Importance of Pre-Milking Udder Hygiene to Reduce Transfer of Clostridial Spores from Teat Skin to Raw Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Overview
2.1.1. Teat Swab samples
2.1.2. Pooled Quarter Milk Samples
2.1.3. Bulk Tank Milk Samples
2.2. Quantification of Clostridial Spores
2.2.1. Analysis of Raw Milk Samples: PQM and BTM
2.2.2. Analysis of Teat Swab Samples and Negative Controls
2.3. Farm Management Data, Average Cow Cleanliness, Somatic Cell Count, and Colony Count
2.4. Statistical Analyses
3. Results and Discussion
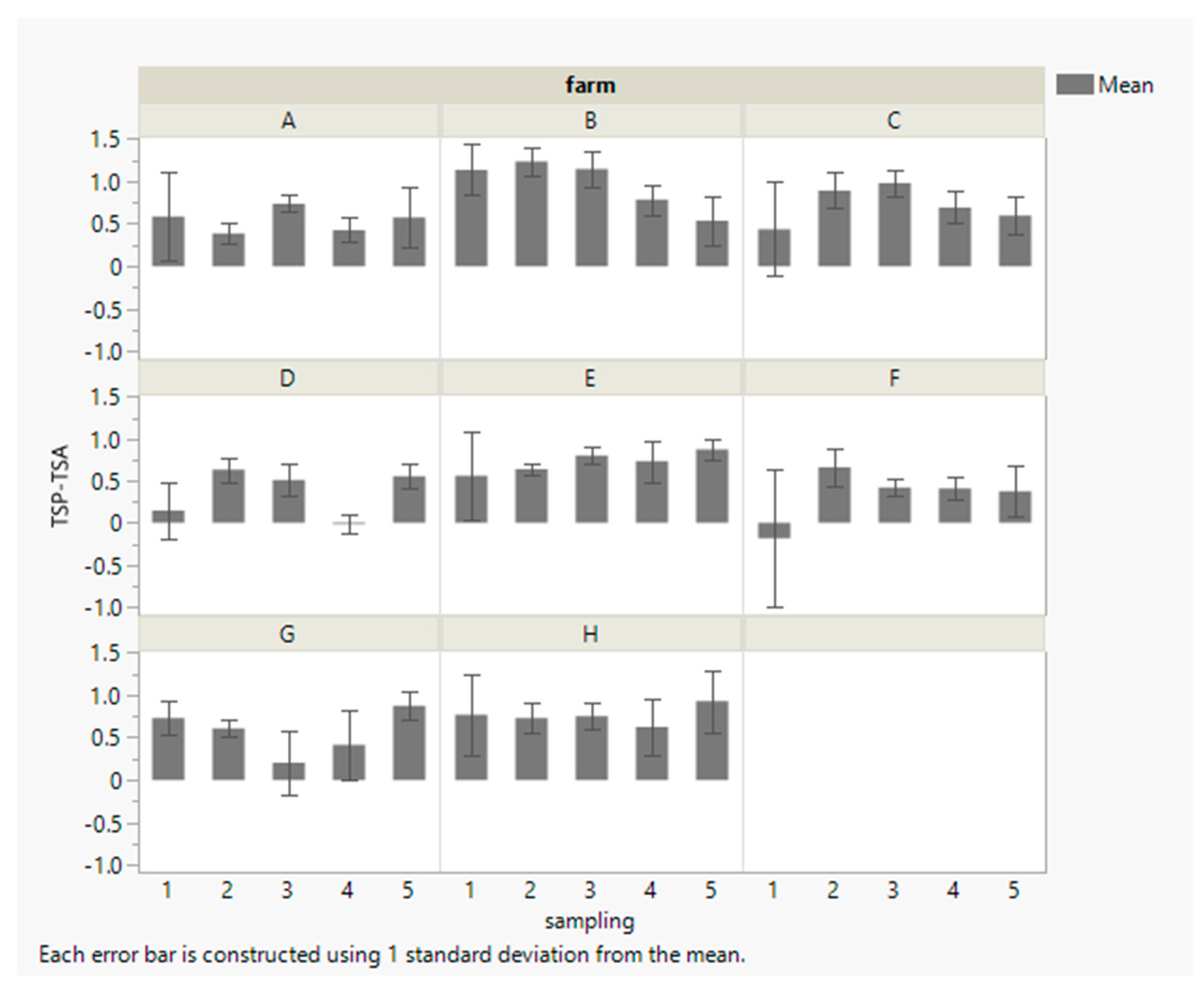
3.1. Teat Cleaning Efficacy
3.2. BAPC Spore Counts in Milk
3.2.1. BAPC Spore Counts in PQM
3.2.2. BAPC Spore Counts in BTM
3.3. Correlations of BAPC Spore Counts in BTM with Somatic Cell Count (SCC) and Colony Count (CC)
3.4. Correlations of BAPC Spore Counts in PQM with Farm Management Data
3.5. Correlations of BAPC Spore Counts in Raw Milk with the Average Cow Cleanliness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klijn, N.; Nieuwenhof, F.F.J.; Hoolwerf, J.D.; Van der Waals, C.B.; Weerkamp, A.H. Identification of Clostridium tyrobutyricum as the causative agent of late blowing in cheese by species-specific PCR amplification. Appl. Environ. Microbiol. 1995, 61, 2919–2924. [Google Scholar] [CrossRef]
- Brändle, J.; Domig, K.J.; Kneifel, W. Relevance and analysis of butyric acid producing clostridia in milk and cheese. Food Control 2016, 67, 96–113. [Google Scholar] [CrossRef]
- Vissers, M.M.M.; Driehuis, F.; Te Giffel, M.C.; De Jong, P.; Lankveld, J.M.G. Minimizing the Level of Butyric Acid Bacteria Spores in Farm Tank Milk. J. Dairy Sci. 2007, 90, 3278–3285. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.M.M.; Driehuis, F.; Te Giffel, M.C.; De Jong, P.; Lankveld, J.M.G. Short communication: Quantification of the transmission of microorganisms to milk via dirt attached to the exterior of teats. J. Dairy Sci. 2007, 90, 3579–3582. [Google Scholar] [CrossRef] [PubMed]
- Bava, L.; Colombini, S.; Zucali, M.; Decimo, M.; Morandi, S.; Silvetti, T.; Brasca, M.; Tamburini, A.; Crovetto, G.M.; Sandrucci, A. Efficient milking hygiene reduces bacterial spore contamination in milk. J. Dairy Res. 2017, 84, 322–328. [Google Scholar] [CrossRef]
- Vissers, M.M.M.; Driehuis, F.; Te Giffel, M.C.; De Jong, P.; Lankveld, J.M.G. Improving farm management by modeling the contamination of farm tank milk with butyric acid bacteria. J. Dairy Sci. 2006, 89, 850–858. [Google Scholar] [CrossRef]
