Phytoplankton Size Structure and Diversity in the Transitional System of the Aquatina Lagoon (Southern Adriatic Sea, Mediterranean)
Abstract
:1. Introduction
2. Materials and Methods
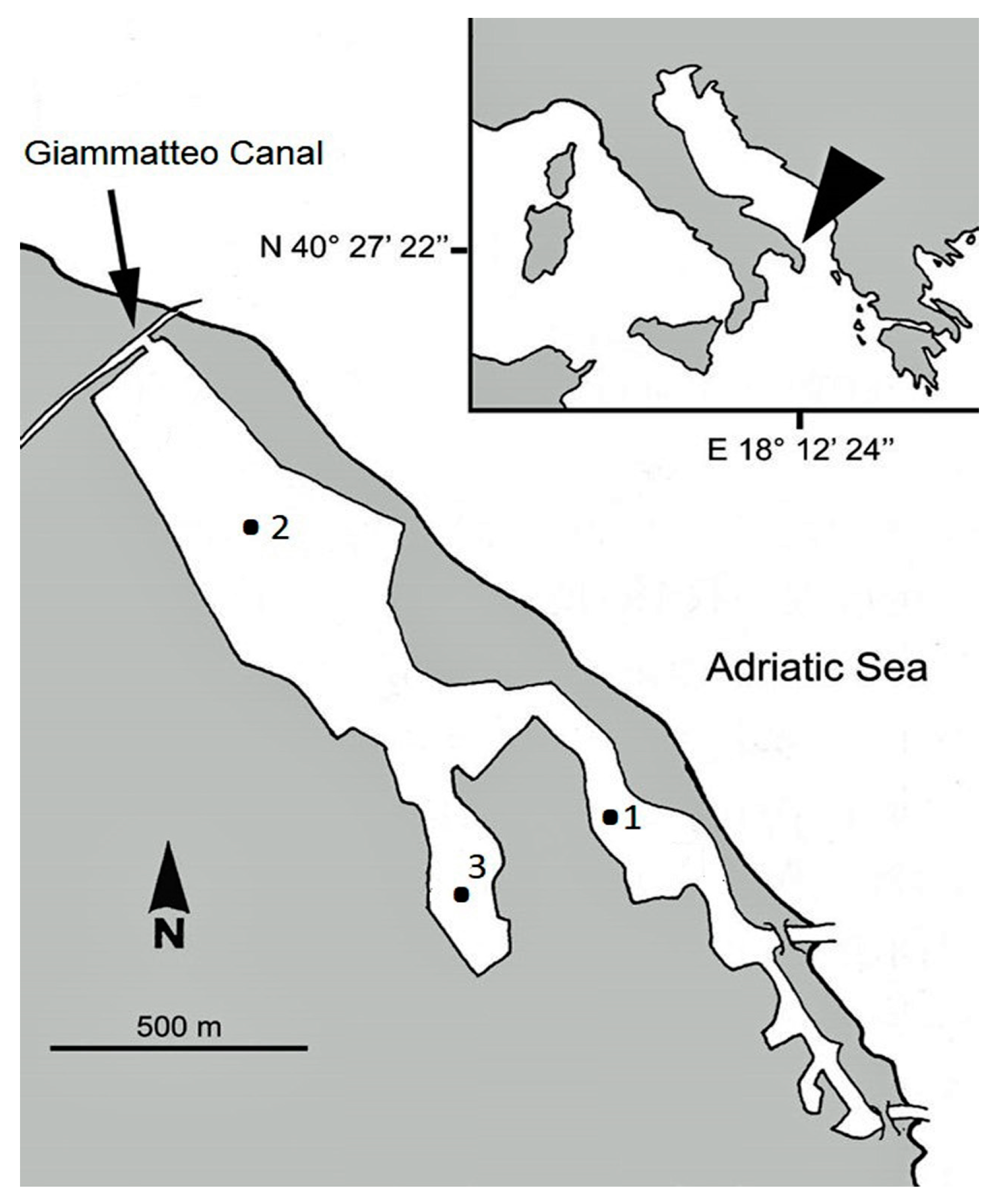
2.1. Study Area
2.2. Sample Collection and Abiotic Factors
2.3. Phytoplankton Communities
2.3.1. Picophytoplankton (PPP)
2.3.2. Nanophytoplankton (NPP)
2.3.3. Microphytoplankton (MPP)
2.4. Data Analyses
3. Results
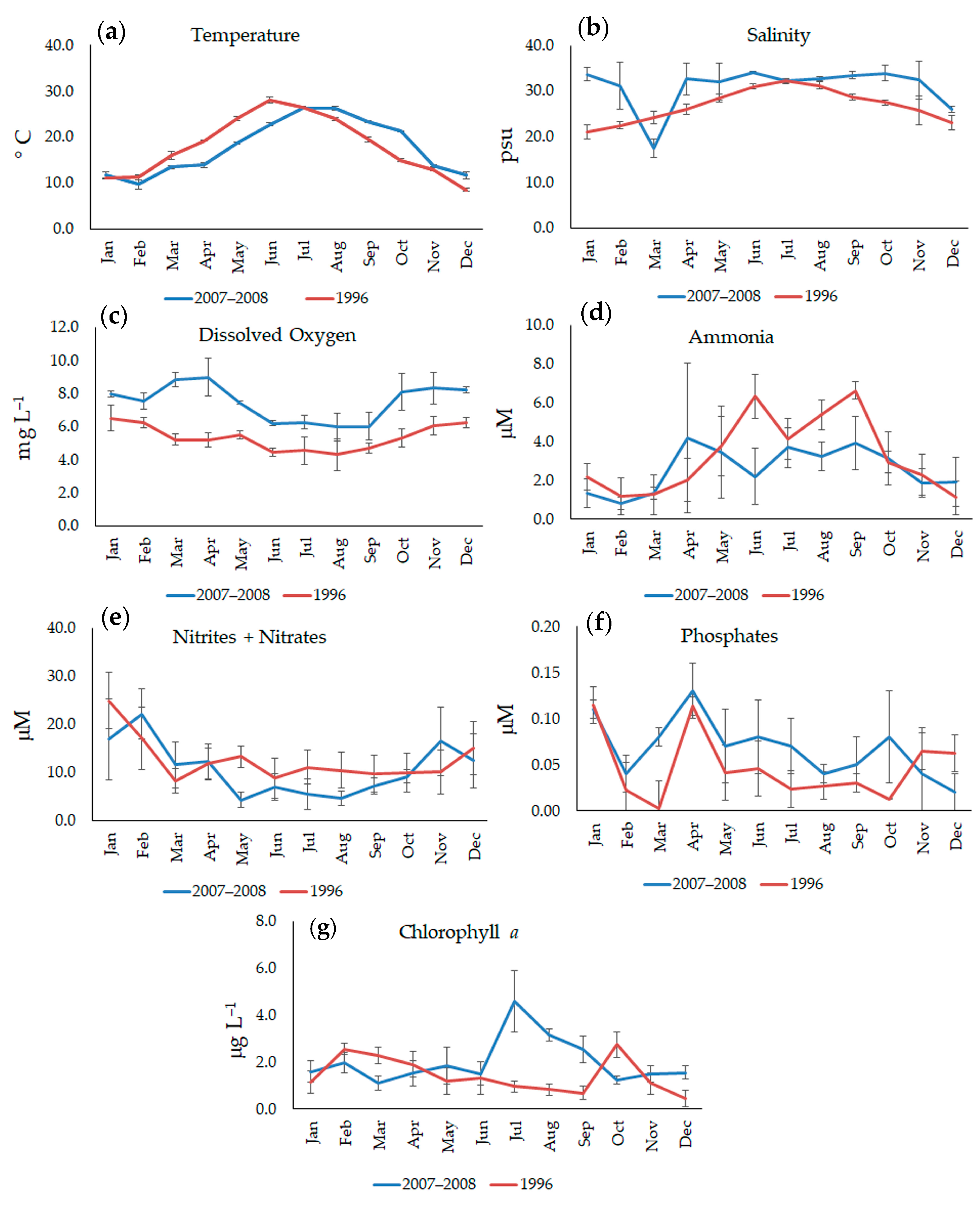
3.1. Environmental Background
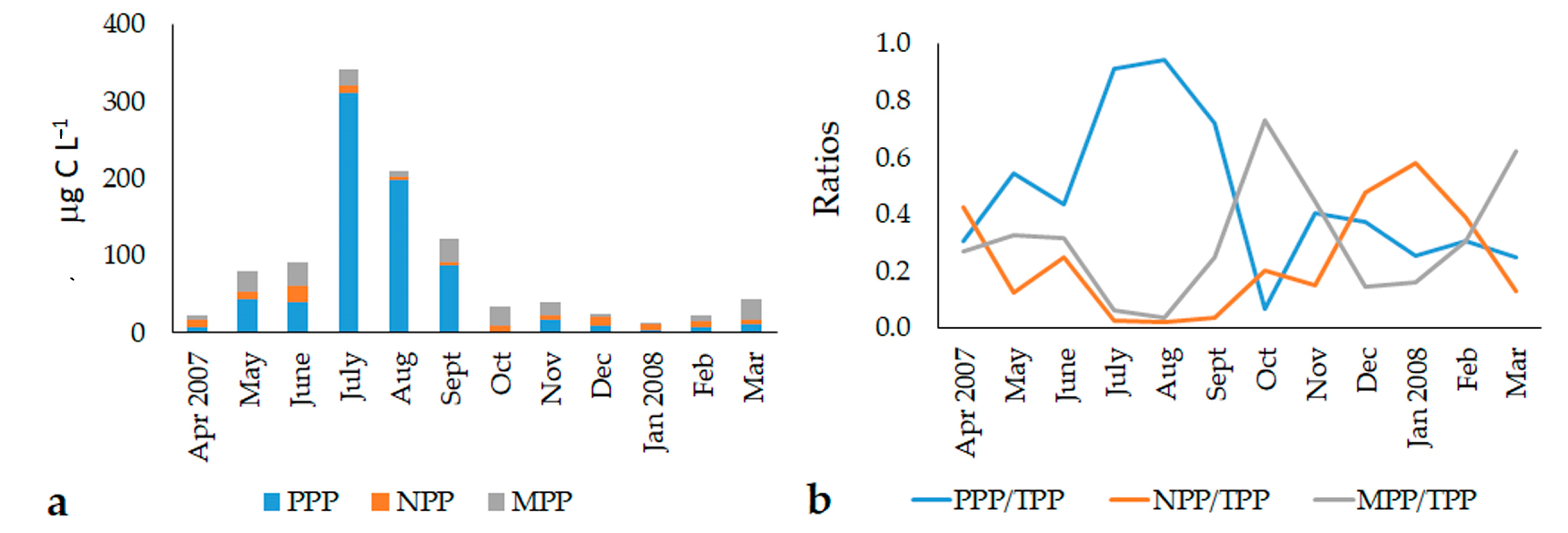
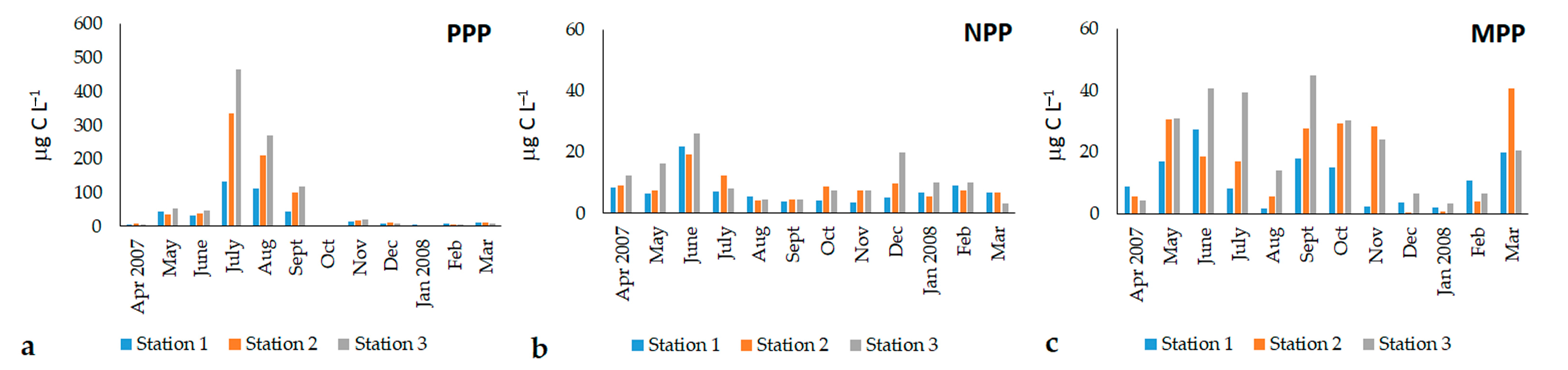
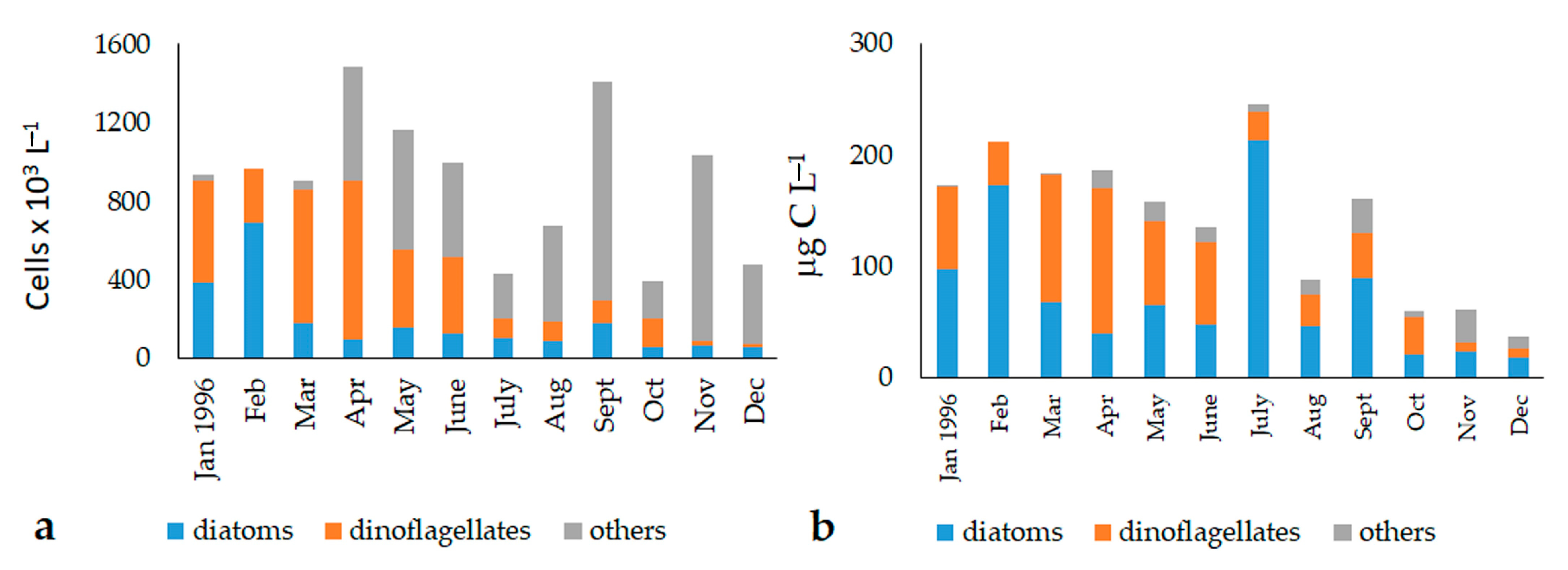
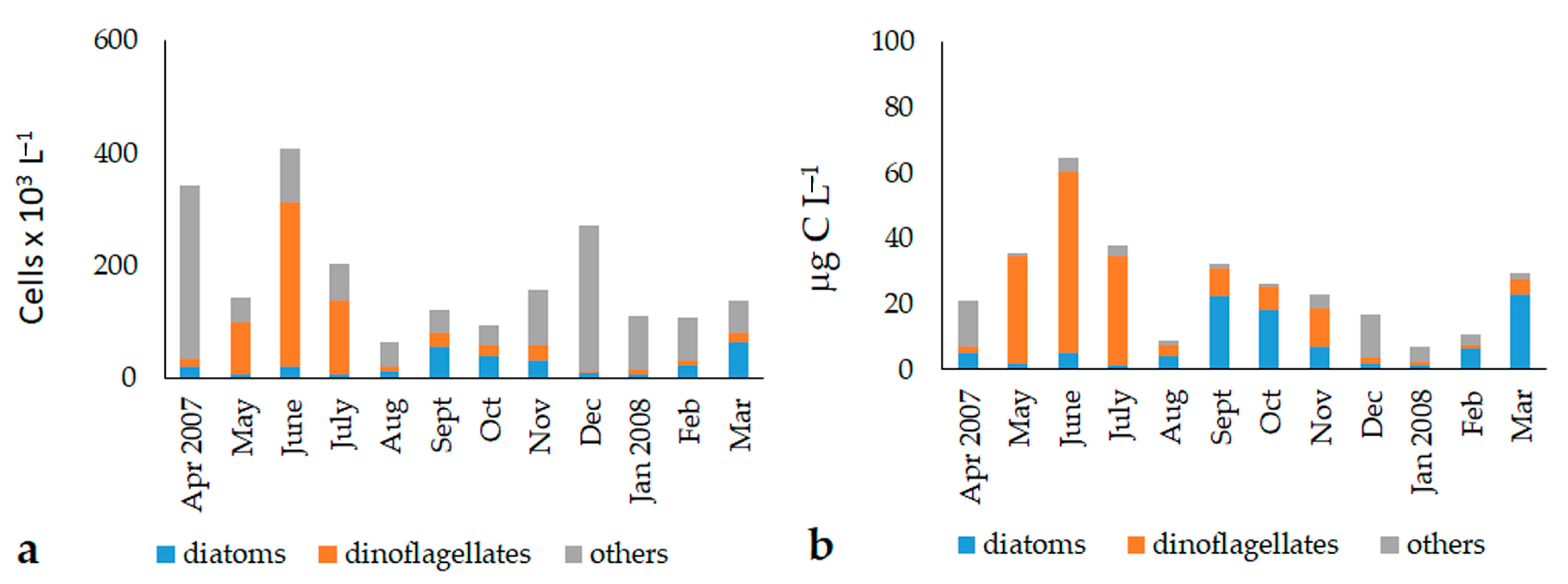
3.2. Abundance and Biomass of the Phytoplankton Fractions (2007–2008)
3.3. Phytoplankton Community Structure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vadrucci, M.R.; Sabetta, L.; Fiocca, A.; Mazziotti, C.; Silvestri, C.; Cabrini, M.; Guardiani, B.; Konjka, E.; Evangelos, A.; Koutsoubas, D.; et al. Statistical evaluation of differences in phytoplankton richness and abundance as constrained by environmental drivers in transitional waters of the Mediterranean Basin. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, S88–S104. [Google Scholar] [CrossRef]
- Cloern, J.E.; Jassby, A.D. Complex seasonal patterns of primary producers at the land-sea interface. Ecol. Lett. 2008, 11, 1294–1303. [Google Scholar] [CrossRef]
