Evaluation of Rouxiella badensis Subsp Acadiensis (Canan SV-53) as a Potential Probiotic Bacterium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria Strains
2.2. Animals and Diet Supplementation
2.3. Scanning and Transmission Electron Microscopy
2.4. Analysis of Some Total Populations of the Intestinal Microbiota
2.5. Bacterial Translocation to Spleen and Liver
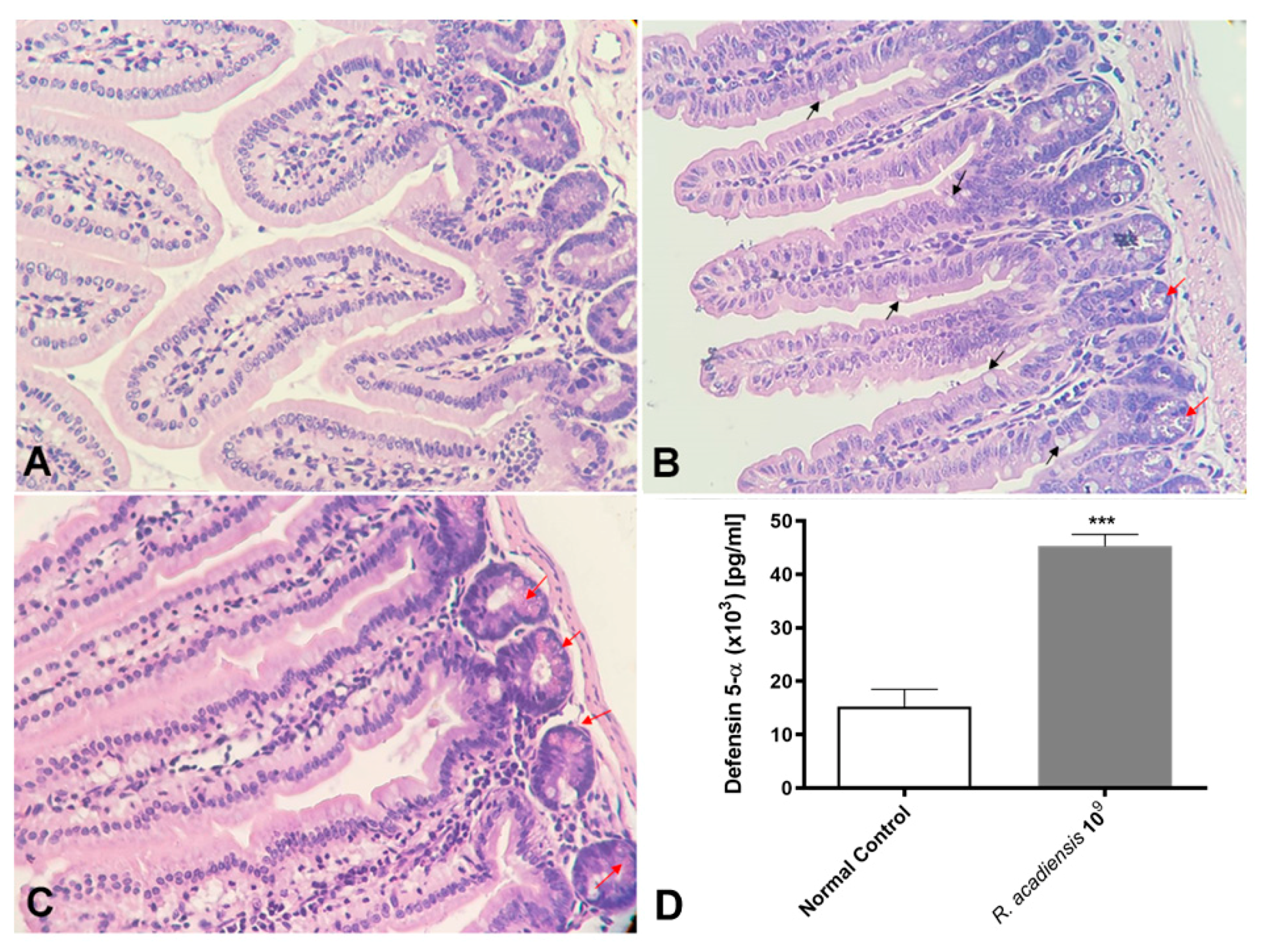
2.6. Histology of Small Intestine
2.7. Immunohistochemically Analysis
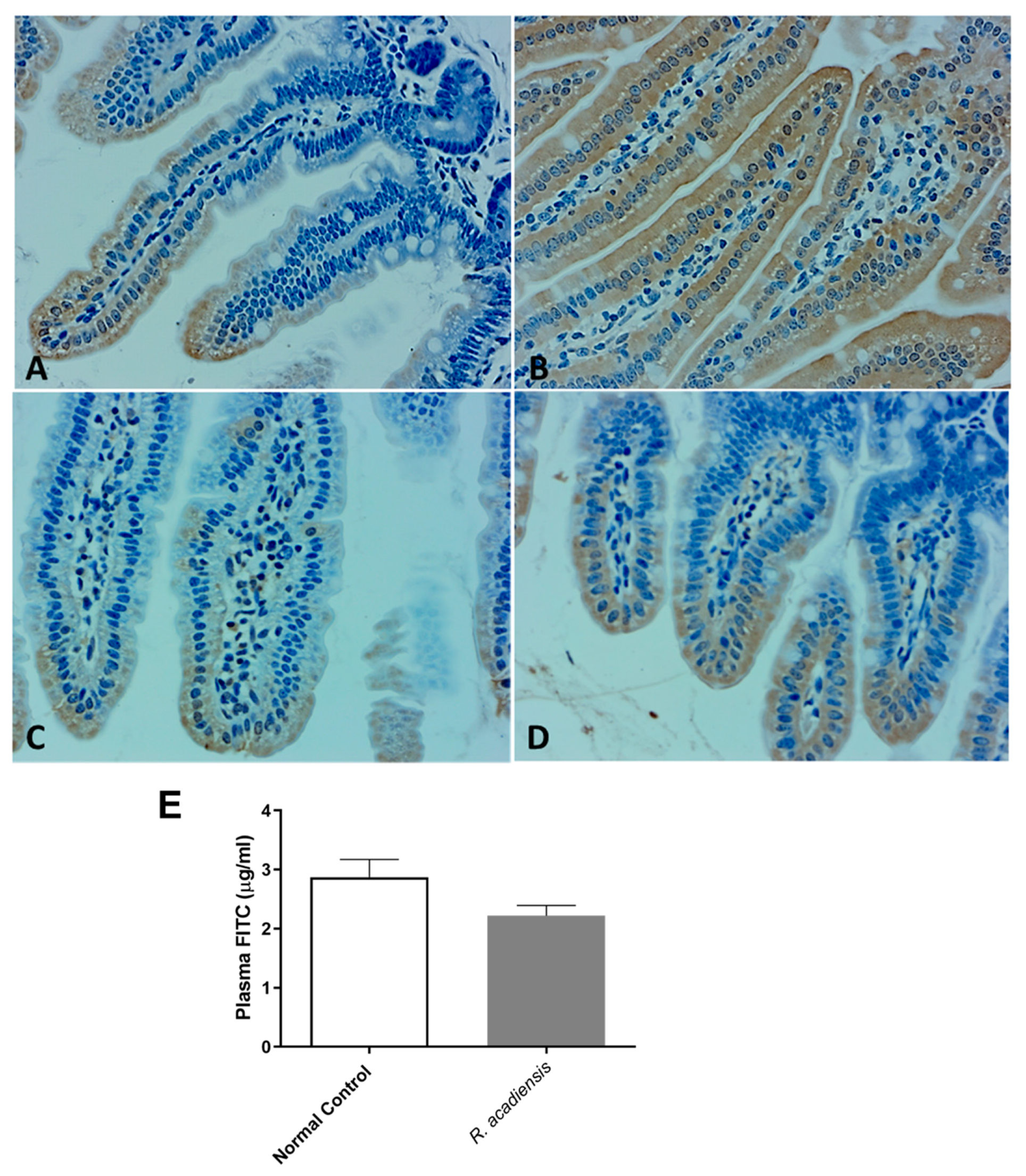
2.8. Intestinal Permeability
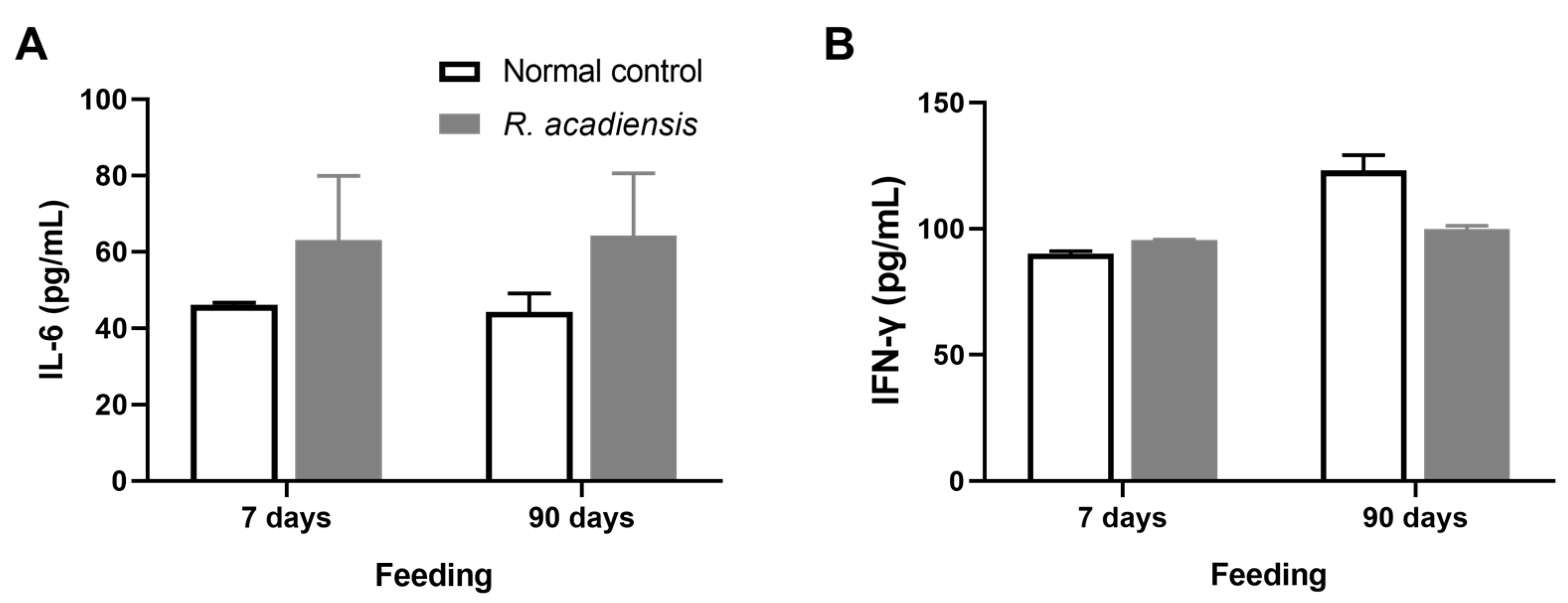
2.9. Intestinal Epithelial Cells Isolation and Cytokines Determination
2.10. Determination of Antimicrobial Activity from the Intestinal Fluid
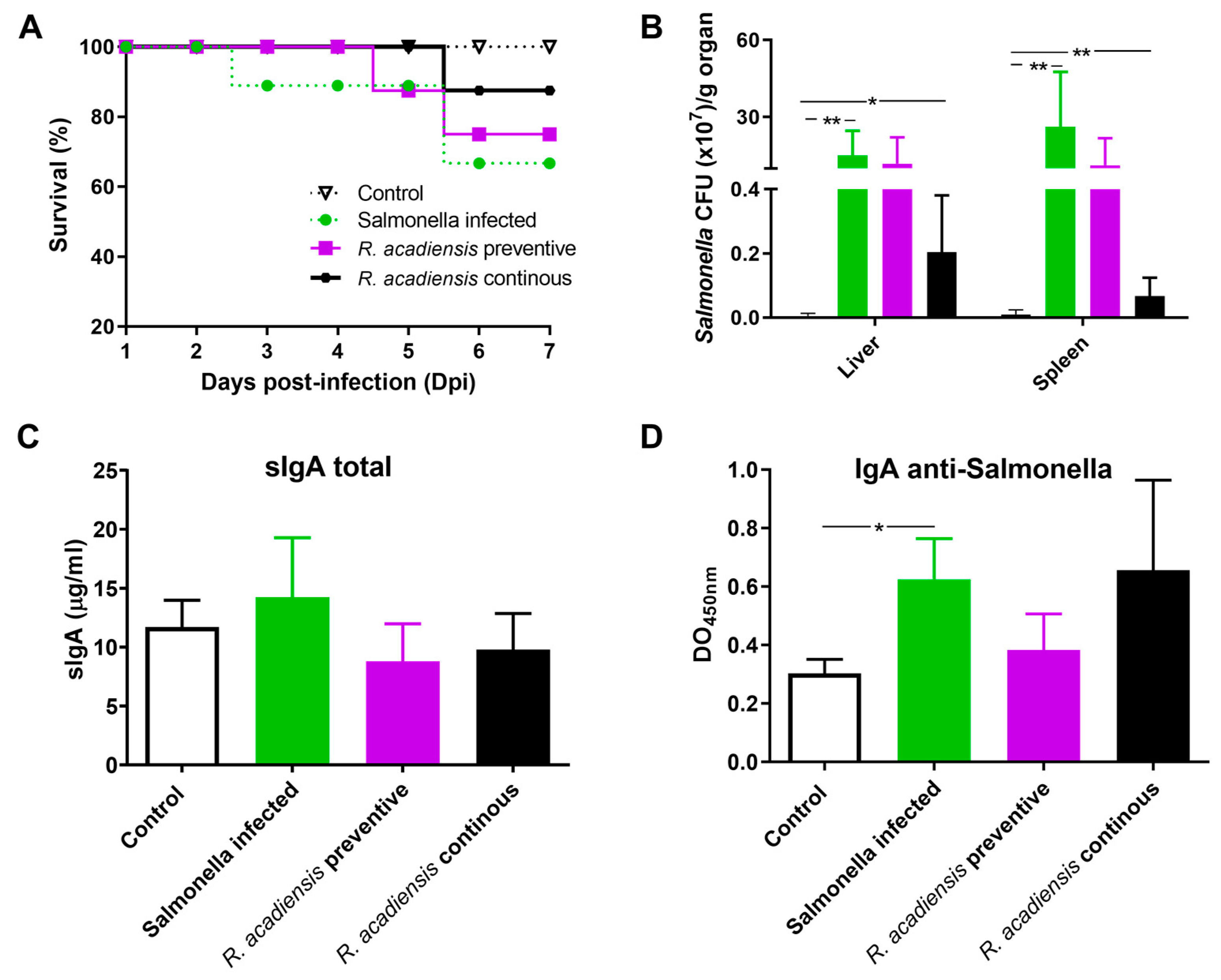
2.11. Salmonella Typhimurium Infection
2.12. Total and Specific Anti-Salmonella Secretory IgA (S-IgA)
2.13. Statistical Analyses
3. Results
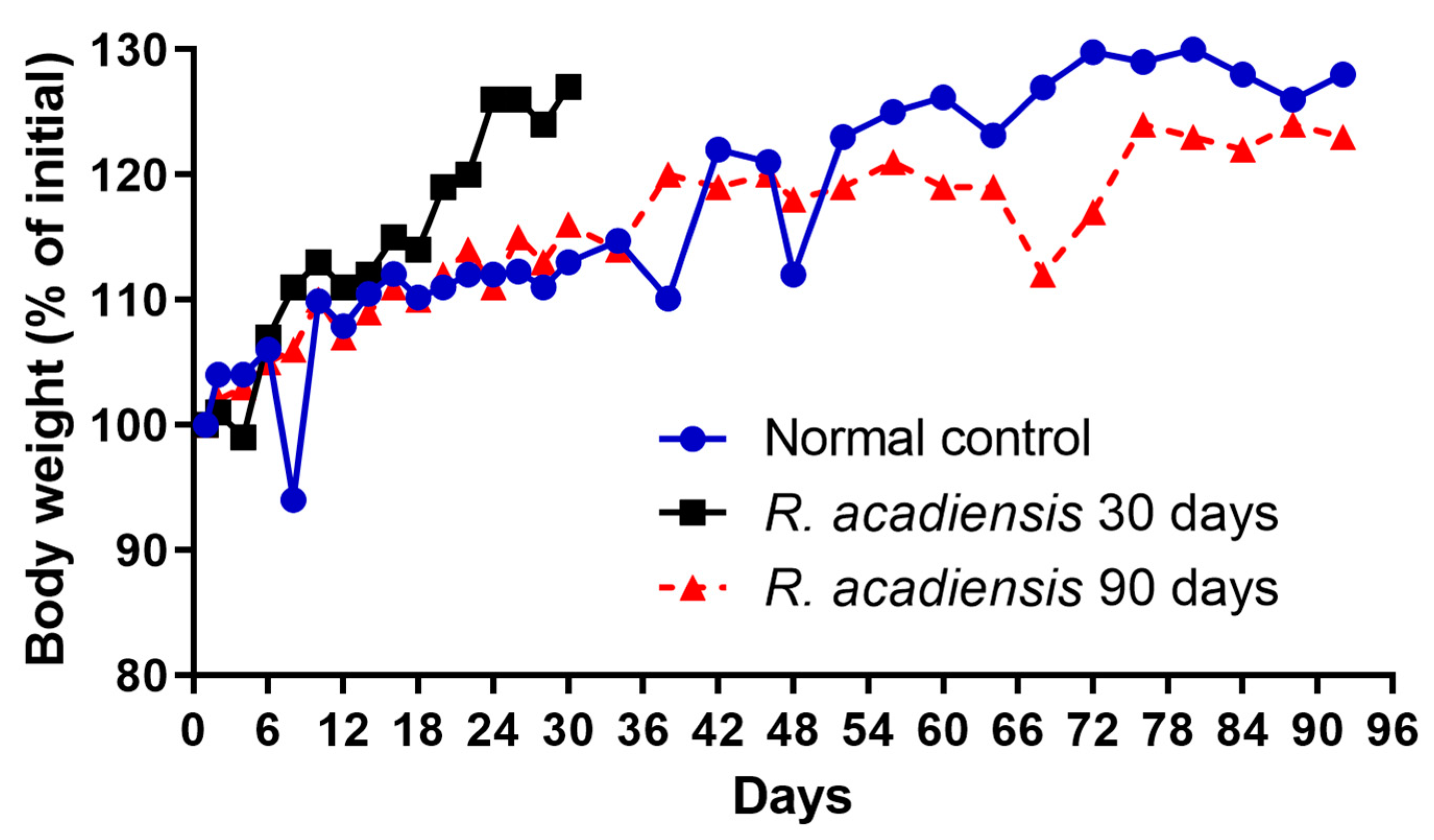
3.1. Effect of R. acadiensis Administration on Body Weight
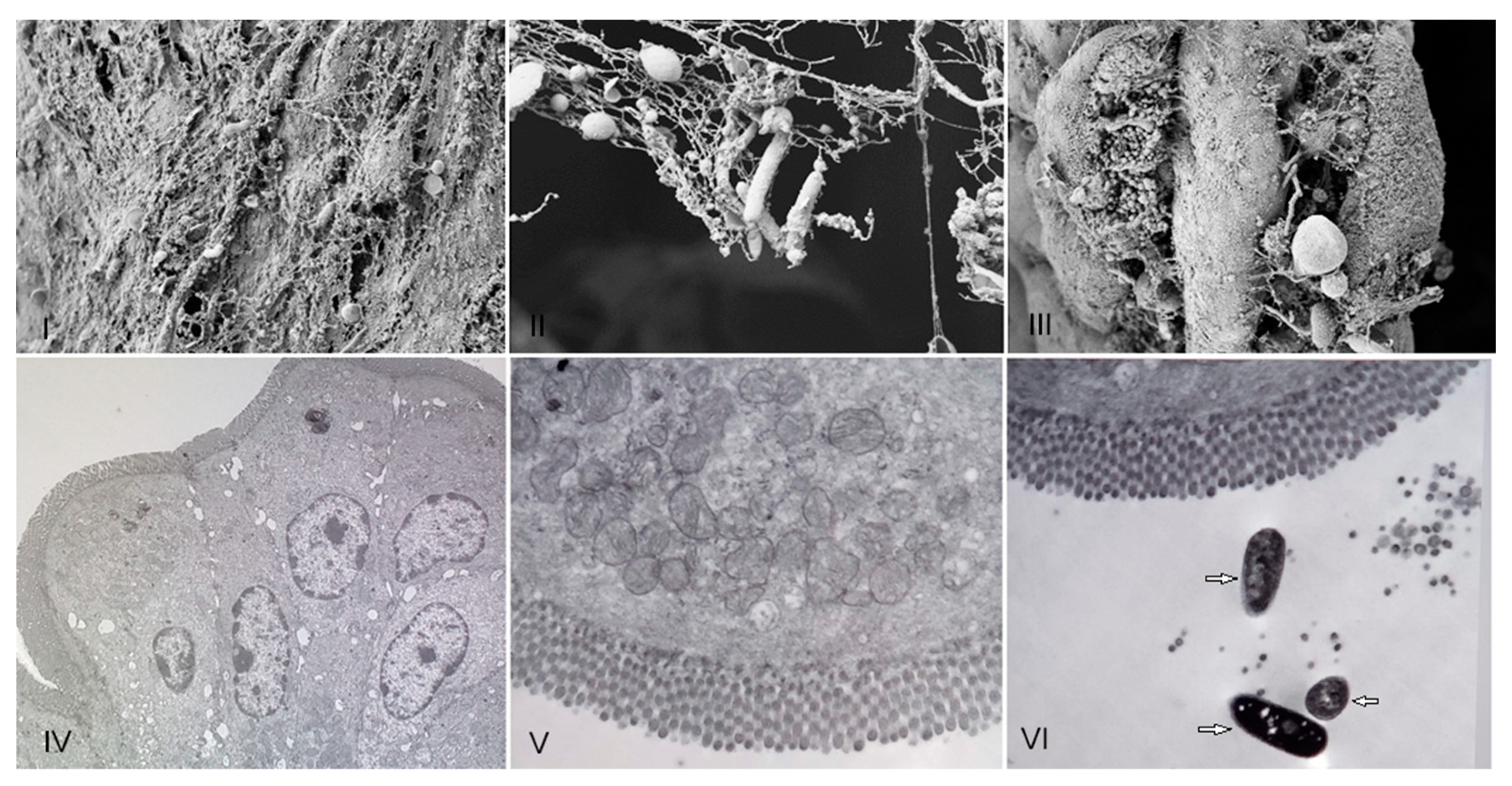
3.2. Evaluation of the In Vivo Adherence of R. acadiensis to the Intestinal Epithelium
3.3. R. acadiensis Oral Supplementation Did Not Disturb the Large Intestinal Homeostasis
