O-Polysaccharides of LPS Modulate E. coli Uptake by Acanthamoeba castellanii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids and Chemicals
2.2. Purification of LPS
2.3. Cultivation and Harvesting of A. castellanii
2.4. Chromosomal Gene Inactivation in ATCC11775
2.5. Predation Assay
2.6. Competition Assay with Purified LPS
2.7. Statistical Methods
3. Results
3.1. O1 Antigen Blocks Bacterial Consumption by A. castellanii
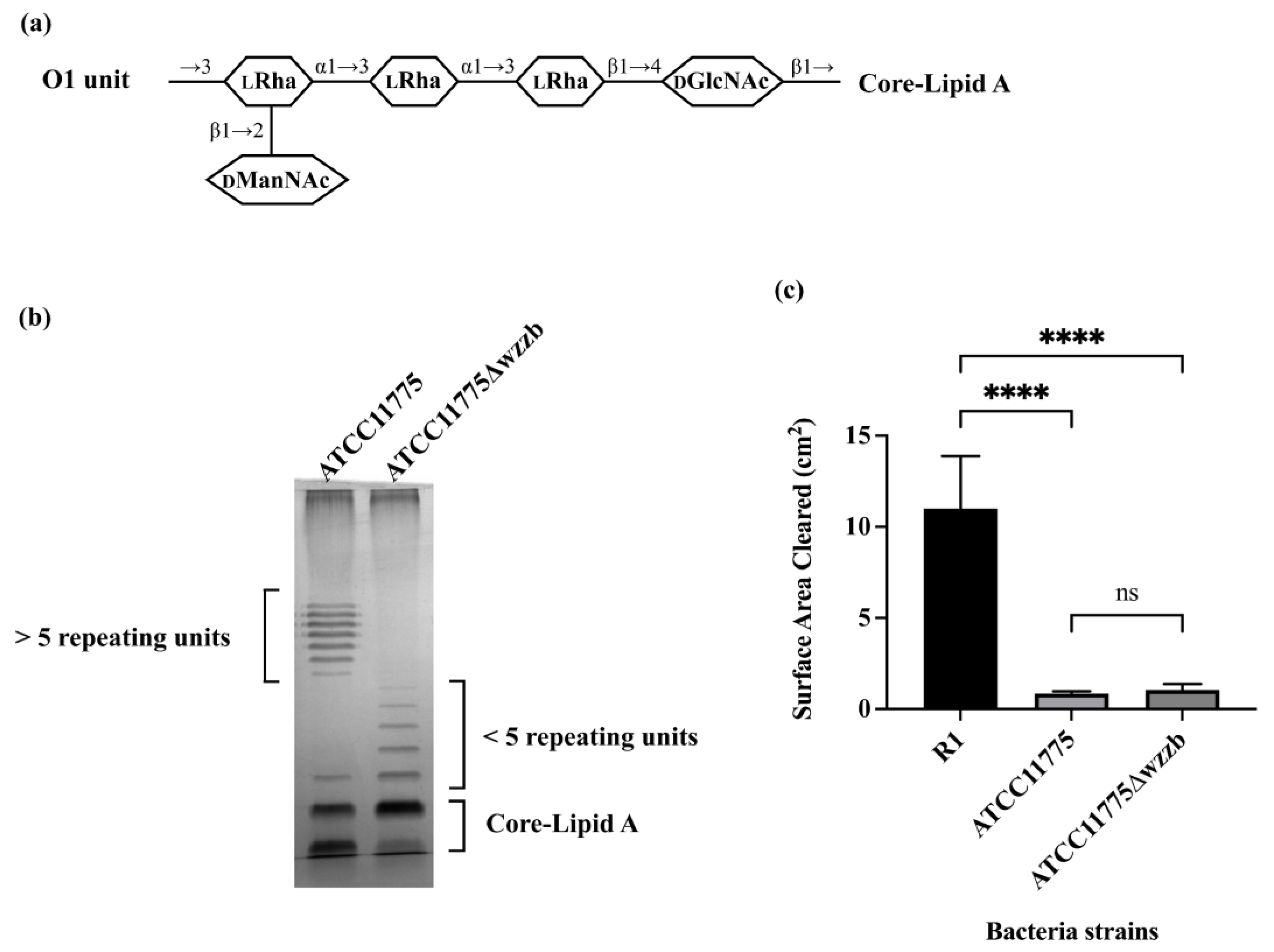
3.2. O8 and O9 Antigens Enhance Bacterial Consumption by A. castellanii

3.3. Role of O-Antigen Structure in Regulating Efficiency of Bacterial Predation by A. castellanii

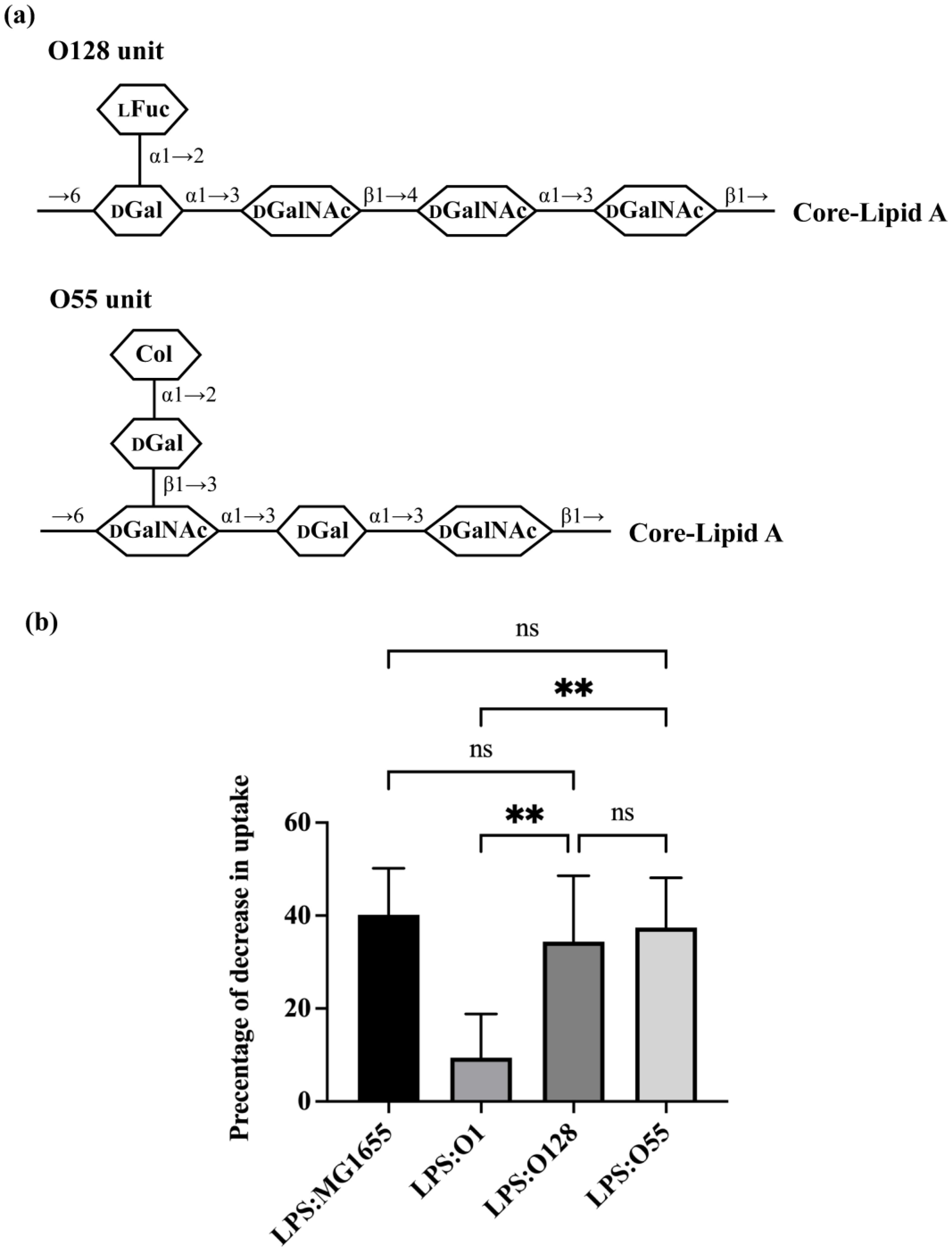
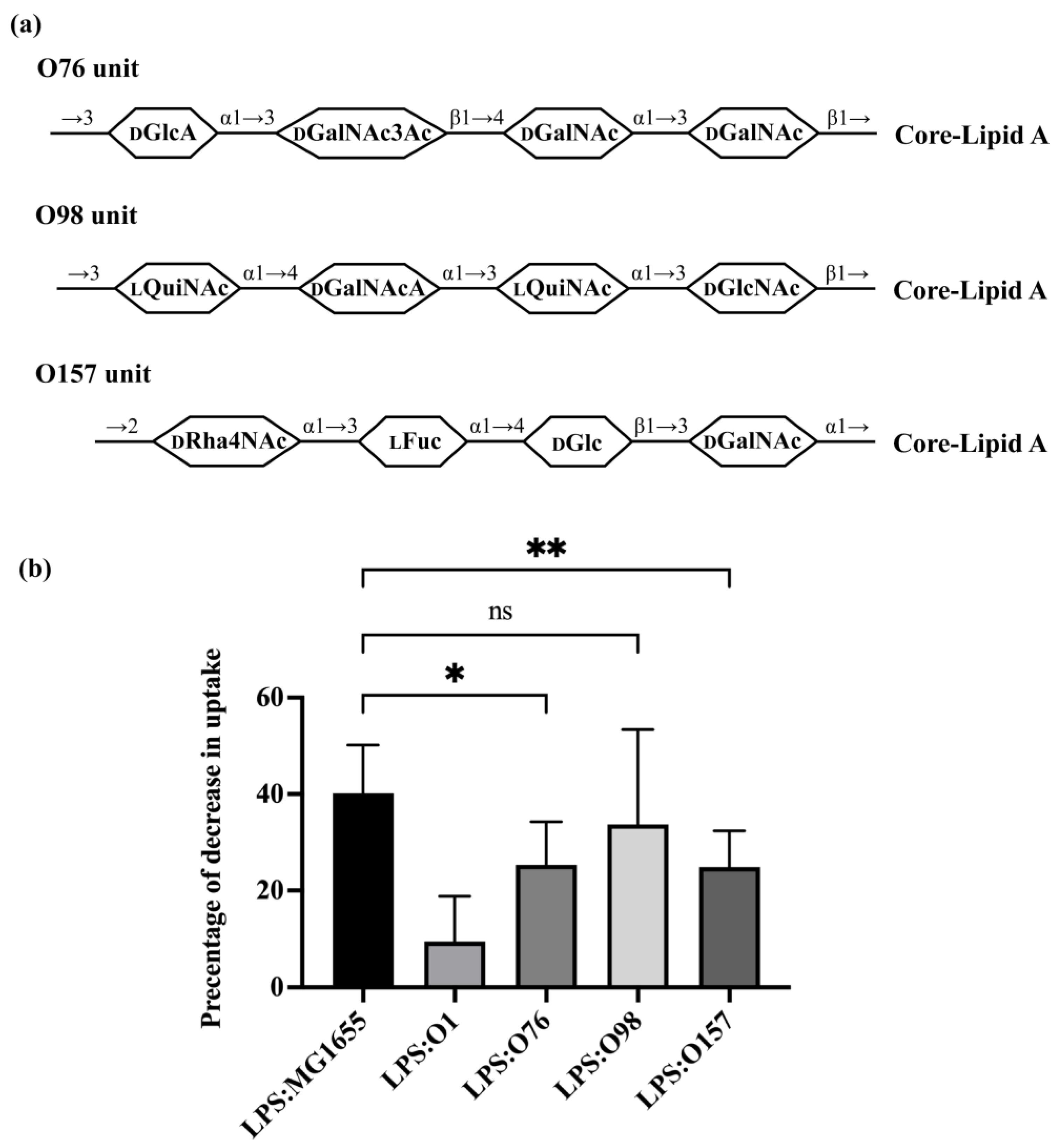
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bladen, H.A.; Mergenhagen, S.E. Ultrastructure of Veillonella and morphological correlation of an outer membrane with particles associated with endotoxic activity. J. Bacteriol. 1964, 88, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Kamio, Y.; Nikaido, H. Outer membrane of Salmonella typhimurium: Accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry 1976, 15, 2561–2570. [Google Scholar] [CrossRef] [PubMed]
- Caroff, M.; Karibian, D. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 2003, 338, 2431–2447. [Google Scholar] [CrossRef]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Liu, B.; Furevi, A.; Perepelov, A.V.; Guo, X.; Cao, H.; Wang, Q.; Reeves, P.R.; Knirel, Y.A.; Wang, L.; Widmalm, G. Structure and genetics of Escherichia coli O antigens. FEMS Microbiol. Rev. 2020, 44, 655–683. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Knirel, Y.A.; Feng, L.; Perepelov, A.V.; Senchenkova, S.Y.N.; Reeves, P.R.; Wang, L. Structural diversity in Salmonella O antigens and its genetic basis. FEMS Microbiol. Rev. 2014, 38, 56–89. [Google Scholar] [CrossRef]
- Galloway, S.M.; Raetz, C.R. A mutant of Escherichia coli defective in the first step of endotoxin biosynthesis. J. Biol. Chem. 1990, 265, 6394–6402. [Google Scholar] [CrossRef]