- Zucali, M.; Bava, L.; Colombini, S.; Brasca, M.; Decimo, M.; Morandi, S.; Tamburini, A.; Crovetto, G.M. Management practices and forage quality affecting the contamination of milk with anaerobic spore-forming bacteria. J. Sci. Food Agric. 2015, 95, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.H.; Kent, D.J.; Evanowski, R.L.; Zuber Hrobuchak, T.J.; Wiedmann, M. Bacterial spore levels in bulk tank raw milk are influenced by environmental and cow hygiene factors. J. Dairy Sci. 2019, 102, 9689–9701. [Google Scholar] [CrossRef]
- Doyle, C.J.; Gleeson, D.; Jordan, K.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. Anaerobic sporeformers and their significance with respect to milk and dairy products. Int. J. Food Microbiol. 2015, 197, 77–87. [Google Scholar] [CrossRef]
- Magnusson, M.; Christiansson, A.; Svensson, B.; Kolstrup, C. Effect of different premilking manual teat-cleaning methods on bacterial spores in milk. J. Dairy Sci. 2006, 89, 3866–3875. [Google Scholar] [CrossRef]
- Schreiner, D.A.; Ruegg, P.L. Relationship between udder and leg hygiene scores and subclinical mastitis. J. Dairy Sci. 2003, 86, 3460–3465. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, E.; Arnesson, A.; Bengtsson, A. Investigation of Clostridial Spores in Swedish Dairy Herds. In Proceedings of the 14th International Symposium of Forage Conservation, Brno, Czech Republic, 17–19 March 2010; pp. 79–82. [Google Scholar]
- Cook, N.; Reinemann, D. A Tool Box for Assessing Cow, Udder and Teat Hygiene. 46th Annual Meeting of the National Mastitis Council. San Antonio, Texas. 2007. Available online: https://www.researchgate.net/publication/237580204_A_Tool_Box_for_Assessing_Cow_Udder_and_Teat_Hygiene/citations (accessed on 26 March 2023).
- AgrarMarkt Austria. Kennzahlen der österreichischen Milchwirtschaft (bis 2020). Available online: https://www.ama.at/getattachment/d1948ea0-a131-4404-acb9-ab66938cda6d/Kennzahlen_AT_1995-19.pdf (accessed on 24 March 2023).
- Brändle, J.; Heinzle, L.; Fraberger, V.; Berta, J.; Zitz, U.; Schinkinger, M.; Stocker, W.; Kneifel, W.; Domig, K.J. Novel approach to enumerate clostridial endospores in milk. Food Control 2018, 85, 318–326. [Google Scholar] [CrossRef]
- ISO.6887:5:2010; Microbiology of Food and Animal Feeding Stuffs–Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. ISO: Geneva, Switzerland, 2010.
