Mutualistic Relationships between Microorganisms and Eusocial Wasps (Hymenoptera, Vespidae)
Abstract
1. Introduction
2. Defence against Pathogens: Antimicrobial Secretions
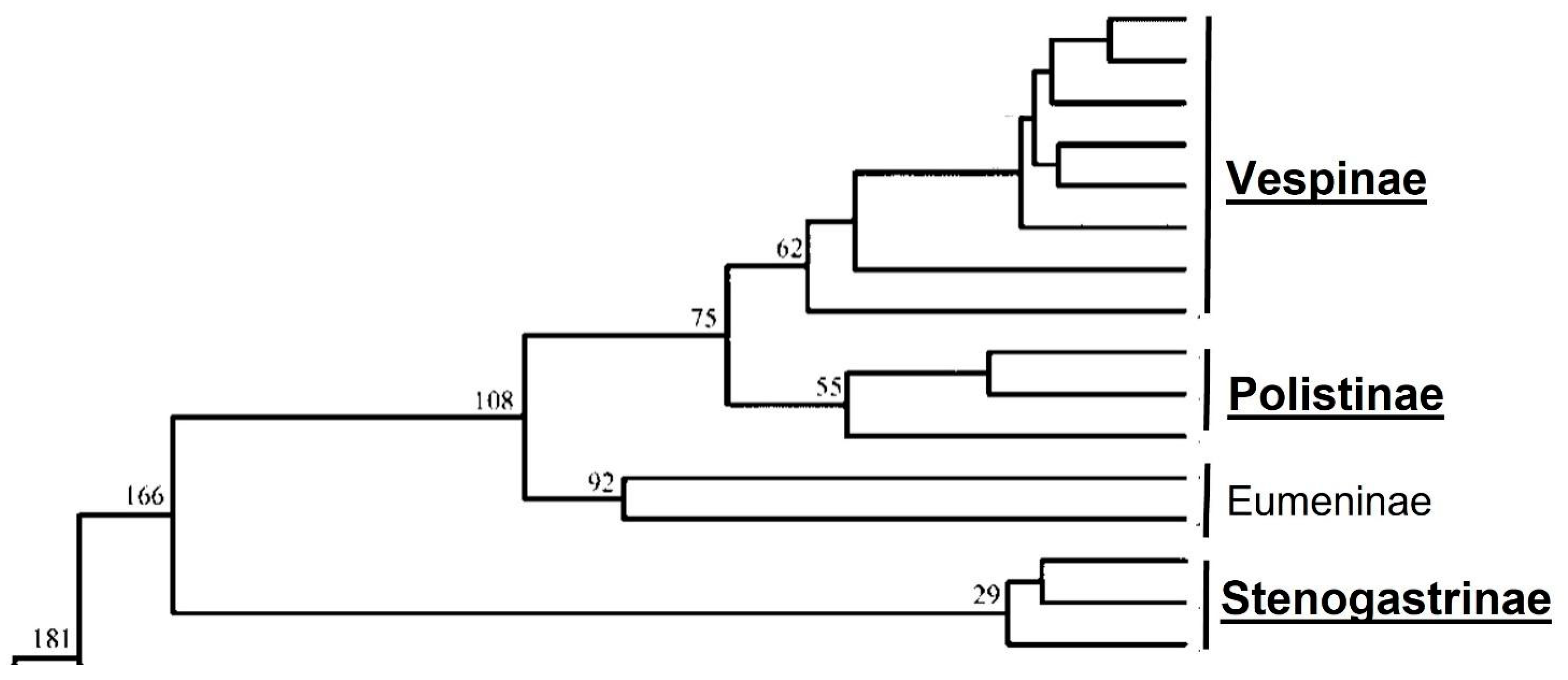
3. The Eusocial Wasps
3.1. The Stenogastrinae: The Primitively Eusocial Wasps
3.2. The Polistinae and Vespinae: The More Evolute Eusocial Wasps
3.2.1. Viruses
3.2.2. Bacteria
3.2.3. Fungi
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilson, E.O. Genesis: The Deep Origin of Societies; Penguin: London, UK, 2019. [Google Scholar]
- Hines, H.M.; Hunt, J.H.; O’Connor, T.K.; Gillespie, J.J.; Cameron, S.A. Multigene phylogeny reveals eusociality evolved twice in vespid wasps. Proc. Natl. Acad. Sci. USA 2007, 104, 3295–3299. [Google Scholar] [CrossRef]
- Piekarski, P.K.; Carpenter, J.M.; Lemmon, A.R.; Moriarty Lemmon, E.; Sharanowski, B.J. Phylogenomic evidence overturns current conceptions of social evolution in wasps (Vespidae). Mol. Biol. Evol. 2018, 35, 2097–2109. [Google Scholar] [CrossRef]
- Huang, P.; Carpenter, J.M.; Chen, B.; Li, T.J. The first divergence time estimation of the subfamily Stenogastrinae (Hymenoptera: Vespidae) based on mitochondrial phylogenomics. Int. J. Biol. Macromol. 2019, 137, 767–773. [Google Scholar] [CrossRef]
- Boomsma, J.J.; Schmid-Hempel, P. Pressure across the Major Groups of Social Insects. In Insect Evolutionary Ecology, Proceedings of the Royal Entomological Society’s 22nd Symposium, Reading, UK, 2005; CABI International: Wallingford, UK, 2005; p. 139. [Google Scholar]
- Hughes, D.P.; Pierce, N.E.; Boomsma, J.J. Social insect symbionts: Evolution in homeostatic fortresses. Trends Ecol. Evol. 2008, 23, 672–677. [Google Scholar] [CrossRef]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution ndof animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef]
- Rosenberg, E. Evolution of holobionts: The hologenome concept. In Microbiomes: Current Knowledge and Unanswered Questions; Springer: Cham, Switzerland, 2021; pp. 317–352. [Google Scholar]
- Shigenobu, S.; Yorimoto, S. Aphid hologenomics: Current status and future challenges. Curr. Opin. Insect Sci. 2022, 50, 100882. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Hu, H.; Wang, G.H. Nasonia–microbiome associations: A model for evolutionary hologenomics research. Trends Parasitol. 2022, 39, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Guerrero, R. The holobiont concept: The case of xylophagous termites and cockroaches. Symbiosis 2016, 68, 49–60. [Google Scholar] [CrossRef]
- Guerrero, R.; Margulis, L.; Berlanga, M. Symbiogenesis: The holobiont as a unit of evolution. Int. Microbiol. 2013, 16, 133–143. [Google Scholar]
- Mayorga-Ch, D.; Rodríguez-C, C.; Ortíz-Reyes, A.; Romero-Tabarez, M.; Sarmiento, C.E. Interactions of Social Wasps in Microorganisms. In Neotropical Social Wasps: Basic and Applied Aspects; Prezoto, F., Nascimento, F.S., Barbosa, B.C., Somavilla, A., Eds.; Springer: Cham, Switzerland, 2021; pp. 405–434. [Google Scholar]
