Bovine Udder Health: From Standard Diagnostic Methods to New Approaches—A Practical Investigation of Various Udder Health Parameters in Combination with 16S rRNA Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Committee Approval
2.2. Animals and Farm Conditions
2.3. Scoring
2.4. Udder and Milk Sample Collection
2.5. Conventional Bacteriological Culturing and Somatic Cell Count
2.6. Evaluation of the Condition of the Udder Health
- Healthy animals in terms of the udder displaying normal secretion. The udder quarters display no external pathological changes; the milk is free of pathogenic microorganisms (“negative”) and has a normal average cell count (≤ 100,000 cells/mL).
- In latent infection, pathogens are detected without an increase in the somatic cell count (≤ 100,000 cells/mL).
- Subclinical mastitis is described as an inflammation of the udder without externally visible clinical signs. However, the average SCC of the milk is increased (> 100,000 cells/mL) and mastitis pathogens can be detected.
- Animals with acute clinical mastitis display inflammation on the udder, such as increased temperature and swelling. The milk is macroscopically altered, and the animals often show fever. Mastitis pathogens and an increased SCC are detectable (> 100,000 cells/mL).
2.7. Data Analysis Scoring and CMS
2.8. DNA Extraction—Teat Canal Sample
2.9. DNA Extraction—Cisternal Milk Samples
2.10. Bacterial 16S rRNA Gene Sequencing
2.11. Data Analysis Microbiome Data
3. Results
3.1. Reproduction and Production Parameters of the Cows
3.2. Scoring
3.3. Conventional Bacteriological Culturing and Somatic Cell Count
3.4. Evaluation of the Condition of the Udder Health
3.5. General Results of the 16S rRNA Gene Amplicon Sequencing
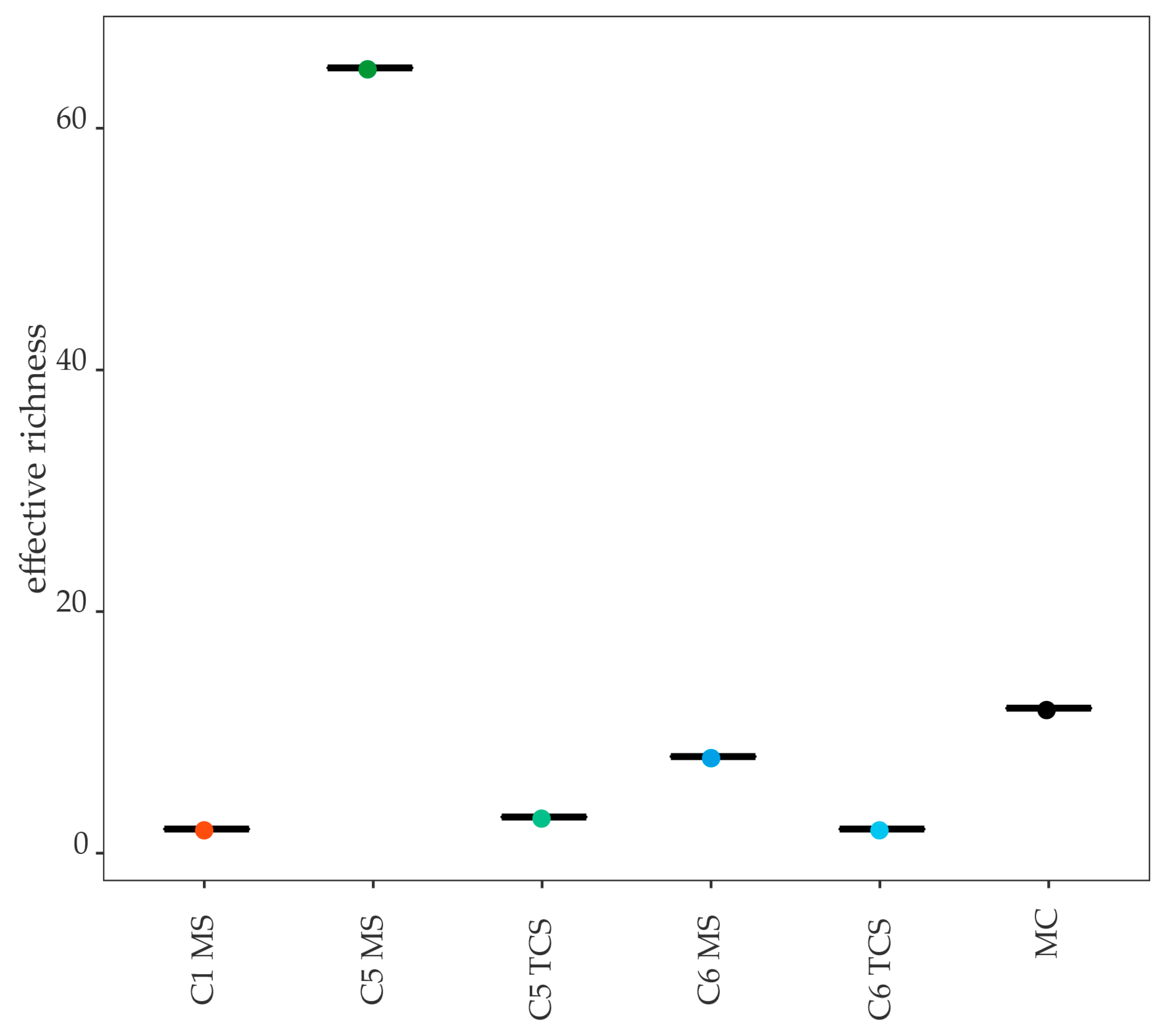
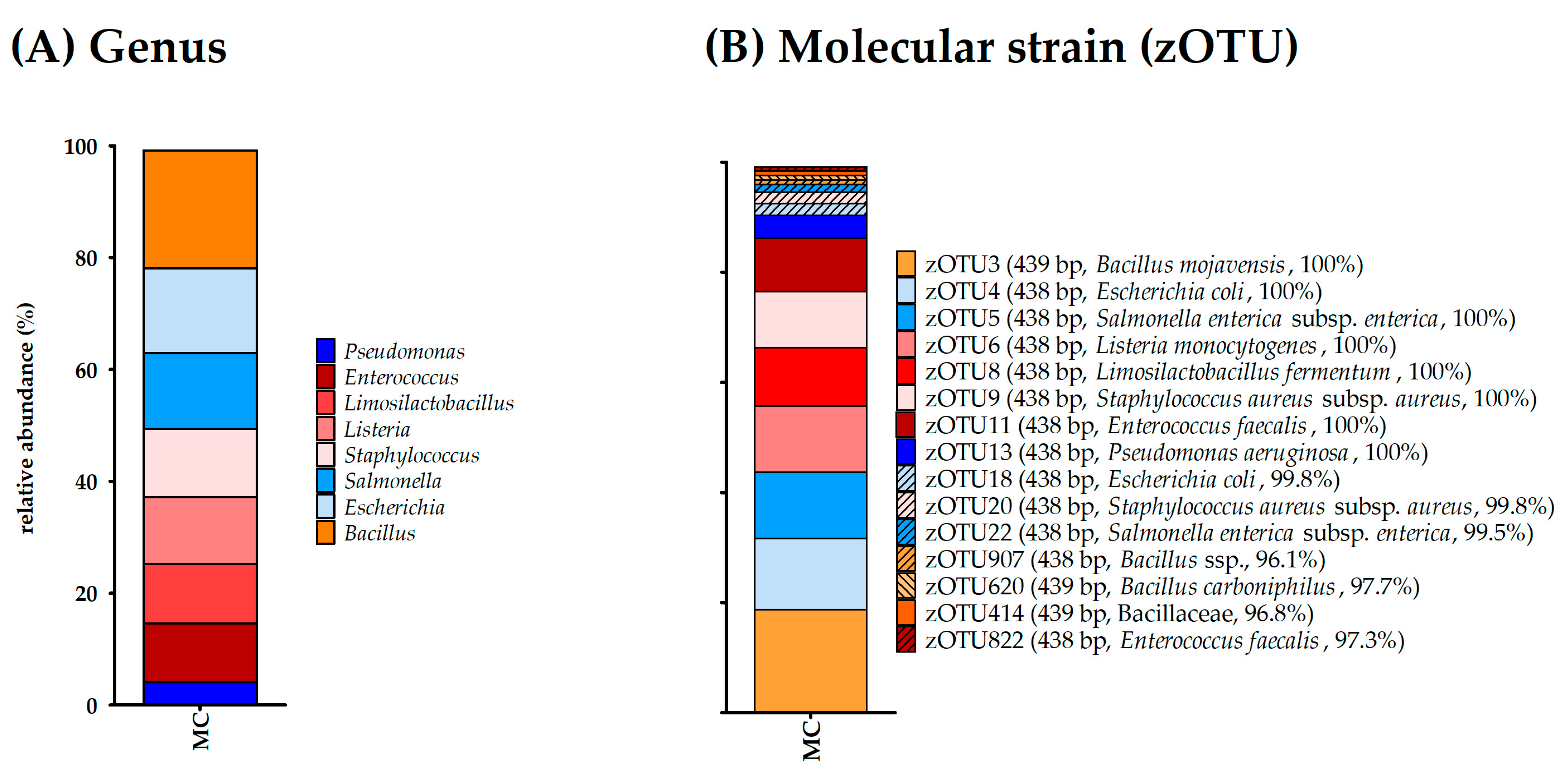
3.5.1. Mock Community
3.5.2. Negative Controls
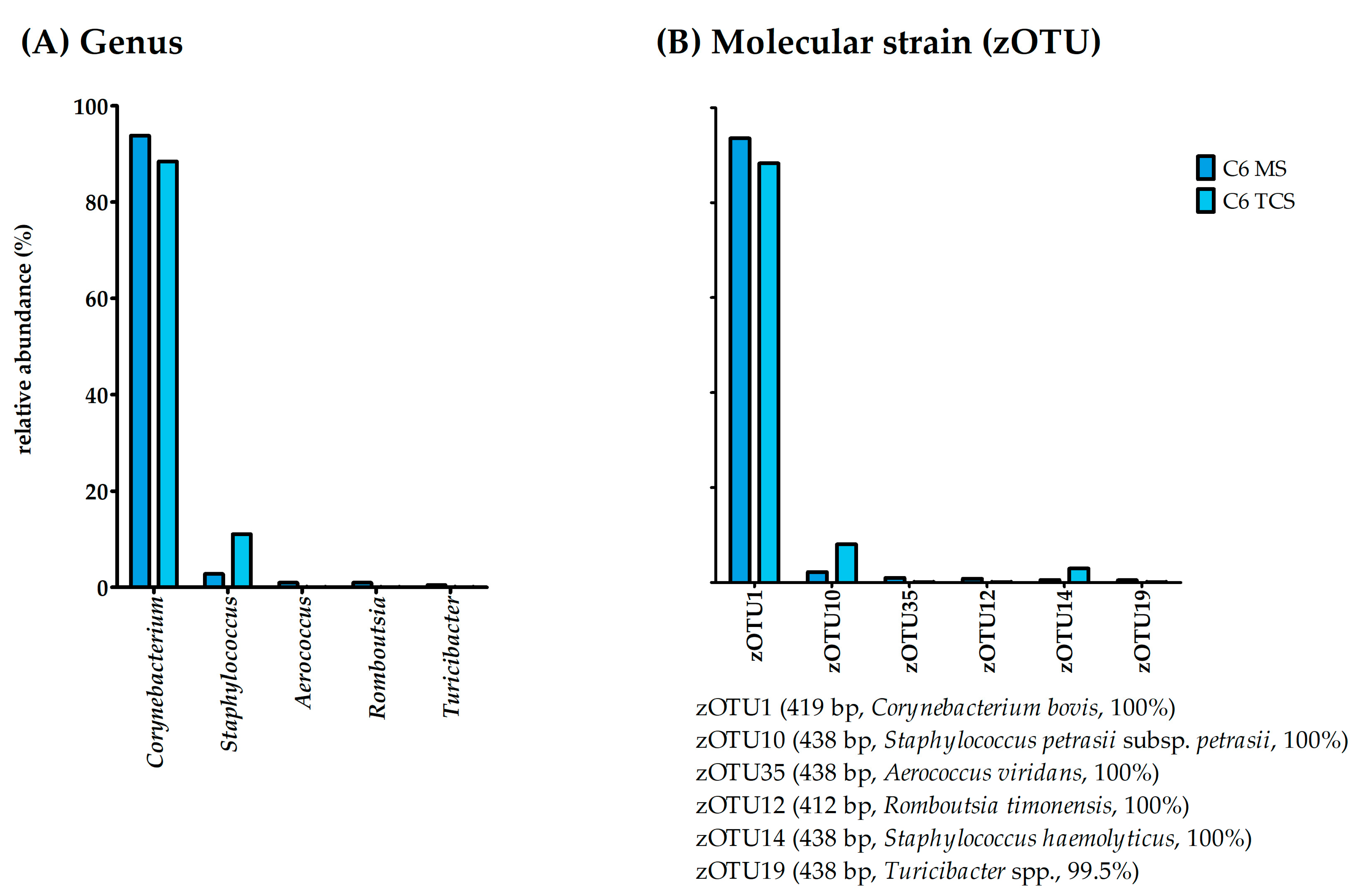
3.6. Condition of the Udder—Healthy
3.7. Condition of the Udder—Latent
3.8. Condition of the Udder—Subclinical
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kemp, M.H.; Nolan, A.M.; Cripps, P.J.; Fitzpatrick, J.L. Animal-based measurements of the severity of mastitis in dairy cows. Vet. Rec. 2008, 163, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Barbano, D.M.; Ma, Y.; Santos, M.V. Influence of raw milk quality on fluid milk shelf life. J. Dairy Sci. 2006, 89 (Suppl. S1), E15–E19. [Google Scholar] [CrossRef] [PubMed]