3.4. R. acadiensis Reinforces the Intestinal Epithelial Barrier without Disturbing the Small Intestinal Homeostasis, Even after Oral Long-Term Consumption
3.5. Determination of Antimicrobial Activity from the Intestinal Fluid
3.6. Continuous Administration of R. acadiensis Protects against Salmonella Typhimurium Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Pédron, T.; Sansonetti, P. Commensals, bacterial pathogens and intestinal inflammation: An intriguing ménage à trois. Cell Host Microbe 2008, 3, 344–347. [Google Scholar] [CrossRef]
- Neal, M.D.; Sodhi, C.P.; Jia, H.; Dyer, M.; Egan, C.E.; Yazji, I.; Good, M.; Afrazi, A.; Marino, R.; Slagle, D.; et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J. Biol. Chem. 2012, 287, 37296–37308. [Google Scholar] [CrossRef] [PubMed]
- Gaboriau-Routhiau, V.; Cerf-Bensussan, N. Gut microbiota and development of the immune system. Med. Sci. 2016, 32, 961–967. [Google Scholar] [CrossRef]
- Gallo, R.L.; Hooper, L.V. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 2012, 12, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Sjovall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Reid, G.; Gadir, A.A.; Dhir, R. Probiotics: Reiterating What They Are and What They Are Not. Front. Microbiol. 2019, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar]