- Cremer, S.; Armitage, S.A.; Schmid-Hempel, P. Social immunity. Curr. Biol. 2007, 17, R693–R702. [Google Scholar] [CrossRef] [PubMed]
- Baracchi, D.; Mazza, G.; Turillazzi, S. From individual to collective immunity: The role of the venom as antimicrobial agent in the Stenogastrinae wasp societies. J. Insect Physiol. 2012, 58, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Erturk, Ö.; Bagdatli, E. A comprehensive study on nest materials of Vespa crabro and Polistes dominula: Chemical properties and biological characterization with antioxidant and antimicrobial activity. Biologia 2019, 74, 797–812. [Google Scholar] [CrossRef]
- Gambino, P. Antibiotic activity of larval saliva of Vespula wasps. J. Invertebr. Pathol. 1993, 61, 110. [Google Scholar] [CrossRef]
- Turillazzi, S.; Perito, B.; Pazzagli, L.; Pantera, B.; Gorfer, S.; Tancredi, M. Antibacterial activity of larval saliva of the European paper wasp Polistes dominulus (Hymenoptera, Vespidae). Insectes Sociaux 2004, 51, 339–341. [Google Scholar] [CrossRef]
- Hirai, Y.; Yasuhara, T.; Yoshida, H.; Nakajima, T.; Fujino, M.; Kitada, C. A New Mast Cell Degranulating Peptide “Mastoparan” in the Venom of Vespula lewisii. Chem. Pharm. Bull. 1979, 27, 1942–1944. [Google Scholar] [CrossRef]
- Choi, M.B.; Lee, Y.H. The structure and antimicrobial potential of wasp and hornet (Vespidae) mastoparans: A review. Entomol. Res. 2020, 50, 369–376. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Lee, W.H.; Zhang, Y. Antimicrobial peptides from the venom gland of the social wasp Vespa tropica. Toxicon 2013, 74, 151–157. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.; Lee, Y.J.; Kim, H.R.; Nam, J.O.; Choi, M.B.; Hahn, D. Antibacterial potential of Nidus vespae built by invasive alien hornet, Vespa velutina nigrithorax, against food-borne pathogenic bacteria. Entomol. Res. 2020, 50, 28–33. [Google Scholar] [CrossRef]
- Kim, Y.; Son, M.; Noh, E.-Y.; Kim, S.; Kim, C.; Yeo, J.-H.; Park, C.; Lee, K.W.; Bang, W.Y. MP-V1 from the Venom of Social Wasp Vespula vulgaris Is a de Novo Type of Mastoparan that Displays Superior Antimicrobial Activities. Molecules 2016, 21, 512. [Google Scholar] [CrossRef]
- Ertürk, Ö.; Başkan, C.; Koleren, Z. Antimicrobial Activities of Nest Materials Dolichovespula saxonica (Fabricius, 1793) (Hymenoptera: Vespidae) in Turkey. Arıcılık Araştırma Derg. 2018, 10, 9–14. [Google Scholar]
- Wang, K.; Dang, W.; Xie, J.; Zhu, R.; Sun, M.; Jia, F.; Zhao, Y.; An, X.; Qiu, S.; Li, X.; et al. Antimicrobial peptide protonectin (Agelaya pallipes pallipes) disturbs the membrane integrity and induces ROS production in yeast cells. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 2365–2373. [Google Scholar] [CrossRef]
- Ribeiro, S.P.; Mendes, M.A.; Dos Santos, L.D.; de Souza, B.M.; Marques, M.R.; de Azevedo, W.F., Jr.; Palma, M.S. Structural and functional characterization of N-terminally blocked peptides isolated from the venom of the social wasp Polybia paulista. Peptides 2004, 25, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, R.C.; Trentini, M.M.; de Castro e Silva, J.; Simon, K.S.; Bocca, A.L.; Silva, L.P.; Mortari, M.R.; Kipnis, A.; Junqueira-Kipnis, A.P. Antimycobacterial activity of a new peptide polydim-I isolated from neotropical social wasp Polybia dimorpha. PLoS ONE 2016, 11, e0149729. [Google Scholar] [CrossRef] [PubMed]