- Winder, M.; Cloern, J.E. The annual cycles of phytoplankton biomass. Phil. Trans. R. Soc. B 2010, 365, 3215–3226. [Google Scholar] [CrossRef]
- Newton, A.; Icely, J.; Cristina, S.; Brito, A.; Cardoso, A.C.; Colijn, F.; Dalla Riva, S.; Gertz, F.; Hansen, J.W.; Holmer, M.; et al. An overview of ecological status, vulnerability and future perspectives of European large shallow, semi-enclosed coastal systems, lagoons and transitional waters. Estuar. Coast. Shelf Sci. 2014, 140, 95–122. [Google Scholar] [CrossRef]
- Brito, A.C.; Newton, A.; Tett, P.; Fernandes, T.F. How will shallow coastal lagoons respond to climate change? A modelling investigation. Estuar. Coast. Shelf Sci. 2012, 112, 98–104. [Google Scholar] [CrossRef]
- Padedda, B.M.; Pulina, S.; Magni, P.; Sechi, N.; Lugliè, A. Phytoplankton dynamics in relation to environmental changes in a phytoplankton-dominated Mediterranean lagoon (Cabras Lagoon, Italy). Adv. Oceanogr. Limnol. 2012, 3, 147–169. [Google Scholar] [CrossRef]
- Davies-Vollum, K.S.; Puttick, S.; Doherty, F.; Agyekumhene, A.; Aneyo, I.; Appeaning Addo, K.; Boateng, I.; Danby, A.; Danso-Wiredu, E.; Degbe, G.; et al. Resilient lagoons? Climate change, sustainability and adaptation. Geography 2022, 107, 153–157. [Google Scholar] [CrossRef]
- Van den Hoek, C.; Mann, D.G.; Jahns, H.M. Algae: An Introduction to Phycology; Cambridge University Press: Cambridge, UK, 1997; pp. 1–627. [Google Scholar]
