Surface bound plasmin promotes migration of Streptococcus pneumoniae through reconstituted basement membranes. Microb. Pathog. 1999, 26, 175–181. [Google Scholar] [CrossRef]
- Kuusela, P.; Ullberg, M.; Saksela, O.; Kronvall, G. Tissue-type plasminogen activator-mediated activation of plasminogen on the surface of group A, C, and G streptococci. Infect. Immun. 1992, 60, 196–201. [Google Scholar] [CrossRef]
- Hirayama, S.; Domon, H.; Hiyoshi, T.; Isono, T.; Tamura, H.; Sasagawa, K.; Takizawa, F.; Terao, Y. Triosephosphate isomerase of Streptococcus pneumoniae is released extracellularly by autolysis and binds to host plasminogen to promote its activation. FEBS Open Bio 2022, 12, 1206–1219. [Google Scholar] [CrossRef]
- Rodríguez-Bolaños, M.; Perez-Montfort, R. Medical and veterinary importance of the moonlighting functions of triosephosphate isomerase. Curr. Protein Pept. Sci. 2019, 20, 304–315. [Google Scholar] [CrossRef]
- Roland, B.P.; Stuchul, K.A.; Larsen, S.B.; Amrich, C.G.; Vandemark, A.P.; Celotto, A.M.; Palladino, M.J. Evidence of a triosephosphate isomerase non-catalytic function crucial to behavior and longevity. J. Cell Sci. 2013, 126, 3151–3158. [Google Scholar] [CrossRef]
- Roland, B.P.; Zeccola, A.M.; Larsen, S.B.; Amrich, C.G.; Talsma, A.D.; Stuchul, K.A.; Heroux, A.; Levitan, E.S.; VanDemark, A.P.; Palladino, M.J. Structural and genetic studies demonstrate neurologic dysfunction in triosephosphate isomerase deficiency is associated with impaired synaptic vesicle dynamics. PLoS Genet. 2016, 12, e1005941. [Google Scholar] [CrossRef]
- Kawabata, S.; Tamura, Y.; Murakami, J.; Terao, Y.; Nakagawa, I.; Hamada, S. A novel, anchorless streptococcal surface protein that binds to human immunoglobulins. Biochem. Biophys. Res. Commun. 2002, 296, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, S.; Nakao, R. Glycine significantly enhances bacterial membrane vesicle production: A powerful approach for isolation of LPS-reduced membrane vesicles of probiotic Escherichia coli. Microb. Biotechnol. 2020, 13, 1162–1178. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Ozuna, J.F.; Hernández-García, M.S.; Brieba, L.G.; Benítez-Cardoza, C.G.; Ortega-López, J.; González-Robles, A.; Arroyo, R. The Glycolytic enzyme triosephosphate isomerase of trichomonas vaginalis is a surface-associated protein induced by glucose that functions as a laminin- and fibronectin-binding protein. Infect. Immun. 2016, 84, 2878–2894. [Google Scholar] [CrossRef] [PubMed]
- Terao, Y.; Mori, Y.; Yamaguchi, M.; Shimizu, Y.; Ooe, K.; Hamada, S.; Kawabata, S. Group A streptococcal cysteine protease degrades C3 (C3b) and contributes to evasion of innate immunity. J. Biol. Chem. 2008, 283, 6253–6260. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Domon, H.; Maekawa, T.; Oda, M.; Hiyoshi, T.; Tamura, H.; Yonezawa, D.; Arai, Y.; Yokoji, M.; Tabeta, K.; et al. Pneumococcal DNA-binding proteins released through autolysis induce the production of proinflammatory cytokines via toll-like receptor 4. Cell. Immunol. 2018, 325, 14–22. [Google Scholar] [CrossRef]
- Furuya, H.; Ikeda, R. Interaction of triosephosphate isomerase from Staphylococcus aureus with plasminogen. Microbiol. Immunol. 2011, 55, 855–862. [Google Scholar] [CrossRef]
- Hirayama, S.; Yasui, Y.; Sasagawa, K.; Domon, H.; Terao, Y. Pneumococcal proteins ClpC and UvrC as novel host plasminogen binding factors. Microbiol. Immunol. 2022, 67, 99–104. [Google Scholar] [CrossRef]
- de Jong, W.W.; Zweers, A.; Cohen, L.H. Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes. Biochem. Biophys. Res. Commun. 1978, 82, 532–539. [Google Scholar] [CrossRef]
- Strauss, E.G.; Kaesberg, P. Acrylamide gel electrophoresis of bacteriophage Q beta: Electrophoresis of the intact virions and of the viral proteins. Virology 1970, 42, 437–452. [Google Scholar] [CrossRef]