- van Schaik, G.; Lotem, M.; Schukken, Y.H. Trends in somatic cell counts, bacterial counts, and antibiotic residue violations in New York State during 1999-2000. J. Dairy Sci. 2002, 85, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, H.; Huijps, K.; Lam, T.J.G.M. Economic aspects of mastitis: New developments. N. Z. Vet. J. 2011, 59, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Nickerson, S.C. Morphologic changes in the bovine mammary gland during involution and lactogenesis. Am. J. Vet. Res. 1988, 49, 1112–1120. [Google Scholar]
- Bradley, A.J.; Green, M.J. The importance of the nonlactating period in the epidemiology of intramammary infection and strategies for prevention. Vet. Clin. North Am. Food Anim. Pr. 2004, 20, 547–568. [Google Scholar] [CrossRef]
- Winter, P.M.; Burvenich, C.; Hogeeven, H.; Nijenhuis, F.; Dem Rasmussen, M.; Schweigert, F.J.; Spiegeleer, B.; de Zehle, H.H. Praktischer Leitfaden Mastitis: Vorgehen beim Einzeltier und im Bestand; Parey: Stuttgart, Germany, 2009; ISBN 9783830441687. [Google Scholar]
- Constable, P.D.; Pyörälä, S.; Smith, G.W. Guidelines for Antimicrobial Use in Cattle. In Guide to Antimicrobial Use in Animals; Guardabassi, L., Jensen, L.B., Kruse, H., Eds.; Blackwell Pub: Oxford, UK; Ames, IA, USA, 2008; pp. 143–160. ISBN 9781405150798. [Google Scholar]
- Fehlings, K.; Zschöck, M.; Baumgärtner, B.; Geringer, M.; Hamann, J.; Knappstein, K. Leitlinien Entnahme von Milchproben unter antiseptischen Bedingungen und Isolierung und Identifizierung von Mastitiserregern, 3rd ed.; Verlag der Deutschen Veterinärmedizinischen Gesellschaft e.V: Gießen, Germany, 2009; ISBN 9783941703223. [Google Scholar]
- Hitch, T.C.A.; Riedel, T.; Oren, A.; Overmann, J.; Lawley, T.D.; Clavel, T. Automated analysis of genomic sequences facilitates high-throughput and comprehensive description of bacteria. ISME Commun. 2021, 1, 16. [Google Scholar] [CrossRef]
- Almeida, A.; Nayfach, S.; Boland, M.; Strozzi, F.; Beracochea, M.; Shi, Z.J.; Pollard, K.S.; Sakharova, E.; Parks, D.H.; Hugenholtz, P.; et al. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat. Biotechnol. 2021, 39, 105–114. [Google Scholar] [CrossRef]
- Nyman, A.-K.; Persson Waller, K.; Emanuelson, U.; Frössling, J. Sensitivity and specificity of PCR analysis and bacteriological culture of milk samples for identification of intramammary infections in dairy cows using latent class analysis. Prev. Vet. Med. 2016, 135, 123–131. [Google Scholar] [CrossRef]
- Taponen, S.; Salmikivi, L.; Simojoki, H.; Koskinen, M.T.; Pyörälä, S. Real-time polymerase chain reaction-based identification of bacteria in milk samples from bovine clinical mastitis with no growth in conventional culturing. J. Dairy Sci. 2009, 92, 2610–2617. [Google Scholar] [CrossRef]