- Onishi, H.R.; Pelak, B.A.; Gerckens, L.S.; Silver, L.L.; Kahan, F.M.; Chen, M.-H.; Patchett, A.A.; Galloway, S.M.; Hyland, S.A.; Anderson, M.S. Antibacterial agents that inhibit lipid A biosynthesis. Science 1996, 274, 980–982. [Google Scholar] [CrossRef]
- Steeghs, L.; de Cock, H.; Evers, E.; Zomer, B.; Tommassen, J.; van der Ley, P. Outer membrane composition of a lipopolysaccharide-deficient Neisseria meningitidis mutant. EMBO J. 2001, 20, 6937–6945. [Google Scholar] [CrossRef]
- Meredith, T.; Aggarwal, P.; Mamat, U.; Lindner, B.; Woodard, R. Redefining the requisite lipopolysaccharide structure in Escherichia coli. ACS Chem. Biol. 2006, 1, 33–42. [Google Scholar] [CrossRef]
- Wildschutte, H.; Wolfe, D.M.; Tamewitz, A.; Lawrence, J.G. Protozoan predation, diversifying selection, and the evolution of antigenic diversity in Salmonella. Proc. Natl. Acad. Sci. USA 2004, 101, 10644–10649. [Google Scholar] [CrossRef]
- Alsam, S.; Jeong, S.R.; Sissons, J.; Dudley, R.; Kim, K.S.; Khan, N.A. Escherichia coli interactions with Acanthamoeba: A symbiosis with environmental and clinical implications. J. Med. Microbiol. 2006, 55, 689–694. [Google Scholar] [CrossRef]
- Arnold, J.W.; Spacht, D.; Koudelka, G.B. Determinants that govern the recognition and uptake of Escherichia coli O157: H7 by Acanthamoeba castellanii. Cell. Microbiol. 2016, 18, 1459–1470. [Google Scholar] [CrossRef]
- Daniels, C.; Vindurampulle, C.; Morona, R. Overexpression and topology of the Shigella flexneri O-antigen polymerase (Rfc/Wzy). Mol. Microbiol. 1998, 28, 1211–1222. [Google Scholar] [CrossRef]
- Bronner, D.; Clarke, B.R.; Whitfield, C. Identification of an ATP-binding cassette transport system required for translocation of lipopolysaccharide O-antigen side-chains across the cytoplasmic membrane of Klebsiella pneumoniae serotype O1. Mol. Microbiol. 1994, 14, 505–519. [Google Scholar] [CrossRef]