- Caroppo, C.; Roselli, L.; Di Leo, A. Hydrological conditions and phytoplankton community in the Lesina Lagoon (Southern Adriatic Sea, Mediterranean). Environ. Sci. Pollut. Res. 2018, 25, 1784–1799. [Google Scholar] [CrossRef]
- Bernardi Aubry, F.; Acri, F.; Finotto, S.; Pugnetti, A. Phytoplankton Dynamics and Water Quality in the Venice Lagoon. Water 2021, 13, 2780. [Google Scholar] [CrossRef]
- Bernardi Aubry, F.; Acri, F.; Bastianini, M.; Finotto, S.; Pugnetti, A. Differences and similarities in the phytoplankton communities of two coupled transitional and marine ecosystems (the Lagoon of Venice and the Gulf of Venice-Northern Adriatic Sea). Front. Mar. Sci. 2022, 9, 974967. [Google Scholar] [CrossRef]
- Roselli, L.; Stanca, E.; Ludovisi, A.; Durante, G.; Souza, J.S.D.; Dural, M.; Alp, T.; Bulent, S.; Gjoni, V.; Ghinis, S.; et al. Multi-scale biodiversity patterns in phytoplankton from coastal lagoons: The Eastern Mediterranean. Transit. Waters Bull. 2013, 7, 202–219. [Google Scholar] [CrossRef]
- Behrenfeld, M.; O’Malley, R.; Siegel, D.; McClain, C.R.; Sarmiento, J.L.; Feldman, G.C.; Milligan, A.J.; Falkowski, P.G.; Letelier, R.M.; Boss, E.S. Climate-driven trends in contemporary ocean productivity. Nature 2006, 444, 752–755. [Google Scholar] [CrossRef]
- Brown, P.D.; Craine, J.M.; Richards, D.; Chapman, A.; Marden, B. DNA metabarcoding of the phytoplankton of Great Salt Lake’s Gilbert Bay: Spatio-temporal assemblage changes and comparisons to microscopy. J. Great Lakes Res. 2022, 48, 110–124. [Google Scholar] [CrossRef]