- Gillespie, B.E.; Oliver, S.P. Simultaneous detection of mastitis pathogens, Staphylococcus aureus, Streptococcus uberis, and Streptococcus agalactiae by multiplex real-time polymerase chain reaction. J. Dairy Sci. 2005, 88, 3510–3518. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, M.T.; Holopainen, J.; Pyörälä, S.; Bredbacka, P.; Pitkälä, A.; Barkema, H.W.; Bexiga, R.; Roberson, J.; Sølverød, L.; Piccinini, R.; et al. Analytical specificity and sensitivity of a real-time polymerase chain reaction assay for identification of bovine mastitis pathogens. J. Dairy Sci. 2009, 92, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Paradis, M.-È.; Haine, D.; Gillespie, B.; Oliver, S.P.; Messier, S.; Comeau, J.; Scholl, D.T. Bayesian estimation of the diagnostic accuracy of a multiplex real-time PCR assay and bacteriological culture for four common bovine intramammary pathogens. J. Dairy Sci. 2012, 95, 6436–6448. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, G.; Addis, M.F.; Chassard, C.; Nader-Macias, M.E.F.; Grant, I.; Delbès, C.; Bogni, C.I.; Le Loir, Y.; Even, S. Milk Microbiota: What Are We Exactly Talking About? Front. Microbiol. 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.; Lewis, K.; Cooke, R. Mycoparasitism and plant disease control. In Fungi in Biological Control Systems; Burge, M., Ed.; Manchester University Press: Manchester, UK, 1988; pp. 161–187. [Google Scholar]
- Rainard, P. Mammary microbiota of dairy ruminants: Fact or fiction? Vet. Res. 2017, 48, 25. [Google Scholar] [CrossRef]
- Metzger, S.A.; Hernandez, L.L.; Suen, G.; Ruegg, P.L. Understanding the Milk Microbiota. Vet. Clin. North Am. Food Anim. Pr. 2018, 34, 427–438. [Google Scholar] [CrossRef]
- Derakhshani, H.; Fehr, K.B.; Sepehri, S.; Francoz, D.; de Buck, J.; Barkema, H.W.; Plaizier, J.C.; Khafipour, E. Invited review: Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. J. Dairy Sci. 2018, 101, 10605–10625. [Google Scholar] [CrossRef]
- Addis, M.F.; Tanca, A.; Uzzau, S.; Oikonomou, G.; Bicalho, R.C.; Moroni, P. The bovine milk microbiota: Insights and perspectives from -omics studies. Mol. Biosyst. 2016, 12, 2359–2372. [Google Scholar] [CrossRef]
- Taponen, S.; McGuinness, D.; Hiitiö, H.; Simojoki, H.; Zadoks, R.; Pyörälä, S. Bovine milk microbiome: A more complex issue than expected. Vet. Res. 2019, 50, 44. [Google Scholar] [CrossRef]
- Hiitiö, H.; Simojoki, H.; Kalmus, P.; Holopainen, J.; Pyörälä, S.; Taponen, S. The effect of sampling technique on PCR-based bacteriological results of bovine milk samples. J. Dairy Sci. 2016, 99, 6532–6541. [Google Scholar] [CrossRef]
- Metzger, S.A.; Hernandez, L.L.; Skarlupka, J.H.; Suen, G.; Walker, T.M.; Ruegg, P.L. Influence of sampling technique and bedding type on the milk microbiota: Results of a pilot study. J. Dairy. Sci. 2018, 101, 6346–6356. [Google Scholar] [CrossRef] [PubMed]