- Linton, K.J.; Higgins, C.F. The Escherichia coli ATP-binding cassette (ABC) proteins. Mol. Microbiol. 1998, 28, 5–13. [Google Scholar] [CrossRef]
- Keenleyside, W.J.; Whitfield, C. A novel pathway for O-polysaccharide biosynthesis in Salmonella enterica serovar Borreze. J. Biol. Chem. 1996, 271, 28581–28592. [Google Scholar] [CrossRef]
- Mulford, C.A.; Osborn, M.J. An intermediate step in translocation of lipopolysaccharide to the outer membrane of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 1983, 80, 1159–1163. [Google Scholar] [CrossRef]
- McGrath, B.C.; Osborn, M.J. Localization of the terminal steps of O-antigen synthesis in Salmonella typhimurium. J. Bacteriol. 1991, 173, 649–654. [Google Scholar] [CrossRef]
- Pernthaler, J. Predation on prokaryotes in the water column and its ecological implications. Nat. Rev. Microbiol. 2005, 3, 537–546. [Google Scholar] [CrossRef]
- Griffiths, B.S. Microbial-feeding nematodes and protozoa in soil: Their effectson microbial activity and nitrogen mineralization in decomposition hotspots and the rhizosphere. Plant Soil 1994, 164, 25–33. [Google Scholar] [CrossRef]
- Ronn, R.; McCaig, A.E.; Griffiths, B.S.; Prosser, J.I. Impact of protozoan grazing on bacterial community structure in soil microcosms. Appl. Environ. Microbiol. 2002, 68, 6094–6105. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.; Bonsall, M.B.; Buckling, A.; Whiteley, A.S.; Goodall, T.; Griffiths, R.I. Protists have divergent effects on bacterial diversity along a productivity gradient. Biol. Lett. 2010, 6, 639–642. [Google Scholar] [CrossRef]
- Clarke, B.R.; Cuthbertson, L.; Whitfield, C. Nonreducing terminal modifications determine the chain length of polymannose O antigens of Escherichia coli and couple chain termination to polymer export via an ATP-binding cassette transporter. J. Biol. Chem. 2004, 279, 35709–35718. [Google Scholar] [CrossRef]