- Bazin, P.; Jouenne, F.; Friedl, T.; Deton-Cabanillas, A.-F.; Le Roy, B.; Véron, B. Phytoplankton Diversity and Community Composition along the Estuarine Gradient of a Temperate Macrotidal Ecosystem: Combined Morphological and Molecular Approaches. PLoS ONE 2014, 9, e94110. [Google Scholar] [CrossRef]
- Zangaro, F.; Marini, G.; Specchia, V.; De Luca, M.; Visintin, F.; Bullo, G.; Richard, J.; Šalaja, N.; Rakar, B.; Lipej, B.; et al. Building a transnational biodiversity geo-database of the protected areas in the Adriatic-Ionian Macro-Region: Approaches and results from the IMPRECO Project. Biodivers. Data J. 2021, 9, e67169. [Google Scholar] [CrossRef]
- DEIMS-SDR (Dynamic Ecological Information Management System-Site and Dataset Registry). Site: Acquatina-Italy. Available online: https://deims.org/8e1909ae-afc0-4207-9314-68e234d57405 (accessed on 20 April 2023).
- LTER-Italy. Italian Long-Term Ecological Research (LTER) Network. Available online: www.lteritalia.it (accessed on 20 April 2023).
- Morabito, G.; Mazzocchi, M.G.; Salmaso, N.; Zingone, A.; Bergami, C.; Flaim, G.; Accoroni, S.; Basset, A.; Bastianini, M.; Belmonte, G.; et al. Plankton dynamics across the freshwater, transitional and marine research sites of the LTER-Italy Network. Patterns, fluctuations, drivers. Sci. Total Environ. 2018, 627, 373–387. [Google Scholar] [CrossRef]