- Noel, D.; Nikaido, K.; Ames, G.F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry 1979, 18, 4159–4165. [Google Scholar] [CrossRef] [PubMed]
- Ayón-Núñez, D.A.; Fragoso, G.; Bobes, R.J.; Laclette, J.P. Plasminogen-binding proteins as an evasion mechanism of the host’s innate immunity in infectious diseases. Biosci. Rep. 2018, 38, BSR20180705. [Google Scholar] [CrossRef]
- Figuera, L.; Gómez-Arreaza, A.; Avilán, L. Parasitism in optima forma: Exploiting the host fibrinolytic system for invasion. Acta Trop. 2013, 128, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.B.; Djordjevic, S. Exploitation of plasmin(ogen) by bacterial pathogens of veterinary significance. Vet. Microbiol. 2015, 178, 1–13. [Google Scholar] [CrossRef] [PubMed]
- González-Miguel, J.; Siles-Lucas, M.; Kartashev, V.; Morchón, R.; Simón, F. Plasmin in Parasitic Chronic Infections: Friend or Foe? Trends Parasitol. 2016, 32, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Counihan, N.A.; Chisholm, S.A.; Bullen, H.E.; Srivastava, A.; Sanders, P.R.; Jonsdottir, T.K.; Weiss, G.E.; Ghosh, S.; Crabb, B.S.; Creek, D.J.; et al. parasites deploy RhopH2 into the host erythrocyte to obtain nutrients, grow and replicate. elife 2017, 6, e23217. [Google Scholar] [CrossRef]
- Navarrete-Perea, J.; Toledano-Magaña, Y.; De la Torre, P.; Sciutto, E.; Bobes, R.J.; Soberón, X.; Laclette, J.P. Role of porcine serum haptoglobin in the host-parasite relationship of Taenia solium cysticercosis. Mol. Biochem. Parasitol. 2016, 207, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, J.R.; Jennette, M.A.; Kuhn, R.E. Uptake and secretion of host proteins by Taenia crassiceps metacestodes. J. Parasitol. 2006, 92, 1101–1102. [Google Scholar] [CrossRef]
- Salazar, N.; Souza, M.C.; Biasioli, A.G.; Silva, L.B.; Barbosa, A.S. The multifaceted roles of Leptospira enolase. Res. Microbiol. 2017, 168, 157–164. [Google Scholar] [CrossRef]
- Hsiao, K.C.; Shih, N.Y.; Fang, H.L.; Huang, T.S.; Kuo, C.C.; Chu, P.Y.; Hung, Y.M.; Chou, S.W.; Yang, Y.Y.; Chang, G.C.; et al. Surface α-enolase promotes extracellular matrix degradation and tumor metastasis and represents a new therapeutic target. PLoS ONE 2013, 8, e69354. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ploplis, V.A.; Castellino, F.J. Bacterial plasminogen receptors utilize host plasminogen system for effective invasion and dissemination. J. Biomed. Biotechnol. 2012, 2012, 482096. [Google Scholar] [CrossRef]
- Plow, E.F.; Herren, T.; Redlitz, A.; Miles, L.A.; Hoover-Plow, J.L. The cell biology of the plasminogen system. FASEB J. 1995, 9, 939–945. [Google Scholar] [CrossRef]
- Ehinger, S.; Schubert, W.D.; Bergmann, S.; Hammerschmidt, S.; Heinz, D.W. Plasmin(ogen)-binding alpha-enolase from Streptococcus pneumoniae: Crystal structure and evaluation of plasmin(ogen)-binding sites. J. Mol. Biol. 2004, 343, 997–1005. [Google Scholar] [CrossRef]
- Sehl, L.C.; Castellino, F.J. Thermodynamic properties of the binding of alpha-, omega-amino acids to the isolated kringle 4 region of human plasminogen as determined by high sensitivity titration calorimetry. J. Biol. Chem. 1990, 265, 5482–5486. [Google Scholar] [CrossRef]
- Menhart, N.; Sehl, L.C.; Kelley, R.F.; Castellino, F.J. Construction, expression, and purification of recombinant kringle 1 of human plasminogen and analysis of its interaction with omega-amino acids. Biochemistry 1991, 30, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Menhart, N.; McCance, S.G.; Sehl, L.C.; Castellino, F.J. Functional independence of the kringle 4 and kringle 5 regions of human plasminogen. Biochemistry 1993, 32, 8799–8806. [Google Scholar] [CrossRef] [PubMed]