- Friman, M.; Hiitiö, H.; Niemi, M.; Holopainen, J.; Pyörälä, S.; Simojoki, H. The effect of a cannula milk sampling technique on the microbiological diagnosis of bovine mastitis. Vet. J. 2017, 226, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.J.; Sabour, P.M.; Gong, J.; Yu, H.; Leslie, K.E.; Griffiths, M.W. Characterization of bacterial populations recovered from the teat canals of lactating dairy and beef cattle by 16S rRNA gene sequence analysis. FEMS Microbiol. Ecol. 2006, 56, 471–481. [Google Scholar] [CrossRef]
- Falentin, H.; Rault, L.; Nicolas, A.; Bouchard, D.S.; Lassalas, J.; Lamberton, P.; Aubry, J.-M.; Marnet, P.-G.; Le Loir, Y.; Even, S. Bovine Teat Microbiome Analysis Revealed Reduced Alpha Diversity and Significant Changes in Taxonomic Profiles in Quarters with a History of Mastitis. Front. Microbiol. 2016, 7, 480. [Google Scholar] [CrossRef]
- Derakhshani, H.; Plaizier, J.C.; de Buck, J.; Barkema, H.W.; Khafipour, E. Composition of the teat canal and intramammary microbiota of dairy cows subjected to antimicrobial dry cow therapy and internal teat sealant. J. Dairy Sci. 2018, 101, 10191–10205. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.M.; de Goffau, M.C.; Perez-Muñoz, M.E.; Arrieta, M.-C.; Bäckhed, F.; Bork, P.; Braun, T.; Bushman, F.D.; Dore, J.; de Vos, W.M.; et al. Questioning the fetal microbiome illustrates pitfalls of low-biomass microbial studies. Nature 2023, 613, 639–649. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Correction to: Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 119. [Google Scholar] [CrossRef]
- Dirksen, G.; Gründer, H.-D.; Stöber, M. Die klinische Untersuchung des Rindes, 3rd ed.; neubearb. u. erw. Aufl.; Parey: Berlin/Heidelberg, Germany, 1998; ISBN 3-489-56516-9. [Google Scholar]
- Donat, K.; Gärtner, T.; Gernand, E. Pansenfüllung ante partum: Ein Parameter mit prognostischem Wert für Krankheiten und Leistungen in der Folgelaktation. In Proceedings of the bpT—Kongress DIGITAL 2020, Germany, 19 November 2020. [Google Scholar]
- Schreiner, D.A.; Ruegg, P.L. Effects of Tail Docking on Milk Quality and Cow Cleanliness. J. Dairy Sci. 2002, 85, 2503–2511. [Google Scholar] [CrossRef]
- Cook, N.; Reinemann, D.A. Tool Box for Assessing Cow, Udder and Teat Hygiene. In Proceedings of the 46th Annual MeetingNational Mastitis Council, San Antonio, TX, USA, 21–24 January 2007; pp. 31–43. [Google Scholar]
- Götze, K.; Crivellaro, P.; Pieper, L.; Engelhard, T.; Staufenbiel, R. Bewertung der Pansenfüllung von Milchkühen zur Beurteilung der individuellen Futteraufnahme in der Bestandsbetreuung. Tierarztl Prax Ausg G Grosstiere Nutztiere 2019, 47, 5–13. [Google Scholar] [CrossRef]