- Tolomio, C.; Andreoli, C.; Darin, M.; Bortolotto, M. Le phytoplancton de surface dans la lagune d’Acquatina-Frigule (mer Adriatique meridionale). Mar. Life 1992, 2, 47–52. [Google Scholar]
- Vadrucci, M.R.; Fiocca, A.; Decembrini, F.; Magazzù, G. Biomassa e produzione fitoplanctonica frazionate in un ambiente salmastro meso-ologotrofo del Salento (Stagno di Acquatina 1991–1994). In Proceedings of the VII Congress of the Italian Society of Ecology, Naples, Italy, 11–14 September 1996; Volume 17, pp. 455–459. (In Italian). [Google Scholar]
- Vadrucci, M.R.; Puglisi, A.; Maimone, G.; Giacobbe, M.G.; Marra, P.; Magazzù, G. Struttura della comunità fitoplanctonica in un ecosistema salmastro dell’Adriatico meridionale. Biol. Mar. Medit. 1999, 6, 674–680. (In Italian) [Google Scholar]
- Caroppo, C. Le comunità fitoplanctoniche del Lago di Acquatina (Mar Adriatico meridionale). Thalass. Salentina 1999, 31, 29–36. (In Italian) [Google Scholar]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Official Journal of the European Communities. 2000. Available online: https://www.researchgate.net/profile/Atomic-Spectroscopy/publication/342348154_Direct_Determination_of_Arsenic_and_Chromium_in_Seawater_Samples_Using_On-Line_Dilution_and_ICP-MS_Analysis/links/5eef868ca6fdcc73be90c899/Direct-Determination-of-Arsenic-and-Chromium-in-Seawater-Samples-Using-On-Line-Dilution-and-ICP-MS-Analysis.pdf (accessed on 20 February 2023).