- Menhart, N.; Castellino, F.J. The importance of the hydrophobic components of the binding energies in the interaction of omega-amino acid ligands with isolated kringle polypeptide domains of human plasminogen. Int. J. Pept. Protein Res. 1995, 46, 464–470. [Google Scholar] [CrossRef]
- Marti, D.N.; Schaller, J.; Llinás, M. Solution structure and dynamics of the plasminogen kringle 2-AMCHA complex: 3(1)-helix in homologous domains. Biochemistry 1999, 38, 15741–15755. [Google Scholar] [CrossRef] [PubMed]
- Wistedt, A.C.; Kotarsky, H.; Marti, D.; Ringdahl, U.; Castellino, F.J.; Schaller, J.; Sjöbring, U. Kringle 2 mediates high affinity binding of plasminogen to an internal sequence in streptococcal surface protein PAM. J. Biol. Chem. 1998, 273, 24420–24424. [Google Scholar] [CrossRef]
- Berge, A.; Sjöbring, U. PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J. Biol. Chem. 1993, 268, 25417–25424. [Google Scholar] [CrossRef]
- Bergmann, S.; Rohde, M.; Chhatwal, G.S.; Hammerschmidt, S. Alpha-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 2001, 40, 1273–1287. [Google Scholar] [CrossRef]
- Whiting, G.C.; Evans, J.T.; Patel, S.; Gillespie, S.H. Purification of native alpha-enolase from Streptococcus pneumoniae that binds plasminogen and is immunogenic. J. Med. Microbiol. 2002, 51, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Wild, D.; Diekmann, O.; Frank, R.; Bracht, D.; Chhatwal, G.S.; Hammerschmidt, S. Identification of a novel plasmin(ogen)-binding motif in surface displayed alpha-enolase of Streptococcus pneumoniae. Mol. Microbiol. 2003, 49, 411–423. [Google Scholar] [CrossRef]
- Bergmann, S.; Rohde, M.; Chhatwal, G.S.; Hammerschmidt, S. Characterization of plasmin(ogen) binding to Streptococcus pneumoniae. Indian J. Med. Res. 2004, 119, 29–32. [Google Scholar] [PubMed]
- Kolberg, J.; Aase, A.; Bergmann, S.; Herstad, T.K.; Rødal, G.; Frank, R.; Rohde, M.; Hammerschmidt, S. Streptococcus pneumoniae enolase is important for plasminogen binding despite low abundance of enolase protein on the bacterial cell surface. Microbiology 2006, 152, 1307–1317. [Google Scholar] [CrossRef]
- Mori, Y.; Yamaguchi, M.; Terao, Y.; Hamada, S.; Ooshima, T.; Kawabata, S. α-Enolase of Streptococcus pneumoniae induces formation of neutrophil extracellular traps. J. Biol. Chem. 2012, 287, 10472–10481. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Rohde, M.; Hammerschmidt, S. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pneumoniae is a surface-displayed plasminogen-binding protein. Infect. Immun. 2004, 72, 2416–2419. [Google Scholar] [CrossRef]
- Attali, C.; Frolet, C.; Durmort, C.; Offant, J.; Vernet, T.; Di Guilmi, A.M. Streptococcus pneumoniae choline-binding protein E interaction with plasminogen/plasmin stimulates migration across the extracellular matrix. Infect. Immun. 2008, 76, 466–476. [Google Scholar] [CrossRef]
- Papasergi, S.; Garibaldi, M.; Tuscano, G.; Signorino, G.; Ricci, S.; Peppoloni, S.; Pernice, I.; Lo Passo, C.; Teti, G.; Felici, F.; et al. Plasminogen- and fibronectin-binding protein B is involved in the adherence of Streptococcus pneumoniae to human epithelial cells. J. Biol. Chem. 2010, 285, 7517–7524. [Google Scholar] [CrossRef]
- Agarwal, V.; Kuchipudi, A.; Fulde, M.; Riesbeck, K.; Bergmann, S.; Blom, A.M. Streptococcus pneumoniae endopeptidase O (PepO) is a multifunctional plasminogen- and fibronectin-binding protein, facilitating evasion of innate immunity and invasion of host cells. J. Biol. Chem. 2013, 288, 6849–6863. [Google Scholar] [CrossRef]