- Zaaijer, D.; Noordhuizen, J. A novel scoring system for monitoring the relationship between nutritional efficiency and fertility in dairy cows. Ir. Vet. J. 2003, 56, 145–152. [Google Scholar]
- Fehlings, K.; Hamann, J.; Klawonn, W.; Knappstein, K.; Mansfeld, R.; Wittkowski, G.; Zschöck, M. Bekämpfung der Mastitis des Rindes als Bestandsproblem: Leitlinien, 5th ed.; überarb. Aufl.; DVG: Gießen, Germany, 2012; ISBN 9783863450748. [Google Scholar]
- Siebert, A.; Hofmann, K.; Staib, L.; Doll, E.V.; Scherer, S.; Wenning, M. Amplicon-sequencing of raw milk microbiota: Impact of DNA extraction and library-PCR. Appl. Microbiol. Biotechnol. 2021, 105, 4761–4773. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic. Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, A.; Wenderlein, J.; Ulrich, S.; Hiereth, S.; Chitimia-Dobler, L.; Straubinger, R.K. Revealing the Tick Microbiome: Insights into Midgut and Salivary Gland Microbiota of Female Ixodes ricinus Ticks. Int. J. Mol. Sci. 2023, 24, 1100. [Google Scholar] [CrossRef] [PubMed]
- Lagkouvardos, I.; Joseph, D.; Kapfhammer, M.; Giritli, S.; Horn, M.; Haller, D.; Clavel, T. IMNGS: A comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Sci. Rep. 2016, 6, 33721. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic. Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 5056. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Fischer, S.; Kumar, N.; Clavel, T. Rhea: A transparent and modular R pipeline for microbial profiling based on 16S rRNA gene amplicons. PeerJ 2017, 5, e2836. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, D.A.; Ruegg, P.L. Relationship Between Udder and Leg Hygiene Scores and Subclinical Mastitis. J. Dairy Sci. 2003, 86, 3460–3465. [Google Scholar] [CrossRef] [PubMed]
- Abellan-Schneyder, I.; Matchado, M.S.; Reitmeier, S.; Sommer, A.; Sewald, Z.; Baumbach, J.; List, M.; Neuhaus, K. Primer, Pipelines, Parameters: Issues in 16S rRNA Gene Sequencing. mSphere 2021, 6, e01202–e01220. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, G.; Machado, V.S.; Santisteban, C.; Schukken, Y.H.; Bicalho, R.C. Microbial diversity of bovine mastitic milk as described by pyrosequencing of metagenomic 16S rDNA. PLoS ONE 2012, 7, e47671. [Google Scholar] [CrossRef]
- Kuehn, J.S.; Gorden, P.J.; Munro, D.; Rong, R.; Dong, Q.; Plummer, P.J.; Wang, C.; Phillips, G.J. Bacterial community profiling of milk samples as a means to understand culture-negative bovine clinical mastitis. PLoS ONE 2013, 8, e61959. [Google Scholar] [CrossRef]
- Bhatt, V.D.; Ahir, V.B.; Koringa, P.G.; Jakhesara, S.J.; Rank, D.N.; Nauriyal, D.S.; Kunjadia, A.P.; Joshi, C.G. Milk microbiome signatures of subclinical mastitis-affected cattle analysed by shotgun sequencing. J. Appl. Microbiol. 2012, 112, 639–650. [Google Scholar] [CrossRef]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef]