- Chaucheyras-Durand, F.; Durand, H. Probiotics in animal nutrition and health. Benef. Microbes 2010, 1, 3–9. [Google Scholar] [CrossRef]
- Kumar, M.; Verma, V.; Nagpal, R.; Kumar, A.; Behare, P.V.; Singh, B.; Aggarwal, P.K. Anticarcinogenic effect of probiotic fermented milk and chlorophyllin on aflatoxin-B-induced liver carcinogenesis in rats. Br. J. Nutr. 2012, 107, 1006. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E. Probiotics in 2015: Their Scope and Use. J. Clin. Gastroenterol. 2015, 49 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Velez, E.M.; Galdeano, C.M.; Carmuega, E.; Weill, R.; Bibas-Bonet, M.E.; Perdigón, G. Probiotic fermented milk consumption modulates the allergic process induced by ovoalbumin in mice. Br. J. Nutr. 2015, 114, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.S. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016, 37, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Fábrega, M.J.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Algieri, F.; Badia, J.; Gimenez, R.; Gálvez, J.; Baldomà, L. Intestinal Anti-inflammatory Effects of Outer Membrane Vesicles from Escherichia Coli Nissle 1917 in DSS-Experimental Colitis in Mice. Front. Microbiol. 2017, 11, 1274. [Google Scholar] [CrossRef]
