Relationship between Human Papillomavirus Status and the Cervicovaginal Microbiome in Cervical Cancer
Abstract
:1. Introduction
2. Human Papillomavirus
2.1. Structure and Genome
2.2. Life Cycle of HPV
2.3. HPV and Host Immune Responses
2.3.1. HPV and Innate Immune Response
2.3.2. HPV and the Adaptive Immune Response
2.3.3. HPV and Immune Suppression
3. Cervicovaginal Microbiome
3.1. Cervicovaginal Microbiome in Healthy Women
3.2. Cervicovaginal Microbiome, Host Response, and Cervical Cancer
4. HPV Infection, Cervicovaginal Microbiome, and Cervical Carcinogenesis
4.1. HPV Infection and Cervicovaginal Microbiome
4.2. Cervical Carcinogenesis in the Relationship between HPV Infection and the Cervicovaginal Microbiome
5. Bacteriotherapy in Cervical Cancer Treatment
5.1. Probiotic Bacteriotherapy in Cervical Cancer Treatment
5.2. Novel Approaches Using Bacteria in Cervical Cancer Treatment
5.2.1. Vaginal Suppositories
5.2.2. Probiotic Injection
5.2.3. Probiotics and Modulating the Gastrointestinal Problem of Cervical Cancer
5.2.4. Vaginal Microbiota Transplantation (VMT)
| Studies | Participate | Probiotics | Administration | Results |
|---|---|---|---|---|
| Sun et al. (2022) [114] | 200 HPV-infected women (90 control group, 110 research group) | Lacidophilin + rhIFN-α2b | Vaginal capsules | HPV-positive decreased, and the vaginal microecology recovered higher in the research group than using rhIFN-α2b alone. |
| Palma et al. (2018) [112] | 117 women (BV or vaginitis with concomitant HPV infections) | L. rhamnosus BMX 54 (after treatment with metronidazole for 7 days or fluconazole for 2 consecutive days) | Vaginal tablets, short-term (3 months) and long-term (6 months), follow-up for 9–30 months | HPV-related cytological abnormalities decreased in the long-term vaginal probiotic group. HPV clearance increased after using vaginal probiotics. |
| Negi D. et al. (2020) [116] | Mouse model of CC | L. rhamnosus + cisplastin -loaded pessaries | Vaginal pessaries | Reduced the tumor volume in mice with CC and has a low side effect of cisplatin. |
| Haghighi et al. (2022) [117] | Mouse model of CC with TC1 cells | L. casei combined to α-GalCer | Heat-killed extracts of L. casei and α-GalCer (subcutaneous injections) | Increasing splenocyte proliferation and cell death and decreasing IL-4 and TGF-β. The combination can be efficacious in the CC treatment model. |
| Linn et al. (2019) [119] | 57 CC patients with diarrhea after radiotherapy | Lactobacillus acidophilus LA-5 and Bifidobacterium animalis subsp. lactis BB-12 | Oral capsule (Biogurt®) | The diarrhea symptoms were significantly reduced. |
| Le-Sagie et al. (2019) [121] | 5 patients with intractable and recurrent BV | Vaginal microbiota transplantation | Vaginal health fluid from donors | Four patients improved their symptoms, the Amsel score normalized, and microscopic vaginal fluid appeared. |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global Estimates of Incidence and Mortality of Cervical Cancer in 2020: A Baseline Analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef] [PubMed]