- Watts, J.L.; Lowery, D.E.; Teel, J.F.; Rossbach, S. Identification of Corynebacterium bovis and other coryneforms isolated from bovine mammary glands. J. Dairy. Sci. 2000, 83, 2373–2379. [Google Scholar] [CrossRef]
- Zadoks, R.N.; Gillespie, B.E.; Barkema, H.W.; Sampimon, O.C.; Oliver, S.P.; Schukken, Y.H. Clinical, epidemiological and molecular characteristics of Streptococcus uberis infections in dairy herds. Epidemiol. Infect. 2003, 130, 335–349. [Google Scholar] [CrossRef]
- Davies, P.L.; Leigh, J.A.; Bradley, A.J.; Archer, S.C.; Emes, R.D.; Green, M.J. Molecular Epidemiology of Streptococcus uberis Clinical Mastitis in Dairy Herds: Strain Heterogeneity and Transmission. J. Clin. Microbiol. 2016, 54, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Selbitz, H.-J.; Truyen, U.; Valentin-Weigand, P. Tiermedizinische Mikrobiologie, Infektions- und Seuchenlehre: Teil III Spezielle Bakteriologie; Grampositive Kokken; Georg Thieme Verlag: Stuttgart, Germany, 2015; ISBN 9783830412625. [Google Scholar]
- Diaz-Cao, J.M.; Barreal, M.L.; Pombo, B.; Prieto, A.; Alonso, J.M.; Iglesias, A.; Lorenzana, R.; López-Novo, C.; Díez-Baños, P.; Fernández, G. Evaluation and cluster analysis of inflammatory reactions of dairy cattle mastitis pathogens in milk samples submitted for microbiological examination. Span. J. Agric. Res. 2020, 17, e0505. [Google Scholar] [CrossRef]
- Bonsaglia, E.C.R.; Gomes, M.S.; Canisso, I.F.; Zhou, Z.; Lima, S.F.; Rall, V.L.M.; Oikonomou, G.; Bicalho, R.C.; Lima, F.S. Milk microbiome and bacterial load following dry cow therapy without antibiotics in dairy cows with healthy mammary gland. Sci. Rep. 2017, 7, 8067. [Google Scholar] [CrossRef] [PubMed]
- Ganda, E.K.; Bisinotto, R.S.; Lima, S.F.; Kronauer, K.; Decter, D.H.; Oikonomou, G.; Schukken, Y.H.; Bicalho, R.C. Longitudinal metagenomic profiling of bovine milk to assess the impact of intramammary treatment using a third-generation cephalosporin. Sci. Rep. 2016, 6, 37565. [Google Scholar] [CrossRef] [PubMed]
- Ganda, E.K.; Gaeta, N.; Sipka, A.; Pomeroy, B.; Oikonomou, G.; Schukken, Y.H.; Bicalho, R.C. Normal milk microbiome is reestablished following experimental infection with Escherichia coli independent of intramammary antibiotic treatment with a third-generation cephalosporin in bovines. Microbiome 2017, 5, 74. [Google Scholar] [CrossRef]
- Vangroenweghe, F.; Dosogne, H.; Mehrzad, J.; Burvenich, C. Effect of milk sampling techniques on milk composition, bacterial contamination, viability and functions of resident cells in milk. Vet. Res. 2001, 32, 565–579. [Google Scholar] [CrossRef]