- Hurley, M.A.; Roscoe, M.E. Automated statistical analysis of microbial enumeration by dilution series. J. Appl. Bacteriol. 1983, 55, 159–164. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Melin, M.; Wiktorsson, H.; Christiannsson, A.; McLean, J.; Sinclair, M.; West, B. Teat cleaning efficiency before milking in DeLaval VMSTM versus conventional manual cleaning using Clostridium tyrobutyricum spores as marker. In Proceedings of the First North American Conference on robotic milking, Toronto, ON, Canada, 20–22 March 2002. [Google Scholar]
- Stadhouders, J.; Jorgensen, K. Prevention of the contamination of raw milk by a hygienic milk production. Bull. Int. Dairy Fed. 1990, 251, 32–36. [Google Scholar]
- Rasmussen, M.D.; Galton, D.M.; Petersson, L.G. Effects of Premilking Teat Preparation on Spores of Anaerobes, Bacteria, and Iodine Residues in Milk. J. Dairy Sci. 1991, 74, 2472–2478. [Google Scholar] [CrossRef]
- Ruf, R.; Bolt, R.; Hässig, M. Vergleich von Papier und Holzwolle zur Euterreinigung. Klauentierpraxis 2015, 23, 111–116. [Google Scholar]
- Frey, H. Werkstoffmonografie Holzwolle Daten, Fakten; 2011, Editionylichtensteig. Available online: https://www.lignum.ch/files/_migrated/content_uploads/Werkstoff_Holzwolle.PDF (accessed on 26 March 2023).
- Summer, A.; Franceschi, P.; Formaggioni, P.; Malacarne, M. Characteristics of raw milk produced by free-stall or tie-stall cattle herds in the Parmigiano-Reggiano cheese production area. Dairy Sci. Technol. 2014, 94, 581–590. [Google Scholar] [CrossRef]
- Feligini, M.; Brambati, E.; Panelli, S.; Ghitti, M.; Sacchi, R.; Capelli, E.; Bonacina, C. One-year investigation of Clostridium spp. occurrence in raw milk and curd of Grana Padano cheese by the automated ribosomal intergenic spacer analysis. Food Control 2014, 42, 71–77. [Google Scholar] [CrossRef]
- FAO; IDF. Guide to Good Dairy Farming Practice. In Animal Production and Health Guidelines; FAO: Rome, Italy; IDF: Springfield, MO, USA, 2011; No. 8. [Google Scholar]
- Secchi, G.; Amalfitano, N.; Carafa, I.; Franciosi, E.; Gallo, L.; Schiavon, S.; Sturaro, E.; Tagliapietra, F.; Bittante, G. Milk metagenomics and cheese-making properties as affected by indoor farming and summer highland grazing. J. Dairy Sci. 2023, 106, 96–116. [Google Scholar] [CrossRef]
- Barkema, H.W.; Schukken, Y.H.; Lam, T.J.G.M.; Beiboer, M.L.; Benedictus, G.; Brand, A. Management Practices Associated with Low, Medium, and High Somatic Cell Counts in Bulk Milk. J. Dairy Sci. 1998, 81, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.I.; Kent, D.; Martin, N.H.; Evanowski, R.L.; Patel, K.; Godden, S.M.; Wiedmann, M. Bedding and bedding management practices are associated with mesophilic and thermophilic spore levels in bulk tank raw milk. J. Dairy Sci. 2019, 102, 6885–6900. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.L. Standards for somatic cells in milk: Physiological and regulatory. IDF Mastit. News 1996, 21, 7–9. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burtscher, J.; Rudavsky, T.; Zitz, U.; Neubauer, V.; Domig, K.J. Importance of Pre-Milking Udder Hygiene to Reduce Transfer of Clostridial Spores from Teat Skin to Raw Milk. Microorganisms 2023, 11, 1337. https://doi.org/10.3390/microorganisms11051337
Burtscher J, Rudavsky T, Zitz U, Neubauer V, Domig KJ. Importance of Pre-Milking Udder Hygiene to Reduce Transfer of Clostridial Spores from Teat Skin to Raw Milk. Microorganisms. 2023; 11(5):1337. https://doi.org/10.3390/microorganisms11051337
Chicago/Turabian StyleBurtscher, Johanna, Tamara Rudavsky, Ulrike Zitz, Viktoria Neubauer, and Konrad J. Domig. 2023. "Importance of Pre-Milking Udder Hygiene to Reduce Transfer of Clostridial Spores from Teat Skin to Raw Milk" Microorganisms 11, no. 5: 1337. https://doi.org/10.3390/microorganisms11051337
APA StyleBurtscher, J., Rudavsky, T., Zitz, U., Neubauer, V., & Domig, K. J. (2023). Importance of Pre-Milking Udder Hygiene to Reduce Transfer of Clostridial Spores from Teat Skin to Raw Milk. Microorganisms, 11(5), 1337. https://doi.org/10.3390/microorganisms11051337