- Lopes, K.S.; Campos, G.A.A.; Camargo, L.C.; de Souza, A.C.B.; Ibituruna, B.V.; Magalhães, A.C.M.; da Rocha, L.F.; Garcia, A.B.; Rodrigues, M.C.; Ribeiro, D.M.; et al. Characterization of two peptides isolated from the venom of social wasp Chartergellus communis (Hymenoptera: Vespidae): Influence of multiple alanine residues and C-terminal amidation on biological effects. Peptides 2017, 95, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Freire, D.O.; da Cunha, N.B.; Leite, M.L.; Kostopoulos, A.G.; da Silva, S.N.; de Souza, A.C.; Nolasco, D.O.; Franco, O.L.; Mortari, M.R.; Dias, S.C. Wasp venom peptide, synoeca-MP, from Synoeca surinama shows antimicrobial activity against human and animal pathogenic microorganisms. Pept. Sci. 2020, 112, e24141. [Google Scholar] [CrossRef]
- Turillazzi, S.; Mastrobuoni, G.; Dani, F.R.; Moneti, G.; Pieraccini, G.; la Marca, G.; Nolasco, D.O.; Franco, O.L.; Mortari, M.R.; Dias, S.C. Dominulin A and B: Two new antibacterial peptides identified on the cuticle and in the venom of the social paper wasp Polistes dominulus using MALDI-TOF, MALDI-TOF/TOF, and ESI-ion trap. J. Am. Soc. Mass Spectrom. 2006, 17, 376–383. [Google Scholar] [CrossRef]
- Čeřovský, V.; Slaninová, J.; Fučík, V.; Hulačová, H.; Borovičková, L.; Ježek, R.; Bednárová, L. New potent antimicrobial peptides from the venom of Polistinae wasps and their analogs. Peptides 2008, 29, 992–1003. [Google Scholar] [CrossRef]
- Al-Shammery, K.; Hozzein, W.N. Antibacterial activities of two potential peptides extracted from Polistes wattii Cameron, 1900 (Vespidae: Polistinae) wasp venom collected at Eastern Province, Saudi Arabia. PLoS ONE 2022, 17, e0264035. [Google Scholar] [CrossRef]
- Hoggard, S.J.; Wilson, P.D.; Beattie, A.J.; Stow, A.J. Social complexity and nesting habits are factors in the evolution of antimicrobial defences in wasps. PLoS ONE 2011, 6, e21763. [Google Scholar] [CrossRef]
- Turillazzi, S. The Biology of Hover Wasps; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Krombein, K.V. Biosystematic Studies of Ceylonese Wasps, XIX: Natural History Notes in Several Families (Hymenoptera: Eumenidae, Vespidae, Pompilidae and Crabronidae); Smithsonian Contributions to Zoology; Smithsonian Institution Press: Washington, DC, USA, 1991. [Google Scholar]
- Hansell, M.H.; Turillazzi, S. Nest structure and building material of three species of Anischnogaster (Vespidae Stenogastrinae) from Papua New Guinea. Trop. Zool. 1995, 8, 203–219. [Google Scholar] [CrossRef]
- Turillazzi, S. Function and characteristics of the abdominal substance secreted by wasps of the genus Parischnogaster (Hymenoptera Stenogastrinae). Monit. Zool. Ital. J. Zool. 1985, 19, 91–99. [Google Scholar]