| Subject of Examination | Examination Method | Evaluation Key | Source |
|---|---|---|---|
| General examination | |||
| Rectal temperature | Rectal thermometer | [°C] | [32] |
| Breathing rate | Counting respiratory movements at the costal arch and flank | Breaths per minute | [32] |
| Rumen filling | Rumen filling score | 1, 3, 5 | [33] |
| Further examination of the udder | |||
| Hygiene of the udder skin | Hygiene score | 1–4 | [7,34,35] |
| Abnormalities in texture, condition, temperature | Palpation | Yes/No | [32] |
| Functionality of each udder quarter | Palpation | Intact/Blind | [32] |
| Sensory secretion examination | |||
| Consistency of the milk secretion | Visual evaluation | NAD, a–f | [32] |
| Quarter Level | Bacteriological Examination | ||
|---|---|---|---|
| Negative | Positive | ||
| SCC | ≤ 100,000 cells/mL | normal secretion | latent infection |
| > 100,000 cells/mL | unspecific mastitis | mastitis | |
| Cow–ID | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | Average | Median | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reproduction and production parameters of the cows | ||||||||||||
| Number of lactation instances | 2 | 2 | 3 | 4 | 2 | 5 | 2 | 2 | 2.8 | 2 | ||
| Dry period in days | 64 | 51 | 55 | 52 | 59 | 60 | 62 | 64 | 58.4 | 59.5 | ||
| Average amount of milk from previous lactations in kg | 9708 | 9978 | 8436 | 7975 | 9116 | 10,783 | 9943 | 5483 | 8928 | 9412 | ||
| Results of the general examination | ||||||||||||
| Rectal temperature in °C | 38.4 | 38.6 | 38.8 | 38.5 | 38.5 | 38.0 | 38.5 | 38.4 | 38.5 | 38.5 | ||
| Breathing rate in breaths per minute | 22 | 32 | 22 | 24 | 36 | 24 | 24 | 40 | 28 | 24 | ||
| Rumen filling (“1”, “3”, or “5”) | 3 | 3 | 3 | 1 | 3 | 1 | 3 | 3 | 3 | 3 | ||
| Results of the further examination of the udder and the sensory secretion examination | ||||||||||||
| Hygiene of the udder skin (“1”–“4”) | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | ||
| Abnormalities in texture, condition, temperature | Yes (Edema) | No | No | No | No | No | No | No | ||||
| Consistency of the secretion | NAD | NAD | NAD | NAD | NAD | NAD | NAD | NAD | ||||
| Cytological and bacteriological results with an evaluation of the udder health | ||||||||||||
| SCC before drying off (in 1000 cells per mL) | 125 | 16 | 57 | 43 | 34 | 611 | 69 | 162 | 139.6 | 63.0 | ||
| SCC average on seven DIM (in 1000 cells per mL) | 54 | 20 | 11 | 50 | 101 | 26 | 29 | 22 | 39.0 | 27.4 | ||
| Examinations on the quarter level on seven DIM | FL | BC | neg | neg | neg | neg | neg | neg | neg | CNS | ||
| SCC | 9 | 14 | 11 | 131 | 36 | 10 | 26 | 6 | ||||
| FR | BC | neg | neg | neg | neg | neg | neg | neg | neg | |||
| SCC | 32 | 21 | 13 | 10 | 237 | 15 | 38 | 30 | ||||