- He, J.; Zhang, F.; Han, Y. Effect of probiotics on lipid profiles and blood pressure in patients with type 2 diabetes: A meta-analysis of RCTs. Medicine 2017, 96, e9166. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Huys, G.; Botteldoorn, N.; Delvigne, F.; Vuyst, L.D.; Heyndrickx, M.; Pot, B.; Dubois, J.-J.; Daube, G. Microbial characterization of probiotics—Advisory report of the Working Group “8651 Probiotics” of the Belgian Superior Health Council (SHC). Mol. Nut. Food Res. 2013, 57, 1479–1504. [Google Scholar] [CrossRef] [PubMed]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Kołożyn-Krajewska, D. Food-Origin Lactic Acid Bacteria May Exhibit Probiotic Properties: Review. BioMed Res. Int. 2018, 2018, 5063185. [Google Scholar] [CrossRef]
- Saarela, M.; Lähteenmäki, L.; Crittenden, R.; Salminen, S.; Mattila-Sandholm, T. Gut bacteria and health foods the European perspective. Int. J. Food Microbiol. 2002, 15, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Mallet, J.F.; Kulshreshtha, G.; Hincke, M.; Ismail, N.; Matar, C. Immunomodulation and Intestinal Morpho-Functional Aspects of a Novel Gram-Negative Bacterium Rouxiella badensis subsp. acadiensis. Front. Microbiol. 2021, 22, 569119. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, E.; Tremblay, J.; Arbour, M.; Mallet, J.F.; Masson, L.; Matar, C. Complete PacBio Single-Molecule Real-Time Sequence of a Novel Probiotic-Like Bacterium, Rouxiella badensis subsp. acadiensis, Isolated from the Biota of Wild Blueberries in the Acadian Forest. Microbiol. Resour. Announc. 2023, 10, e0134022. [Google Scholar] [CrossRef]
- Tri Vuong, L.M.; Chantal, M. Antioxidant activity of fermented berry juices and their effects on nitric oxide and tumor necrosis factor-alpha production in macrophages 264.7 Gamma NO (–) cell line. J. Food Biochem. 2006, 30, 249–268. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Perdigón, G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine Immunol. 2006, 13, 219–226. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Perdigón, G. Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. J. Appl. Microbiol. 2004, 97, 673–681. [Google Scholar] [CrossRef]