- Della Fera, A.N.; Warburton, A.; Coursey, T.L.; Khurana, S.; McBride, A.A. Persistent Human Papillomavirus Infection. Viruses 2021, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The Vaginal Microbiota, Human Papillomavirus Infection and Cervical Intraepithelial Neoplasia: What Do We Know and Where Are We Going Next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal Microbiota and the Potential of Lactobacillus Derivatives in Maintaining Vaginal Health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef]
- Pino, A.; Bartolo, E.; Caggia, C.; Cianci, A.; Randazzo, C.L. Detection of Vaginal Lactobacilli as Probiotic Candidates. Sci. Rep. 2019, 9, 3355. [Google Scholar] [CrossRef]
- D’Antonio, D.L.; Marchetti, S.; Pignatelli, P.; Piattelli, A.; Curia, M.C. The Oncobiome in Gastroenteric and Genitourinary Cancers. Int. J. Mol. Sci. 2022, 23, 9664. [Google Scholar] [CrossRef]
- Zhou, Z.; Hou, Y.; Qing, W.; Shi, Y.; Zhang, Y.; Chen, R.; Ou, J.; Zhou, H.; Chen, M. The Association of HPV Infection and Vaginal Microbiota of Reproductive Women in China: A Multicenter Cohort Study Protocol. Med. Microecol. 2023, 15, 100072. [Google Scholar] [CrossRef]
- Lebeau, A.; Bruyere, D.; Roncarati, P.; Peixoto, P.; Hervouet, E.; Cobraiville, G.; Taminiau, B.; Masson, M.; Gallego, C.; Mazzucchelli, G.; et al. HPV Infection Alters Vaginal Microbiome through Down-Regulating Host Mucosal Innate Peptides Used by Lactobacilli as Amino Acid Sources. Nat. Commun. 2022, 13, 1076. [Google Scholar] [CrossRef]
- Pourmollaei, S.; Barzegari, A.; Farshbaf-Khalili, A.; Nouri, M.; Fattahi, A.; Shahnazi, M.; Dittrich, R. Anticancer Effect of Bacteria on Cervical Cancer: Molecular Aspects and Therapeutic Implications. Life Sci. 2020, 246, 117413. [Google Scholar] [CrossRef]
- Guo, C.; Dai, W.; Zhou, Q.; Gui, L.; Cai, H.; Wu, D.; Hou, J.; Li, C.; Li, S.; Du, H.; et al. Cervicovaginal Microbiota Significantly Changed for HPV-Positive Women with High-Grade Squamous Intraepithelial Lesion. Front. Cell. Infect. Microbiol. 2022, 12, 973875. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.U.; Jung, D.R.; Lee, Y.H.; Jeon, S.Y.; Han, H.S.; Chong, G.O.; Shin, J.H. Potential Association between Vaginal Microbiota and Cervical Carcinogenesis in Korean Women: A Cohort Study. Microorganisms 2021, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Dellino, M.; Cascardi, E.; Laganà, A.S.; Di Vagno, G.; Malvasi, A.; Zaccaro, R.; Maggipinto, K.; Cazzato, G.; Scacco, S.; Tinelli, R.; et al. Lactobacillus Crispatus M247 Oral Administration: Is It Really an Effective Strategy in the Management of Papillomavirus-Infected Women? Infect. Agent. Cancer 2022, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, V.; Renard, N.; Makar, A.; Van Royen, P.; Bogers, J.P.; Lardon, F.; Peeters, M.; Baay, M. Probiotics Enhance the Clearance of Human Papillomavirus-Related Cervical Lesions: A Prospective Controlled Pilot Study. Eur. J. Cancer Prev. 2013, 22, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Pierro, F.; Criscuolo, A.A.; Giudici, A.D.; Senatori, R.; Sesti, F.; Ciotti, M.; Piccione, E. Oral Administration of Lactobacillus Crispatus M247 to Papillomavirus-Infected Women: Results of a Preliminary, Uncontrolled, Open Trial Francesco. Minerva Obstet. Gynecol. 2021, 73, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef]
- McBride, A.A. Human Papillomaviruses: Diversity, Infection and Host Interactions. Nat. Rev. Microbiol. 2021, 20, 95–108. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; You, J. Targeting Persistent Human Papillomavirus Infection. Viruses 2017, 9, 229. [Google Scholar] [CrossRef]