- Silveira, O.T.; Andena, S.R.; Somavilla, A.; Carpenter, J.M. Phylogeny and classification of the Neotropical social wasps. In Neotropical Social Wasps: Basic and Applied Aspects; Springer: Cham, Switzerland, 2021; pp. 267–291. [Google Scholar]
- Kaltenpoth, M.; Engl, T. Defensive microbial symbionts in Hymenoptera. Funct. Ecol. 2014, 28, 315–327. [Google Scholar] [CrossRef]
- Roossinck, M.J. The good viruses: Viral mutualistic symbioses. Nat. Rev. Microbiol. 2011, 9, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Morel, G.; Fouillaud, M. Presence of microorganisms and viral inclusion bodies in the nests of the paper wasp Polistes hebraeus Fabricius (Hymenoptera, Vespidae). J. Invertebr. Pathol. 1992, 60, 210–212. [Google Scholar] [CrossRef]
- Fouillaud, M.; Morel, G. Characterization of cytoplasmic and nuclear polyhedrosis viruses recovered from the nest of Polistes hebraeus F. (Hymenoptera; Vespidae). J. Invertebr. Pathol. 1994, 64, 89–95. [Google Scholar] [CrossRef]
- Madden, A.A.; Boyden, S.D.; Soriano, J.A.N.; Corey, T.B.; Leff, J.W.; Fierer, N.; Starks, P.T. The emerging contribution of social wasps to grape rot disease ecology. PeerJ 2017, 5, e3223. [Google Scholar] [CrossRef] [PubMed]
- Dalmon, A.; Gayral, P.; Decante, D.; Klopp, C.; Bigot, D.; Thomasson, M.; Herniou, E.A.; Alaux, C.; Le Conte, Y. Viruses in the invasive hornet Vespa velutina. Viruses 2019, 11, 1041. [Google Scholar] [CrossRef]
- Currie, C.R. A community of ants, fungi, and bacteria: A multilateral approach to studying symbiosis. Annu. Rev. Microbiol. 2001, 55, 357–380. [Google Scholar] [CrossRef]
- Little, A.E.; Currie, C.R. Symbiotic complexity: Discovery of a fifth symbiont in the attine ant–microbe symbiosis. Biol. Lett. 2007, 3, 501–504. [Google Scholar] [CrossRef]
- Moreau, C.S. Symbioses among ants and microbes. Curr. Opin. Insect Sci. 2020, 39, 1–5. [Google Scholar] [CrossRef]
- Kim, E.; Seo, J.; Yang, S.H.; Kim, I.S.; Koo, Y. Intestine bacterial microbiota of Asian hornet (Vespa velutina nigrithorax) and honeybee. Korean J. Environ. Agric. 2018, 37, 135–140. [Google Scholar] [CrossRef]
- Fang, C.; Achal, V. Physico-Chemical Aspects and Complete Bacterial Community Composition Analysis of Wasp Nests. Sustainability 2020, 12, 2652. [Google Scholar] [CrossRef]
- Cini, A.; Meriggi, N.; Bacci, G.; Cappa, F.; Vitali, F.; Cavalieri, D.; Cervo, R. Gut microbial composition in different castes and developmental stages of the invasive hornet Vespa velutina nigrithorax. Sci. Total Environ. 2020, 745, 140873. [Google Scholar] [CrossRef] [PubMed]
- Ishay, J.S.; Riabinin, K.; Pertsis, V. Symbiotic bacteria in hornet pupal silk. Naturwissenschaften 2003, 90, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Tempestini, A. Caratterizzazione di Batteri Associati a Larve di Polistes Dominulus; Facoltà di Scienze Matematiche Fisiche Naturali, Corso di Laurea in Scienze Biologiche, Università degli Studi di Firenze: Florence, Italy, 2006; p. 86. [Google Scholar]
- Madden, A.A.; Grassetti, A.; Soriano, J.A.N.; Starks, P.T. Actinomycetes with antimicrobial activity isolated from paper wasp (Hymenoptera: Vespidae: Polistinae) nests. Environ. Entomol. 2013, 42, 703–710. [Google Scholar] [CrossRef]