- Soria, J.; Caniego, G.; Hernández-Sáez, N.; Dominguez-Gomez, J.A.; Erena, M. Phytoplankton Distribution in Mar Menor Coastal Lagoon (SE Spain) during 2017. J. Mar. Sci. Eng. 2020, 8, 600. [Google Scholar] [CrossRef]
- Margiotta, S.; Marini, G.; Fay, S.; D’Onghia, F.M.; Liso, I.S.; Parise, M.; Pinna, M. Hydro-Stratigraphic Conditions and Human Activity Leading to Development of a Sinkhole Cluster in a Mediterranean Water Ecosystem. Hydrology 2021, 8, 111. [Google Scholar] [CrossRef]
- Marrocco, V.; Sicuro, A.; Zangaro, F.; Pinna, M. First record of the protected species Pinna nobilis (Linnaeus, 1758) in the Aquatina Lagoon (NATURA 2000 site IT9150003, South-East Italian coastline). Nat. Conserv. 2018, 28, 51–59. [Google Scholar] [CrossRef]
- Bianchi, C.N. Tipologia ecologica delle lagune costiere italiane. In Le Lagune Costiere Ricerca e Gestione; Carrada, G.C., Cicogna, F., Fresi, E., Eds.; CLEM Pubblicazioni: Massalubrense, Italy, 1988; pp. 57–66. [Google Scholar]
- Petrocelli, A.; Basset, A.; Belmonte, G.; Giangrande, A.; Marchiori, S.; Medagli, P.; Cecere, E. The Lake of Acquatina. In Flora and Vegetation of the Italian Transitional Water Systems; Cecere, E., Petrocelli, A., Izzo, G., Sfriso, A., Eds.; CORILA (Consorzio per la Gestione del Centro di Coordinamento delle Ricerche Inerenti il Sistema Lagunare di Venezia): Venice, Italy, 2009; pp. 173–180. [Google Scholar]
- Cappello, I.; Palmisano, L.; Pomes, A.; Vadrucci, M.R.; Basset, A. Acquatina Lake. In Nutrient Fluxes in Transitional Zones of the Italian Coast; Giordani, G., Viaroli, P., Swaney, D.P., Murray, C.N., Zaldívar, J.M., Marshall Cross-land, J.I., Eds.; LOICZ Reports and Studies: Texel, The Netherlands, 2005; pp. 75–79. [Google Scholar]
- Strickland, Y.D.H.; Parsons, T.R. A practical handbook of seawater analysis. Bull. Fish Res. Board. Canada 1972, 16, 1–311. [Google Scholar]
- Lorenzen, C.J.; Jeffrey, S.W. Determination of chlorophyll in seawater. UNESCO Tech. Pap. Mar. Sci. 1980, 35, 1–20. [Google Scholar]
- Partensky, F.; Blanchot, J.; Vaulot, D. Differential distribution and ecology of Prochlorococcus and Synechococcus: A review. Bull. Inst. Océanog. Monaco 1999, 19, 457–475. [Google Scholar]
- Lund, J.W.G.; Kilpling, C.; Le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimation by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Edler, L. Recommendations for marine biological studies in the Baltic Sea. Phytoplankton and chlorophyll. Balt. Mar. Biol. Publ. 1979, 5, 1–38. [Google Scholar]
- Sun, J.; Liu, D. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef]
- Strathmann, R.R. Estimating the organic carbon content of phytoplankton from cell volume or plasma volume. Limnol. Oceanogr. 1967, 12, 411–418. [Google Scholar] [CrossRef]
- Edler, L.; Elbrächter, M. The Utermöhl method for quantitative phytoplankton analysis. In Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis; Karlson, B., Cusack, C., Bresnan, E., Eds.; UNESCO IOC Manuals and Guides n. 55: Paris, France, 2010; pp. 13–20. [Google Scholar]
- Andersen, P.; Throndsen, J. Estimating cell numbers. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A., Eds.; Unesco Publishing, Monographs on Oceanographic Methodology: Paris, France, 2004; Volume 11, pp. 99–130. [Google Scholar]
- Hillebrand, H.; Durselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms and other protest plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E Ltd.: Plymouth, UK, 2014. [Google Scholar]
- Vadrucci, M.R.; Decembrini, F.; Magazzù, G. Ruolo Limitante del fosforo sulla produzione primaria nello stagno salmastro di Acquatina (Lecce). In Proceedings of the VI Congress of the Italian Society of Ecology, Venice, Italy, 26–29 September 1994; Volume 16, pp. 225–228. (In Italian). [Google Scholar]