- Velez, E.; Castillo, N.; Mesón, O.; Grau, A.; Bibas Bonet, M.E.; Perdigón, G. Study of the effect exerted by fructo-oligosaccharides from yacon (Smallanthus sonchifolius) root flour in an intestinal infection model with Salmonella Typhimurium. Br. J. Nutr. 2013, 109, 1971–1979. [Google Scholar] [CrossRef]
- Rendon, J.L.; Li, X.; Akhtar, S.; Choudhry, M.A. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock 2013, 39, 11–18. [Google Scholar] [CrossRef]
- Najarro, K.M.; Boe, D.M.; Walrath, T.M.; Mullen, J.E.; Paul, M.T.; Frankel, J.H.; Hulsebus, H.J.; Idrovo, J.P.; McMahan, R.H.; Kovacs, E.J. Advanced age exacerbates intestinal epithelial permeability after burn injury in mice. Exp. Gerontol. 2022, 158, 111654. [Google Scholar] [CrossRef]
- Canali, M.M.; Porporatto, C.; Aoki, M.P.; Bianco, I.D.; Correa, S.G. Signals elicited at the intestinal epithelium upon chitosan feeding contribute to immunomodulatory activity and biocompatibility of the polysaccharide. Vaccine 2010, 28, 5718–5724. [Google Scholar] [CrossRef] [PubMed]
- Cazorla, S.I.; Maldonado-Galdeano, C.; Weill, R.; De Paula, J.; Perdigón, G. Oral Administration of Probiotics Increases Paneth Cells and Intestinal Antimicrobial Activity. Front. Microbiol. 2018, 16, 736. [Google Scholar] [CrossRef]