- Cervo, R.; Zacchi, F.; Turillazzi, S. Polistes dominulus (Hymenoptera, Vespidae) invading North America: Some hypotheses for its rapid spread. Insectes Sociaux 2000, 47, 155–157. [Google Scholar] [CrossRef]
- Mhlongwe, T.R. The Search for a Biological Control Agent to Control Invasive Polistes Dominula Wasps in the Western Cape Region, South Africa. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2018. [Google Scholar]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W.; et al. The antimicrobial potential of Streptomyces from insect microbiomes. Nat. Commun. 2019, 10, 516. [Google Scholar] [CrossRef]
- Baranova, A.A.; Zakalyukina, Y.V.; Ovcharenko, A.A.; Korshun, V.A.; Tyurin, A.P. Antibiotics from Insect-Associated Actinobacteria. Biology 2022, 11, 1676. [Google Scholar] [CrossRef]
- Matarrita-Carranza, B.; Moreira-Soto, R.D.; Murillo-Cruz, C.; Mora, M.; Currie, C.R.; Pinto-Tomas, A.A. Evidence for widespread associations between neotropical hymenopteran insects and Actinobacteria. Front. Microbiol. 2017, 8, 2016. [Google Scholar] [CrossRef]
- Chavarría-Pizarro, L. Los insectos y la biotecnología: Avispas sociales como fuente de nuevos compuestos antibióticos. Rev. Tecnol. Marcha 2019, g-114. [Google Scholar] [CrossRef]
- Matarrita-Carranza, B.; Murillo-Cruz, C.; Avendaño, R.; Ríos, M.I.; Chavarría, M.; Gómez-Calvo, M.L.; Tamayo-Castillo, G.; Araya, J.J.; Pinto-Tomás, A.A. Streptomyces sp. M54: An actinobacteria associated with a neotropical social wasp with high potential for antibiotic production. Antonie Van Leeuwenhoek 2021, 114, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Araya, M.; Núñez-Montero, K.; Pizarro-Cerdá, J.; Chavarría-Pizarro, L. Draft Genome Sequences of Saccharopolyspora sp. Strains and Streptomyces sp. Strains, Isolated from Social Wasps (Vespidae; Polistinae: Epiponini). Microbiol. Resour. Announc. 2022, 11, e00935-21. [Google Scholar] [CrossRef] [PubMed]
- Glare, T.R.; Harris, R.J.; Donovan, B.J. Aspergillus flavus as a pathogen of wasps, Vespula spp., in New Zealand. N. Z. J. Zool. 1996, 23, 339–344. [Google Scholar] [CrossRef]
- Harris, R.J.; Harcourt, S.J.; Glare, T.R.; Rose, E.A.F.; Nelson, T.J. Susceptibility of Vespula vulgaris (Hymenoptera: Vespidae) to generalist entomopathogenic fungi and their potential for wasp control. J. Invertebr. Pathol. 2000, 75, 251–258. [Google Scholar] [CrossRef]
- Durrell, L.W. Fungi in nests of paper wasps. Am. Midl. Nat. 1965, 73, 501–503. [Google Scholar] [CrossRef]
- Fouillaud, M.; Morel, G. Fungi associated with nests of the paper wasp Polistes hebraeus (Hymenoptera: Vespidae) on La Reunion Island. Environ. Entomol. 1995, 24, 298–305. [Google Scholar] [CrossRef]
- Jayaprakash, A.; Ebenezer, P. A new report on mycobiota associated with Ropalidia marginata paper nests. Indian J. Sci. Technol. 2010, 3, 6–8. [Google Scholar] [CrossRef]
- Madden, A.A.; Stchigel, A.M.; Guarro, J.; Sutton, D.; Starks, P.T. Mucor nidicola sp. nov., a fungal species isolated from an invasive paper wasp nest. Int. J. Syst. Evol. Microbiol. 2012, 62, 1710–1714. [Google Scholar] [CrossRef]