- Bzhalava, D.; Eklund, C.; Dillner, J. International Standardization and Classification of Human Papillomavirus Types. Virology 2015, 476, 341–344. [Google Scholar] [CrossRef]
- McLaughlin-Daurbin, M.E.; Münger, K. Oncogenic Activities of Human Papillomaviruses Margaret. Virus Res. 2009, 143, 195–208. [Google Scholar] [CrossRef]
- Alizon, S.; Murall, C.L.; Bravo, I.G. Why Human Papillomavirus Acute Infections Matter. Viruses 2017, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.V. The Human Papillomavirus Replication Cycle, and Its Links to Cancer Progression: A Comprehensive Review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef] [PubMed]
- Haręża, D.A.; Wilczyński, J.R.; Paradowska, E. Human Papillomaviruses as Infectious Agents in Gynecological Cancers. Oncogenic Properties of Viral Proteins. Int. J. Mol. Sci. 2022, 23, 1818. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Das, S.S.; Biswal, S.S.; Nath, A.; Das, D.; Basu, A.; Malik, S.; Kumar, L.; Kar, S.; Singh, S.K.; et al. Mechanistic Role of HPV-Associated Early Proteins in Cervical Cancer: Molecular Pathways and Targeted Therapeutic Strategies. Crit. Rev. Oncol. Hematol. 2022, 174, 103675. [Google Scholar] [CrossRef]
- Squarzanti, D.F.; Sorrentino, R.; Landini, M.M.; Chiesa, A.; Pinato, S.; Rocchio, F.; Mattii, M.; Penengo, L.; Azzimonti, B. Human Papillomavirus Type 16 E6 and E7 Oncoproteins Interact with the Nuclear P53-Binding Protein 1 in an in Vitro Reconstructed 3D Epithelium: New Insights for the Virus-Induced DNA Damage Response. Virol. J. 2018, 15, 176. [Google Scholar] [CrossRef]
- Fischer, M.; Uxa, S.; Stanko, C.; Magin, T.M.; Engeland, K. Human Papilloma Virus E7 Oncoprotein Abrogates the P53-P21-DREAM Pathway. Sci. Rep. 2017, 7, 2603. [Google Scholar] [CrossRef]
- Giarrè, M.; Caldeira, S.; Malanchi, I.; Ciccolini, F.; Leão, M.J.; Tommasino, M. Induction of PRb Degradation by the Human Papillomavirus Type 16 E7 Protein Is Essential To Efficiently Overcome P16 INK4a -Imposed G 1 Cell Cycle Arrest. J. Virol. 2001, 75, 4705–4712. [Google Scholar] [CrossRef]
- Racle, J.; de Jonge, K.; Baumgaertner, P.; Speiser, D.E.; Gfeller, D. Simultaneous Enumeration of Cancer and Immune Cell Types from Bulk Tumor Gene Expression Data. eLife 2017, 6, e26476. [Google Scholar] [CrossRef]
- Jeon, S.; Allen-Hoffmann, B.L.; Lambert, P.F. Integration of Human Papillomavirus Type 16 into the Human Genome Correlates with a Selective Growth Advantage of Cells. J. Virol. 1995, 69, 2989–2997. [Google Scholar] [CrossRef]
- Amador-Molina, A.; Hernández-Valencia, J.F.; Lamoyi, E.; Contreras-Paredes, A.; Lizano, M. Role of Innate Immunity against Human Papillomavirus (HPV) Infections and Effect of Adjuvants in Promoting Specific Immune Response. Viruses 2013, 5, 2624–2642. [Google Scholar] [CrossRef]