- Mellmann, A.; Bielaszewska, M.; Köck, R.; Friedrich, A.W.; Fruth, A.; Middendorf, B.; Harmsen, D.; Schmidt, M.A.; Karch, H. Analysis of Collection of Hemolytic Uremic Syndrome–associated Enterohemorrhagic Escherichia coli. Emerg. Infect. Dis. 2008, 14, 1287–1290. [Google Scholar] [CrossRef]
- Davis, M.R., Jr.; Goldberg, J.B. Purification and visualization of lipopolysaccharide from Gram-negative bacteria by hot aqueous-phenol extraction. JoVE (J. Vis. Exp.) 2012, 63, e3916. [Google Scholar]
- Caspers, H. FC Page: An Illustrated Key to Freshwater and Soil Amoebae with Notes on Cultivation and Ecology—With 64 Fig., 155 pp; Freshwater Biological Association, Scientific Publication: Ambleside, UK, 1976; ISBN 900 386 26 6; ISSN 0367-1857. Int. Rev. Hydrobiol. 1978, 63, 289. [Google Scholar]
- Page, F.C. A New Key to Freshwater and Soil Gymnamoebae: With Instructions for Culture; Freshwater Biological Association: Ambleside, UK, 1988. [Google Scholar]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef]
- Arnold, J.W.; Koudelka, G.B. The Trojan Horse of the microbiological arms race: Phage-encoded toxins as a defence against eukaryotic predators. Environ. Microbiol. 2014, 16, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.V.; Liu, D.; Reeves, P.R. The wzz (cld) protein in Escherichia coli: Amino acid sequence variation determines O-antigen chain length specificity. J. Bacteriol. 1998, 180, 2670–2675. [Google Scholar] [CrossRef]
- Osawa, K.; Shigemura, K.; Iguchi, A.; Shirai, H.; Imayama, T.; Seto, K.; Raharjo, D.; Fujisawa, M.; Osawa, R.; Shirakawa, T. Modulation of O-antigen chain length by the wzz gene in Escherichia coli O157 influences its sensitivities to serum complement. Microbiol. Immunol. 2013, 57, 616–623. [Google Scholar]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Baumann, H.; Jansson, P.-E.; Kenne, L.; Widmalm, G. Structural studies of the Escherichia coli O1A O-polysaccharide, using the computer program CASPER. Carbohydr. Res. 1991, 211, 183–190. [Google Scholar] [CrossRef]