- Leblanc, J.; Fliss, I.; Matar, C. Induction of a humoral immune response following an Escherichia coli O157:H7 infection with an immunomodulatory peptidic fraction derived from Lactobacillus helveticus-fermented milk. Clin. Diagn. Lab. Immunol. 2004, 11, 1171–1181. [Google Scholar]
- Floch, M.H. Recommendations for probiotic use in humans-a 2014 update. Pharmaceuticals 2014, 10, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Jiménez, C.; Martínez-Ramírez, M.J.; Gil, A.; Gómez-Llorente, C. Effects of Probiotics on Metabolic Syndrome: A Systematic Review of Randomized Clinical Trials. Nutrients 2020, 12, 124. [Google Scholar] [CrossRef]
- Rittiphairoj, T.; Pongpirul, K.; Janchot, K.; Mueller, N.T.; Li, T. Probiotics Contribute to Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 722–734. [Google Scholar] [CrossRef]
- Velez, E.; Novotny-Nuñez, I.; Correa, S.; Perdigón, G.; Maldonado-Galdeano, C. Modulation of gut immune response by probiotic fermented milk consumption to control IgE in a respiratory allergy model. Benef. Microbes 2021, 12, 175–186. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- FDA. GRAS Notices. 2021. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices (accessed on 3 May 2023).