- Bienkowska-Haba, M.; Zwolinska, K.; Keiffer, T.; Scott, R.S.; Sapp, M. Human Papillomavirus Genome Copy Number Is Maintained by S-Phase Amplification, Genome Loss to the Cytosol during Mitosis, and Degradation in G1 Phase. J. Virol. 2023, 97, e01879-22. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, R.; Nakahama, Y.; Nguyen, V.; Espinoza, J.L. The Host-Microbe Interplay in Human Papillomavirus-Induced Carcinogenesis. Microorganisms 2019, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 Recognizes Cytosolic DsDNA and Forms a Caspase-1-Activating Inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.L.; Woodby, B.L.; Ulicny, J.; Raikhy, G.; Orr, A.W.; Songock, W.K. Human Papillomavirus 16 E5 Inhibits Interferon Signaling and Supports Episomal Viral Maintenance. J. Virol. 2020, 94, e01582-19. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yan, L.; Liu, N.; Xu, M.; Cai, H. IFI16 Promotes Cervical Cancer Progression by Upregulating PD-L1 in Immunomicroenvironment through STING-TBK1-NF-KB Pathway. Biomed. Pharmacother. 2020, 123, 109790. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, Y.; Li, C. The Role of TLRs in Cervical Cancer with HPV Infection: A Review. Signal Transduct. Target. Ther. 2017, 2, 17055. [Google Scholar] [CrossRef]
- Chen, H.; Sun, H.; You, F.; Sun, W.; Zhou, X.; Chen, L.; Yang, J.; Wang, Y.; Tang, H.; Guan, Y.; et al. Activation of STAT6 by STING Is Critical for Antiviral Innate Immunity. Cell 2011, 147, 436–446. [Google Scholar] [CrossRef]
- Cheng, Z.; Dai, T.; He, X.; Zhang, Z.; Xie, F.; Wang, S.; Zhang, L.; Zhou, F. The Interactions between CGAS-STING Pathway and Pathogens. Signal Transduct. Target. Ther. 2020, 5, 91. [Google Scholar] [CrossRef]
- Matsumiya, T.; Prescott, S.M.; Stafforini, D.M. IFN-ε Mediates TNF-α-Induced STAT1 Phosphorylation and Induction of Retinoic Acid-Inducible Gene-I in Human Cervical Cancer Cells. J. Immunol. 2007, 179, 4542–4549. [Google Scholar] [CrossRef]
- Rattay, S.; Hufbauer, M.; Hagen, C.; Putschli, B.; Coch, C.; Akgül, B.; Hartmann, G. Human Beta Papillomavirus Type 8 E1 and E2 Proteins Suppress the Activation of the RIG-I-like Receptor MDA5. Viruses 2022, 14, 1361. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Gallagher, G.; Baurin, V.V.; Lewis-Antes, A.; Shen, M.; Shah, N.K.; Langer, J.A.; Sheikh, F.; Dickensheets, H.; Donnelly, R.P. IFN-Λs Mediate Antiviral Protection through a Distinct Class II Cytokine Receptor Complex. Nat. Immunol. 2003, 4, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Cannella, F.; Scagnolari, C.; Selvaggi, C.; Stentella, P.; Recine, N.; Antonelli, G.; Pierangeli, A. Interferon Lambda 1 Expression in Cervical Cells Differs between Low-Risk and High-Risk Human Papillomavirus-Positive Women. Med. Microbiol. Immunol. 2014, 203, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Hurst, J.; Voges, M.; Krauss, P.; Münch, P.; Iftner, T.; Stubenrauch, F. High-Risk Human Papillomaviruses Repress Constitutive Kappa Interferon Transcription via E6 To Prevent Pathogen Recognition Receptor and Antiviral-Gene Expression. J. Virol. 2011, 85, 11372–11380. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef]
- Richards, K.H.; Wasson, C.W.; Watherston, O.; Doble, R.; Eric Blair, G.; Wittmann, M.; Macdonald, A. The Human Papillomavirus (HPV) E7 Protein Antagonises an Imiquimod-Induced Inflammatory Pathway in Primary Human Keratinocytes. Sci. Rep. 2015, 5, 12922. [Google Scholar] [CrossRef]
- Vanguri, V.K. The Adaptive Immune System. In Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 1–4. ISBN 9780123864567. [Google Scholar]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; De Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic Human Papillomavirus Infection. Nat. Rev. Dis. Prim. 2016, 2, 16086. [Google Scholar] [CrossRef]