- Liu, Y.; Koudelka, G.B. The Oligosaccharide Region of LPS Governs Predation of E. coli by the Bacterivorous Protist, Acanthamoeba castellanii. Microbiol. Spectr. 2023, 11, e02930-22. [Google Scholar] [CrossRef]
- Jansson, P.-E.; Lönngren, J.; Widmalm, G.; Leontein, K.; Slettengren, K.; Svenson, S.B.; Wrangsell, G.; Dell, A.; Tiller, P.R. Structural studies of the O-antigen polysaccharides of Klebsiella O5 and Escherichia coli O8. Carbohydr. Res. 1985, 145, 59–66. [Google Scholar] [CrossRef]
- Prehm, P.; Jann, B.; Jann, K. The O9 antigen of Escherichia coli: Structure of the polysaccharide chain. Eur. J. Biochem. 1976, 67, 53–56. [Google Scholar] [CrossRef]
- Wang, W.; Perepelov, A.V.; Feng, L.; Shevelev, S.D.; Wang, Q.; Sof’ya, N.S.; Han, W.; Li, Y.; Shashkov, A.S.; Knirel, Y.A. A group of Escherichia coli and Salmonella enterica O antigens sharing a common backbone structure. Microbiology 2007, 153, 2159–2167. [Google Scholar] [CrossRef]
- Eklund, K.; Garegg, P.; Kenne, L.; Lindberg, A.; Lindberg, B. Structural studies on the Escherichia coli O111 lipopolysaccharide. In Proceedings of the IXth International Symposium of Carbohydrate Chemistry, London, UK, 10–14 April 1978. [Google Scholar]
- Lindberg, B.; Lindh, F.; Lönngren, J.; Lindberg, A.A.; Svenson, S.B. Structural studies of the O-specific side-chain of the lipopolysaccharide from Escherichia coli O 55. Carbohydr. Res. 1981, 97, 105–112. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.W.; Shao, J.; Shen, J.; Jia, Q.; Yi, W.; Song, J.K.; Woodward, R.; Chow, C.S.; Wang, P.G. Characterization of a novel alpha1,2-fucosyltransferase of Escherichia coli O128:b12 and functional investigation of its common motif. Biochemistry 2008, 47, 378–387. [Google Scholar] [CrossRef]
- Perepelov, A.V.; Wang, Q.; Senchenkova, S.y.N.; Feng, L.; Shashkov, A.S.; Wang, L.; Knirel, Y.A. Structure and gene cluster of the O-antigen of Escherichia coli O76. Carbohydr. Res. 2013, 377, 14–17. [Google Scholar] [CrossRef]