- Gönenç, I.; Wolfin, J. Coastal Lagoons: Ecosystem Processes and Modeling for Sustainable Use and Development; CRC Press: Boca Raton, FL, USA, 2005; pp. 1–500. [Google Scholar]
- Kennish, M.J.; Paerl, H.W. Coastal Lagoons: Critical Habitats of Environmental Change; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Caroppo, C.; Turicchia, S.; Margheri, M.C. Phytoplankton assemblages in coastal waters of the Northern Ionian Sea (eastern Mediterranean), with special reference to cyanobacteria. J. Mar. Biol. Assoc. UK 2006, 86, 927–937. [Google Scholar] [CrossRef]
- Caroppo, C.; Cerino, F.; Auriemma, R.; Cibic, T. Phytoplankton dynamics with a special emphasis on harmful algal blooms in the Mar Piccolo of Taranto (Ionian Sea, Italy). Environ. Sci. Pollut. Res. 2016, 23, 12691–12706. [Google Scholar] [CrossRef] [PubMed]
- Caroppo, C. The contribution of picophytoplankton to community structure in a Mediterranean brackish environment. J. Plankton Res. 2000, 22, 381–397. [Google Scholar] [CrossRef]
- Sathicq, M.B.; Unrein, F.; Gómez, N. Recurrent pattern of picophytoplankton dynamics in estuaries around the world: The case of Río de la Plata. Mar. Environ. Res. 2020, 161, 105136. [Google Scholar] [CrossRef]
- Trombetta, T.; Vidussi, F.; Roques, C.; Scotti, M.; Mostajir, B. Marine microbial food web networks during phytoplankton bloom and non- bloom periods: Warming favors smaller organism interactions and intensifies trophic cascade. Front. Microbiol. 2020, 11, 502336. [Google Scholar] [CrossRef]
- Caroppo, C. Ecology and biodiversity of picoplanktonic cyanobacteria in coastal and brackish environments. Biodivers. Conserv. 2015, 24, 949–971. [Google Scholar] [CrossRef]
- Gaulke, A.K.; Wetz, M.S.; Paerl, H.W. Picophytoplankton: A major contributor to planktonic biomass and primary production in a eutrophic, river-dominated estuary. Estuar. Coast. Shelf Sci. 2010, 90, 45–54. [Google Scholar] [CrossRef]
- Paerl, R.W.; Venezia, R.E.; Sanchez, J.J.; Paerl, H.W. Picophytoplankton dynamics in a large temperate estuary and impacts of extreme storm events. Sci. Rep. 2020, 10, 22026. [Google Scholar] [CrossRef]
- Caroppo, C.; Buttino, I.; Camatti, E.; Caruso, G.; De Angelis, R.; Facca, C.; Giovanardi, F.; Lazzara, L.; Mangoni, O.; Magaletti, E. State of the art and perspectives on the use of planktonic communities as indicators of environmental status in relation to the EU Marine Strategy Framework Directive. Biol. Mar. Medit. 2013, 20, 65–73. [Google Scholar]
- Garmendia, M.; Borja, A.; Franco, J.; Revilla, M. Phytoplankton composition indicators for the assessment of eutrophication in marine waters: Present state and challenges within the European directives. Mar. Pollut. Bull. 2013, 66, 7–16. [Google Scholar] [CrossRef]
- Derolez, V.; Soudant, D.; Malet, N.; Chiantella, C.; Richard, M.; Abadie, E.; Aliaume, C.; Bec, B. Two decades of oligotrophication: Evidence for a phytoplankton community shift in the coastal lagoon of Thau (Mediterranean Sea, France). Estuar. Coast. Shelf Sci. 2020, 241, 106810. [Google Scholar] [CrossRef]
- Carstensen, J.; Klais, R.; Cloern, J.E. Phytoplankton blooms in estuarine and coastal waters: Seasonal patterns and key species. Estuar. Coast. Shelf Sci. 2015, 162, 98–109. [Google Scholar] [CrossRef]
- Glibert, P.M.; Boyer, J.N.; Heil, C.; Madden, C.J.; Sturgis, B.; Wazniak, C.S. Blooms in lagoons: Different from those of river-dominated estuaries. In Coastal Lagoons: Critical Habitats of Environmental Change; Kennish, M., Paerl, H., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 91–114. [Google Scholar]
- Bec, B.; Collos, Y.; Souchu, P.; Vaquer, A.; Lautier, J.; Fiandrino, A.; Benau, L.; Orsoni, V.; Laugier, T. Distribution of picophytoplankton and nanophytoplankton along an anthropogenic eutrophication gradient in French Mediterranean coastal lagoons. Aquat. Microb. Ecol. 2011, 63, 29–45. [Google Scholar] [CrossRef]