- Mohan, S.; Hertweck, C.; Dudda, A.; Hammerschmidt, S.; Skerka, C.; Hallström, T.; Zipfel, P.F. Tuf of Streptococcus pneumoniae is a surface displayed human complement regulator binding protein. Mol. Immunol. 2014, 62, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Fulde, M.; Bernardo-García, N.; Rohde, M.; Nachtigall, N.; Frank, R.; Preissner, K.T.; Klett, J.; Morreale, A.; Chhatwal, G.S.; Hermoso, J.A.; et al. Pneumococcal phosphoglycerate kinase interacts with plasminogen and its tissue activator. Thromb. Haemost. 2014, 111, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Meinel, C.; Spartà, G.; Dahse, H.M.; Hörhold, F.; König, R.; Westermann, M.; Coldewey, S.M.; Cseresnyés, Z.; Figge, M.T.; Hammerschmidt, S.; et al. Streptococcus pneumoniae from patients with hemolytic uremic syndrome binds human plasminogen via the surface protein PspC and uses plasmin to damage human endothelial cells. J. Infect. Dis. 2018, 217, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, V.; Korhonen, T.K. Dancing to another tune-adhesive moonlighting proteins in bacteria. Biology 2014, 3, 178–204. [Google Scholar] [CrossRef] [PubMed]
- Terao, Y.; Yamaguchi, M.; Hamada, S.; Kawabata, S. Multifunctional glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pyogenes is essential for evasion from neutrophils. J. Biol. Chem. 2006, 281, 14215–14223. [Google Scholar] [CrossRef] [PubMed]



| Application | Sequence (5′ to 3′) | |
|---|---|---|
| K4A | Forward | gctCCATTTATCGCTGGTAACTGG |
| Reverse | ACGTGATTTGTCATCGTCATC | |
| K12A | Forward | gctATGAACAAAAATCCAGAAGAAGC |
| Reverse | CCAGTTACCAGCGATAAATGG | |
| K15A | Forward | gctAATCCAGAAGAAGCTAAAGCATTC |
| Reverse | GTTCATTTTCCAGTTACCAGCG | |
| K21A | Forward | gctGCATTCGTTGAAGCAGTTG |
| Reverse | AGCTTCTTCTGGATTTTTGTTC | |
| K30A | Forward | gctCTTCCTTCATCAGATCTTGTTGAAG |
| Reverse | TGATGCAACTGCTTCAACG | |
| K56A | Forward | gctGGCTCAAACCTTAAAGTTGCTG |
| Reverse | TGCAACAGCAAGAACAGTTGTC | |
| K61A | Forward | CTTgctGTTGCTGCTCAAAACTGCTAC |
| Reverse | GTTTGAGCCTTTTGCAACAG | |
| K85A | Forward | gctGAAATCGGTACTGACTACGTTGTTATC |
| Reverse | CAAAACTTGTGGGCTAGTTTCAC | |
| K112A | Forward | gctAAAGCAAAAGCAATCTTTGCG |
| Reverse | GTTGATATCTTCGTCAGTTTCATGG | |
| K113A | Forward | gctGCAAAAGCAATCTTTGCGAAC |
| Reverse | TTTGTTGATATCTTCGTCAGTTTCATG | |
| K115A | Forward | gctGCAATCTTTGCGAACGG |
| Reverse | TGCTTTTTTGTTGATATCTTCGTC | |
| K139A | Forward | gctGCTGCTGAATTCGTAGGTGC |
| Reverse | ACCAGCTTCGTAAGTTTCAAGTG | |
| K177A | Forward | gctTCAGCTTCACAAGACGATGC |
| Reverse | ACCAGTACCGATAGCCCAG | |
| K186A | Forward | gctATGTGTAAAGTTGTTCGTGACGTTG |
| Reverse | TTGTGCATCGTCTTGTGAAG | |
| K189A | Forward | gctGTTGTTCGTGACGTTGTAGCTG |
| Reverse | ACACATTTTTTGTGCATCGTC | |
| K206A | Forward | gctGTTCGTGTTCAATACGGTGG |
| Reverse | GTCTGCGACTTCTTGACCAAAG | |
| K216A | Forward | gctCCTGAAAATGTTGCTTCATACATG |
| Reverse | AACAGAACCACCGTATTGAACAC | |
| K252A | Forward | TAgctTAATCAGTAAGTAGC AAGCTTAACAGGATG |
| Reverse | CAAAGTCAAGCAAAGCCAAGAAG | |
| K112A, K113A, K115A | Forward | gctgctGCAgctGCAATCTTTGCGAACGGTATG |
| Reverse | GTTGATATCTTCGTCAGTTTCATGG | |
| Sequencing | Forward | CGCGATATCAGGATTCGG |
| Reverse | CAATGTAATTGTTCCCTACCTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirayama, S.; Hiyoshi, T.; Yasui, Y.; Domon, H.; Terao, Y. C-Terminal Lysine Residue of Pneumococcal Triosephosphate Isomerase Contributes to Its Binding to Host Plasminogen. Microorganisms 2023, 11, 1198. https://doi.org/10.3390/microorganisms11051198
Hirayama S, Hiyoshi T, Yasui Y, Domon H, Terao Y. C-Terminal Lysine Residue of Pneumococcal Triosephosphate Isomerase Contributes to Its Binding to Host Plasminogen. Microorganisms. 2023; 11(5):1198. https://doi.org/10.3390/microorganisms11051198
Chicago/Turabian StyleHirayama, Satoru, Takumi Hiyoshi, Yoshihito Yasui, Hisanori Domon, and Yutaka Terao. 2023. "C-Terminal Lysine Residue of Pneumococcal Triosephosphate Isomerase Contributes to Its Binding to Host Plasminogen" Microorganisms 11, no. 5: 1198. https://doi.org/10.3390/microorganisms11051198