- Marsden, B.J.; Bundle, D.R.; Perry, M.B. Serological and structural relationships between Escherichia coli O: 98 and Yersinia enterocolitica O: 11,23 and O: 11,24 lipopolysaccharide O-antigens. Biochem. Cell Biol. 1994, 72, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.B.; MacLean, L.; Griffith, D.W. Structure of the O-chain polysaccharide of the phenol-phase soluble lipopolysaccharide of Escherichia coli 0:157:H7. Biochem. Cell Biol. = Biochim. Et Biol. Cell. 1986, 64, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Pluschke, G.; Mayden, J.; Achtman, M.; Levine, R.P. Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect. Immun. 1983, 42, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, J.A.; Najdenski, H.; Skurnik, M. Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol. Microbiol. 2004, 52, 451–469. [Google Scholar] [CrossRef]
- Plainvert, C.; Bidet, P.; Peigne, C.; Barbe, V.; Médigue, C.; Denamur, E.; Bingen, E.; Bonacorsi, S. A new O-antigen gene cluster has a key role in the virulence of the Escherichia coli meningitis clone O45:K1:H7. J. Bacteriol. 2007, 189, 8528–8536. [Google Scholar] [CrossRef]
- Raynaud, C.; Meibom, K.L.; Lety, M.A.; Dubail, I.; Candela, T.; Frapy, E.; Charbit, A. Role of the wbt locus of Francisella tularensis in lipopolysaccharide O-antigen biogenesis and pathogenicity. Infect. Immun. 2007, 75, 536–541. [Google Scholar] [CrossRef]
- March, C.; Cano, V.; Moranta, D.; Llobet, E.; Pérez-Gutiérrez, C.; Tomás, J.M.; Suárez, T.; Garmendia, J.; Bengoechea, J.A. Role of Bacterial Surface Structures on the Interaction of Klebsiella pneumoniae with Phagocytes. PLoS ONE 2013, 8, e56847. [Google Scholar] [CrossRef]
- Sarkar, S.; Ulett, G.C.; Totsika, M.; Phan, M.D.; Schembri, M.A. Role of capsule and O antigen in the virulence of uropathogenic Escherichia coli. PLoS ONE 2014, 9, e94786. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, H.; Huang, X.; Ma, J.; Logue, C.M.; Nolan, L.K.; Li, G. O-specific polysaccharide confers lysozyme resistance to extraintestinal pathogenic Escherichia coli. Virulence 2018, 9, 666–680. [Google Scholar] [CrossRef]