| RR | BC | S. uberis | neg | neg | neg | neg | neg | neg | - | |||
| SCC | 149 | 19 | 12 | 8 | 46 | 27 | 26 | - | ||||
| RL | BC | neg | neg | neg | - | CNS | C. bovis | neg | neg | |||
| SCC | 27 | 24 | 9 | - | 84 | 50 | 27 | 29 | ||||
| Condition of the udder health (i.e., mastitis) * | subclinical | healthy | healthy | subclinical | subclinical, latent | latent | healthy | latent | ||||
| Cow ID | Sample Type | DNA Content (ng/µL) |
|---|---|---|
| C1 | MS | 2.12 |
| TCS | 0.44 | |
| C2 | MS | 0.05 |
| TCS | 0.38 | |
| C3 | MS | 0.04 |
| TCS | 0.31 | |
| C4 | MS | 0.10 |
| TCS | 0.06 | |
| C5 | MS | 5.35 |
| TCS | 2.22 | |
| C6 | MS | 1.34 |
| TCS | 3.85 | |
| C7 | MS | 0.24 |
| TCS | 0.25 | |
| C8 | MS | 0.16 |
| TCS | 0.56 | |
| Mock sample | 0.03 | |
| DNA stool stabilizer | 0.02 | |
| Mock community | 41.23 | |
| NaCl | 0.59 |
| Bacterial Species | Representing OTUs |
|---|---|
| Pseudomonas aeruginosa | OTU11 |
| Escherichia coli | OTU4 |
| Salmonella enterica | OTU5 |
| Lactobacillus fermentum | OTU8 |
| Enterococcus faecalis | OTU9 |
| Staphylococcus aureus | OTU2, OTU645, OTU417 |
| Listeria monocytogenes | OTU6 |
| Bacillus subtilis | OTU3, OTU787, OTU462 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rötzer, V.; Wenderlein, J.; Wiesinger, A.; Versen, F.; Rauch, E.; Straubinger, R.K.; Zeiler, E. Bovine Udder Health: From Standard Diagnostic Methods to New Approaches—A Practical Investigation of Various Udder Health Parameters in Combination with 16S rRNA Sequencing. Microorganisms 2023, 11, 1311. https://doi.org/10.3390/microorganisms11051311
Rötzer V, Wenderlein J, Wiesinger A, Versen F, Rauch E, Straubinger RK, Zeiler E. Bovine Udder Health: From Standard Diagnostic Methods to New Approaches—A Practical Investigation of Various Udder Health Parameters in Combination with 16S rRNA Sequencing. Microorganisms. 2023; 11(5):1311. https://doi.org/10.3390/microorganisms11051311
Chicago/Turabian StyleRötzer, Verena, Jasmin Wenderlein, Anna Wiesinger, Felix Versen, Elke Rauch, Reinhard K. Straubinger, and Eva Zeiler. 2023. "Bovine Udder Health: From Standard Diagnostic Methods to New Approaches—A Practical Investigation of Various Udder Health Parameters in Combination with 16S rRNA Sequencing" Microorganisms 11, no. 5: 1311. https://doi.org/10.3390/microorganisms11051311
APA StyleRötzer, V., Wenderlein, J., Wiesinger, A., Versen, F., Rauch, E., Straubinger, R. K., & Zeiler, E. (2023). Bovine Udder Health: From Standard Diagnostic Methods to New Approaches—A Practical Investigation of Various Udder Health Parameters in Combination with 16S rRNA Sequencing. Microorganisms, 11(5), 1311. https://doi.org/10.3390/microorganisms11051311