- Litwin, T.R.; Irvin, S.R.; Chornock, R.L.; Sahasrabuddhe, V.V.; Stanley, M.; Wentzensen, N. Infiltrating T-Cell Markers in Cervical Carcinogenesis: A Systematic Review and Meta-Analysis. Br. J. Cancer 2021, 124, 831–841. [Google Scholar] [CrossRef]
- Stanley, M.A. Epithelial Cell Responses to Infection with Human Papillomavirus. Clin. Microbiol. Rev. 2012, 25, 215–222. [Google Scholar] [CrossRef]
- Nakahara, T.; Kiyono, T. Interplay between NF-ΚB/Interferon Signaling and the Genome Replication of HPV. Future Virol. 2016, 11, 141–155. [Google Scholar] [CrossRef]
- Tilborghs, S.; Corthouts, J.; Verhoeven, Y.; Arias, D.; Rolfo, C.; Trinh, X.B.; van Dam, P.A. The Role of Nuclear Factor-Kappa B Signaling in Human Cervical Cancer. Crit. Rev. Oncol. Hematol. 2017, 120, 141–150. [Google Scholar] [CrossRef]
- Horta, B.; Pereira, T.; Medeiros, R.; Cerqueira, F. Cervical Cancer Outcome and Tumor-Associated Macrophages: Research Evidence. Immuno 2022, 2, 460–468. [Google Scholar] [CrossRef]
- Kobayashi, A.; Weinberg, V.; Darragh, T.; Smith-McCune, K. Evolving Immunosuppressive Microenvironment during Human Cervical Carcinogenesis. Mucosal Immunol. 2008, 1, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal Microbiota, Women’s Health, and Reproductive Outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, H.; Tsivtsivadze, E.; Verhelst, R.; Marzorati, M.; Jurriaans, S.; Ndayisaba, G.F.; Schuren, F.H.; Van De Wijgert, J.H.H.M. Lactobacillus-Dominated Cervicovaginal Microbiota Associated with Reduced HIV/STI Prevalence and Genital HIV Viral Load in African Women. ISME J. 2014, 8, 1781–1793. [Google Scholar] [CrossRef]
- Delgado-Diaz, D.J.; Jesaveluk, B.; Hayward, J.A.; Tyssen, D.; Alisoltani, A.; Potgieter, M.; Bell, L.; Ross, E.; Iranzadeh, A.; Allali, I.; et al. Lactic Acid from Vaginal Microbiota Enhances Cervicovaginal Epithelial Barrier Integrity by Promoting Tight Junction Protein Expression. Microbiome 2022, 10, 141. [Google Scholar] [CrossRef]
- Jie, Z.; Chen, C.; Hao, L.; Li, F.; Song, L.; Zhang, X.; Zhu, J.; Tian, L.; Tong, X.; Cai, K.; et al. Life History Recorded in the Vagino-Cervical Microbiome Along with Multi-Omes. Genom. Proteom. Bioinform. 2022, 20, 304–321. [Google Scholar] [CrossRef]
- Oliver, A.; Lamere, B.; Weihe, C.; Wandro, S.; Lindsay, K.L.; Wadhwa, P.D.; Mills, D.A.; Pride, D.T.; Fiehn, O.; Northen, T.; et al. Cervicovaginal Microbiome Composition Is Associated with Metabolic Profiles in Healthy Pregnancy. mBio 2020, 11, e01851-20. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.; Zhou, X.; et al. Temporal Dynamics of the Human Vaginal Microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef]
- Vodstrcil, L.A.; Twin, J.; Garland, S.M.; Fairley, C.K.; Hocking, J.S.; Law, M.G.; Plummer, E.L.; Fethers, K.A.; Chow, E.P.F.; Tabrizi, S.N.; et al. The Influence of Sexual Activity on the Vaginal Microbiota and Gardnerella Vaginalis Clade Diversity in Young Women. PLoS ONE 2017, 12, e0171856. [Google Scholar] [CrossRef]
- Zapata, H.J.; Quagliarello, V.J. The Microbiota and Microbiome in Aging: Potential Implications in Health and Age-Related Diseases. J. Am. Geriatr. Soc. 2015, 63, 776–781. [Google Scholar] [CrossRef]