- Davis, T.S.; Boundy-Mills, K.; Landolt, P.J. Volatile emissions from an epiphytic fungus are semiochemicals for eusocial wasps. Microb. Ecol. 2012, 64, 1056–1063. [Google Scholar] [CrossRef]
- Blackwell, M. Made for each other: Ascomycete yeasts and insects. Microbiol. Spectr. 2017, 5, 5.3.13. [Google Scholar] [CrossRef]
- Stefanini, I.; Dapporto, L.; Legras, J.L.; Calabretta, A.; Di Paola, M.; De Filippo, C.; Viola, R.; Capretti, P.; Polsinelli, M.; Turillazzi, S.; et al. Role of social wasps in Saccharomyces cerevisiae ecology and evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 13398–13403. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, I.; Dapporto, L.; Berná, L.; Polsinelli, M.; Turillazzi, S.; Cavalieri, D. Social wasps are a Saccharomyces mating nest. Proc. Natl. Acad. Sci. USA 2016, 113, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Dapporto, L.; Stefanini, I.; Rivero, D.; Polsinelli, M.; Capretti, P.; De Marchi, P.; Viola, R.; Turillazzi, S.; Cavalieri, D. Social wasp intestines host the local phenotypic variability of Saccharomyces cerevisiae strains. Yeast 2016, 33, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Alarco, A.M.; Marcil, A.; Chen, J.; Suter, B.; Thomas, D.; Whiteway, M. Immune-deficient Drosophila melanogaster: A model for the innate immune response to human fungal pathogens. J. Immunol. 2004, 172, 5622–5628. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S. Drosophila and Galleria insect model hosts: New tools for the study of fungal virulence, pharmacology and immunology. Virulence 2011, 2, 521–527. [Google Scholar] [CrossRef]
- Bergin, D.; Murphy, L.; Keenan, J.; Clynes, M.; Kavanagh, K. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microbes Infect. 2006, 8, 2105–2112. [Google Scholar] [CrossRef]
- Meriggi, N.; Di Paola, M.; Vitali, F.; Rivero, D.; Cappa, F.; Turillazzi, F.; Gori, A.; Dapporto, L.; Beani, L.; Turillazzi, S.; et al. Saccharomyces cerevisiae induces immune enhancing and shapes gut microbiota in social wasps. Front. Microbiol. 2019, 10, 2320. [Google Scholar] [CrossRef]
- Rizzetto, L.; Ifrim, D.C.; Moretti, S.; Tocci, N.; Cheng, S.C.; Quintin, J.; Renga, G.; Oikonomou, V.; De Filippo, C.; Weil, T.; et al. Fungal chitin induces trained immunity in human monocytes during cross-talk of the host with Saccharomyces cerevisiae. J. Biol. Chem. 2016, 291, 7961–7972. [Google Scholar] [CrossRef]
- Jimenez, S.I.; Carroll, C.; Babcock, T.; Derstine, N.; Hadwin, A.; Moore, M.; Gries, G. Yeasts harbored by vespine wasps in the Pacific Northwest. Environ. Entomol. 2017, 46, 217–225. [Google Scholar] [CrossRef]
- Valentini, B.; Barbero, F.; Casacci, L.P.; Luganini, A.; Stefanini, I. Forests influence yeast populations vectored by insects into vineyards. Front. Microbiol. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Madden, A.A.; Epps, M.J.; Fukami, T.; Irwin, R.E.; Sheppard, J.; Sorger, D.M.; Dunn, R.R. The ecology of insect–yeast relationships and its relevance to human industry. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172733. [Google Scholar] [CrossRef] [PubMed]