- Iguchi, A.; Iyoda, S.; Kikuchi, T.; Ogura, Y.; Katsura, K.; Ohnishi, M.; Hayashi, T.; Thomson, N.R. A complete view of the genetic diversity of the Escherichia coli O-antigen biosynthesis gene cluster. DNA Res. 2015, 22, 101–107. [Google Scholar] [CrossRef]
- DebRoy, C.; Fratamico, P.M.; Yan, X.; Baranzoni, G.; Liu, Y.; Needleman, D.S.; Tebbs, R.; O’Connell, C.D.; Allred, A.; Swimley, M. Comparison of O-antigen gene clusters of all O-serogroups of Escherichia coli and proposal for adopting a new nomenclature for O-typing. PLoS ONE 2016, 11, e0147434. [Google Scholar]
- Campbell, A. Prophage insertion sites. Res. Microbiol. 2003, 154, 277–282. [Google Scholar] [CrossRef]
- Allison, G.E.; Verma, N.K. Serotype-converting bacteriophages and O-antigen modification in Shigella flexneri. Trends Microbiol. 2000, 8, 17–23. [Google Scholar] [CrossRef]
- Liu, B.; Knirel, Y.A.; Feng, L.; Perepelov, A.V.; Senchenkova, S.y.N.; Wang, Q.; Reeves, P.R.; Wang, L. Structure and genetics of Shigella O antigens. FEMS Microbiol. Rev. 2008, 32, 627–653. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Koudelka, G. O-Polysaccharides of LPS Modulate E. coli Uptake by Acanthamoeba castellanii. Microorganisms 2023, 11, 1377. https://doi.org/10.3390/microorganisms11061377
Liu Y, Koudelka G. O-Polysaccharides of LPS Modulate E. coli Uptake by Acanthamoeba castellanii. Microorganisms. 2023; 11(6):1377. https://doi.org/10.3390/microorganisms11061377
Chicago/Turabian StyleLiu, Ying, and Gerald Koudelka. 2023. "O-Polysaccharides of LPS Modulate E. coli Uptake by Acanthamoeba castellanii" Microorganisms 11, no. 6: 1377. https://doi.org/10.3390/microorganisms11061377
APA StyleLiu, Y., & Koudelka, G. (2023). O-Polysaccharides of LPS Modulate E. coli Uptake by Acanthamoeba castellanii. Microorganisms, 11(6), 1377. https://doi.org/10.3390/microorganisms11061377