- Pachés, M.; Romero, I.; Martínez-Guijarro, R.; Martí, C.M.; Ferrer, J. Changes in phytoplankton composition in a Mediterranean coastal lagoon in the Cullera Estany (Comunitat Valenciana, Spain). Water Environ. J. 2014, 28, 135–144. [Google Scholar] [CrossRef]
- Leruste, A.; Malet, N.; Munaros, D.; Deroles, V.; Hatey, E.; Collos, Y.; De Wit, R. First steps of ecological restoration in Mediterranean lagoons: Shifts in phytoplankton communities. Estuar. Coast. Shelf Sci. 2016, 180, 190–2013. [Google Scholar] [CrossRef]
- Caroppo, C. Autoecology and morphological variability of Dinophysis sacculus (Dinophyceae: Dinophysiaceae) in a Mediterranean lagoon. J. Mar. Biol. Assoc. UK 2001, 81, 11–21. [Google Scholar] [CrossRef]
- Pereira Coutinho, M.T.; Brito, A.C.; Pereira, P.; Gonçalves, A.S.; Moita, M.T. A phytoplankton tool for water quality assessment in semi-enclosed coastal lagoons: Open vs closed regimes. Estuar. Coast. Shelf Sci. 2012, 110, 134–146. [Google Scholar] [CrossRef]

| PPP | NPP | MPP | Total Fractions | |||||
|---|---|---|---|---|---|---|---|---|
| Cells × 106 L−1 | Ug C L−1 | Cells × 103 L−1 | Ug C L−1 | Cells × 103 L−1 | Ug C L−1 | Cells × 106 L−1 | Ug C L−1 | |
| April 2007 | 28.59 ± 5.51 | 7.14 ± 1.38 | 265.42 ± 55.71 | 10.04 ± 2.11 | 22.24 ± 3.18 | 6.33 ± 2.35 | 28.88 ± 5.48 | 23.52 ± 0.69 |
| May | 174.28 ± 35.33 | 43.57 ± 8.83 | 350.78 ± 195.69 | 10.03 ± 5.55 | 32.58 ± 4.35 | 26.12 ± 7.92 | 174.66 ± 35.49 | 79.72 ± 17.67 |
| June | 157.67 ± 34.21 | 39.42 ± 8.55 | 710.87 ± 180.95 | 22.51 ± 3.51 | 76.70 ± 16.83 | 28.82 ± 11.05 | 158.46 ± 34.30 | 90.74 ± 21.34 |
| July | 1247.26 ± 668.94 | 311.81 ± 167.24 | 310.20 ± 86.40 | 9.20 ± 2.68 | 56.89 ± 33.08 | 21.52 ± 15.88 | 1247.62 ± 669.00 | 342.53 ± 182.93 |
| August | 791.07 ± 316.68 | 197.77 ± 79.17 | 163.83 ± 19.32 | 4.68 ± 0.56 | 18.05 ± 16.94 | 7.23 ± 6.29 | 791.25 ± 316.68 | 209.69 ± 50.31 |
| September | 351.49 ± 150.31 | 87.87 ± 37.58 | 154.20 ± 13.07 | 4.26 ± 0.26 | 70.14 ± 29.84 | 30.13 ± 13.57 | 351.72 ± 150.35 | 122.27 ± 50.31 |
| October | 9.46 ± 4.55 | 2.37 ± 1.14 | 180.33 ± 60.23 | 6.82 ± 2.28 | 52.27 ± 15.68 | 24.90 ± 8.54 | 9.69 ± 4.61 | 34.08 ± 11.57 |
| November | 66.22 ± 12.88 | 16.56 ± 3.22 | 210.06 ± 79.55 | 6.10 ± 2.32 | 50.44 ± 31.39 | 18.17 ± 13.97 | 66.48 ± 12.98 | 40.83 ± 19.33 |
| December | 36.16 ± 6.47 | 9.04 ± 1.62 | 381.91 ± 242.12 | 11.59 ± 7.63 | 10.82 ± 7.48 | 3.55 ± 3.19 | 36.55 ± 6.59 | 24.18 ± 10.47 |
| January 2008 | 13.03 ± 7.89 | 3.26 ± 1.97 | 266.47 ± 81.85 | 7.41 ± 2.31 | 8.63 ± 4.76 | 2.05 ± 1.39 | 13.31 ± 7.88 | 12.73 ± 4.11 |
| February | 27.96 ± 5.66 | 6.99 ± 1.42 | 233.65 ± 32.99 | 8.84 ± 1.25 | 25.28 ± 5.11 | 7.02 ± 3.45 | 28.22 ± 5.65 | 22.85 ± 5.22 |
| March | 42.89 ± 4.55 | 10.72 ± 1.14 | 195.76 ± 77.22 | 5.51 ± 2.15 | 70.97 ± 33.68 | 26.98 ± 11.78 | 43.15 ± 4.63 | 43.20 ± 13.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caroppo, C.; Pinna, M.; Vadrucci, M.R. Phytoplankton Size Structure and Diversity in the Transitional System of the Aquatina Lagoon (Southern Adriatic Sea, Mediterranean). Microorganisms 2023, 11, 1277. https://doi.org/10.3390/microorganisms11051277
Caroppo C, Pinna M, Vadrucci MR. Phytoplankton Size Structure and Diversity in the Transitional System of the Aquatina Lagoon (Southern Adriatic Sea, Mediterranean). Microorganisms. 2023; 11(5):1277. https://doi.org/10.3390/microorganisms11051277
Chicago/Turabian StyleCaroppo, Carmela, Maurizio Pinna, and Maria Rosaria Vadrucci. 2023. "Phytoplankton Size Structure and Diversity in the Transitional System of the Aquatina Lagoon (Southern Adriatic Sea, Mediterranean)" Microorganisms 11, no. 5: 1277. https://doi.org/10.3390/microorganisms11051277