- Meriggi, N.; Di Paola, M.; Cavalieri, D.; Stefanini, I. Saccharomyces cerevisiae–insects association: Impacts, biogeography, and extent. Front. Microbiol. 2020, 11, 1629. [Google Scholar] [CrossRef] [PubMed]
- Brock, R.E.; Cini, A.; Sumner, S. Ecosystem services provided by aculeate wasps. Biol. Rev. 2021, 96, 1645–1675. [Google Scholar] [CrossRef] [PubMed]

| Mutual Symbioses between Microorganisms and Social Wasps | |||||
|---|---|---|---|---|---|
| Contribute of Microorganisms | Contribute of Wasps | ||||
| Type of Service | Ref. | Type of Service | Ref. | ||
| Nest walls reinforcement | Fungal sp. Hyphae in nests of some Stenogastrinae and Vespula sp. | [35] | Micro-environment formation and stabilisation | Social wasp colonies constitute perfect environments for microorganisms | [5] |
| [36] | [6] | ||||
| [64] | |||||
| Production of defensive substances against pathogens | Actinomycetes in nests of Polistes dominula and various Epiponini Bacillus sp. against Beauveria in P. dominula | [53] | Horizontal and vertical transmission of microorganisms to colony mates and immature brood | P. dominula and Vespa crabro on Saccharomyces cerevisiae Vespa orientalis on Staphylococcus | [70] |
| [59] | [71] | ||||
| [60] | [51] | ||||
| [55] | |||||
| Production of attractants to food | Aerobasidium pollulas attracts Vespulae to decomposing fruits | [68] | Gut environment induces variability of microorganisms through sexual reproduction | Polistes dominula and Vespa crabro on Saccharomyces cerevisiae | [71] |
| Stimulation of immune system of the hosts | Saccharomyces on Polistes dominula | [76] | Carriers of microorganisms in the environment | Vespula germanica and V. pensylvanica are carriers of the fungus A.pollulans. P. dominula and V.crabro are carriers of Saccharomyces | [68] |
| [70] | |||||
| [71] | |||||
| [72] | |||||
| Defence of the pupae and facilitation of the emergence of the adults | Staphylococcus sp. in nests of Vespa orientalis | [51] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turillazzi, S.; Meriggi, N.; Cavalieri, D. Mutualistic Relationships between Microorganisms and Eusocial Wasps (Hymenoptera, Vespidae). Microorganisms 2023, 11, 1340. https://doi.org/10.3390/microorganisms11051340
Turillazzi S, Meriggi N, Cavalieri D. Mutualistic Relationships between Microorganisms and Eusocial Wasps (Hymenoptera, Vespidae). Microorganisms. 2023; 11(5):1340. https://doi.org/10.3390/microorganisms11051340
Chicago/Turabian StyleTurillazzi, Stefano, Niccolò Meriggi, and Duccio Cavalieri. 2023. "Mutualistic Relationships between Microorganisms and Eusocial Wasps (Hymenoptera, Vespidae)" Microorganisms 11, no. 5: 1340. https://doi.org/10.3390/microorganisms11051340
APA StyleTurillazzi, S., Meriggi, N., & Cavalieri, D. (2023). Mutualistic Relationships between Microorganisms and Eusocial Wasps (Hymenoptera, Vespidae). Microorganisms, 11(5), 1340. https://doi.org/10.3390/microorganisms11051340