- Ruiz, L.; Delgado, S.; Ruas Madiedo, P.; Sanchez, B.; Margolles, A. Bifidobacteria and Their Molecular Communication with the Immune System. Front. Microbiol. 2017, 8, 2345. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme-Dumit, J.M.; Velez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Koutsounas, I.; Kaltsa, G.; Siakavellas, S.I.; Bamias, G. Markers of bacterial translocation in end-stage liver disease. World J. Hepatol. 2015, 7, 2264–2273. [Google Scholar] [CrossRef]
- Zareie, M.; Johnson-Henry, K.; Jury, J.; Yang, P.C.; Ngan, B.Y.; McKay, D.M.; Soderholm, J.-D.; Perdue, M.H.; Sherman, P.M. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 2006, 55, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.R.; Anandharaj, M.; Chandra Ray, R.; Parveen Rani, R. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef]
- Smith, K.; McCoy, K.D.; Macpherson, A.J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin. Immunol. 2007, 19, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 2012, 15, 57–62. [Google Scholar] [CrossRef]
- Wehkamp, J.; Harder, J.; Wehkamp, K.; Wehkamp-von Meissner, B.; Schlee, M.; Enders, C.; Sonnenborn, U.; Nuding, S.; Bengmark, S.; Fellermann, K.; et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: A novel effect of a probiotic bacterium. Infect. Immun. 2004, 72, 5750–5758. [Google Scholar] [CrossRef]
- Ouwerkerk, J.P.; de Vos, W.M.; Belzer, C. Glycobiome: Bacteria and mucus at the epithelial interface. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Shu, Q.; Lin, H.; Rutherfurd, K.J.; Cross, M.L. Protection against translocating Salmonella Typhimurium infection in mice by feeding the immuno-enhancing probiotic Lactobacillus rhamnosus strain HN001. Med. Microbiol. Immunol. 2001, 190, 97–104. [Google Scholar] [CrossRef]
- Asahara, T.; Shimizu, K.; Nomoto, K.; Hamabata, T.; Ozawa, A.; Takeda, Y. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 2004, 72, 2240–2247. [Google Scholar] [CrossRef]
- Lemme Dumit, J.M.; Polti, M.A.; Perdigón, G.; Maldonado-Galdeano, C. Probiotic bacteria cell walls stimulate the activity of the intestinal epithelial cells and macrophage functionality. Benef. Microbes. 2018, 9, 153–164. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novotny-Nuñez, I.; Perdigón, G.; Matar, C.; Martínez Monteros, M.J.; Yahfoufi, N.; Cazorla, S.I.; Maldonado-Galdeano, C. Evaluation of Rouxiella badensis Subsp Acadiensis (Canan SV-53) as a Potential Probiotic Bacterium. Microorganisms 2023, 11, 1347. https://doi.org/10.3390/microorganisms11051347
Novotny-Nuñez I, Perdigón G, Matar C, Martínez Monteros MJ, Yahfoufi N, Cazorla SI, Maldonado-Galdeano C. Evaluation of Rouxiella badensis Subsp Acadiensis (Canan SV-53) as a Potential Probiotic Bacterium. Microorganisms. 2023; 11(5):1347. https://doi.org/10.3390/microorganisms11051347
Chicago/Turabian StyleNovotny-Nuñez, Ivanna, Gabriela Perdigón, Chantal Matar, María José Martínez Monteros, Nour Yahfoufi, Silvia Inés Cazorla, and Carolina Maldonado-Galdeano. 2023. "Evaluation of Rouxiella badensis Subsp Acadiensis (Canan SV-53) as a Potential Probiotic Bacterium" Microorganisms 11, no. 5: 1347. https://doi.org/10.3390/microorganisms11051347
APA StyleNovotny-Nuñez, I., Perdigón, G., Matar, C., Martínez Monteros, M. J., Yahfoufi, N., Cazorla, S. I., & Maldonado-Galdeano, C. (2023). Evaluation of Rouxiella badensis Subsp Acadiensis (Canan SV-53) as a Potential Probiotic Bacterium. Microorganisms, 11(5), 1347. https://doi.org/10.3390/microorganisms11051347