- Sun, N.; Ding, H.; Yu, H.; Ji, Y.; Xifang, X.; Pang, W.; Wang, X.; Zhang, Q.; Li, W. Comprehensive Characterization of Microbial Community in the Female Genital Tract of Reproductive-Aged Women in China. Front. Cell. Infect. Microbiol. 2021, 11, 649067. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Rodriguez, A.C.; Gage, J.C.; Herrero, R.; Hildesheim, A.; Wacholder, S.; Burk, R.; Schiffman, M. A Large, Population-Based Study of Age-Related Associations between Vaginal PH and Human Papillomavirus Infection. BMC Infect. Dis. 2012, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Gao, Y.; Xia, Q.; Wang, H.; Xie, X.; Liu, Y.; Shang, H.; Diao, Y. Reproductive Tract Microbiota of Women in Childbearing Age Shifts upon Gynecological Infections and Menstrual Cycle. BMC Microbiol. 2021, 21, 252. [Google Scholar] [CrossRef] [PubMed]
- Song, S.D.; Acharya, K.D.; Zhu, J.E.; Deveney, C.M.; Walther-Antonio, M.R.; Tetel, M.J.; Chia, N. Daily Vaginal Microbiota Fluctuations Associated with Natural Hormonal Cycle, Contraceptives, Diet, and Exercise. Am. Soc. Microbiol. 2020, 5, e00593-20. [Google Scholar] [CrossRef]
- Weinberg, E.D. Iron Availability and Infection. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 600–605. [Google Scholar] [CrossRef]
- Kaelin, E.A.; Skidmore, P.T.; Łaniewski, P.; Holland, L.A.; Chase, D.M.; Herbst-Kralovetz, M.M.; Lim, E.S. Cervicovaginal DNA Virome Alterations Are Associated with Genital Inflammation and Microbiota Composition. mSystems 2022, 7, e00064-22. [Google Scholar] [CrossRef]
- Sodhani, P.; Gupta, S.; Gupta, R.; Mehrotra, R. Bacterial Vaginosis and Cervical Intraepithelial Neoplasia: Is There an Association or Is Co-Existence Incidental? Asian Pac. J. Cancer Prev. 2017, 18, 1289–1292. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, B.; Lin, Y.; Lin, Y.; Zuo, X. Dysbiosis of Cervical and Vaginal Microbiota Associated with Cervical Intraepithelial Neoplasia. Front. Cell. Infect. Microbiol. 2022, 12, 767693. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Yu, L.; Shi, X.; Min, M.; Xiong, L.; Pan, J.; Liu, P.; Wu, G.; Gao, G. A Cross-Sectional Analysis about Bacterial Vaginosis, High-Risk Human Papillomavirus Infection, and Cervical Intraepithelial Neoplasia in Chinese Women. Sci. Rep. 2022, 12, 6609. [Google Scholar] [CrossRef]
- Onderdonk, A.B.; Delaney, M.L.; Fichorova, R.N. The Human Microbiome during Bacterial Vaginosis. Clin. Microbiol. Rev. 2016, 29, 223–238. [Google Scholar] [CrossRef]
- Torcia, M.G. Interplay among Vaginal Microbiome, Immune Response and Sexually Transmitted Viral Infections. Int. J. Mol. Sci. 2019, 20, 266. [Google Scholar] [CrossRef] [PubMed]
- Łaniewski, P.; Barnes, D.; Goulder, A.; Cui, H.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Linking Cervicovaginal Immune Signatures, HPV and Microbiota Composition in Cervical Carcinogenesis in Non-Hispanic and Hispanic Women. Sci. Rep. 2018, 8, 7593. [Google Scholar] [CrossRef]
- Mitchell, C.; Marrazzo, J. Bacterial Vaginosis and the Cervicovaginal Immune Response. Am. J. Reprod. Immunol. 2014, 71, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, H.; Gautam, R.; Armstrong, S.D.; Xia, D.; Ndayisaba, G.F.; Van Teijlingen, N.H.; Geijtenbeek, T.B.H.; Wastling, J.M.; Van De Wijgert, J.H.H.M. Cervicovaginal Microbiome Dysbiosis Is Associated with Proteome Changes Related to Alterations of the Cervicovaginal Mucosal Barrier. Mucosal Immunol. 2016, 9, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef]
- Łaniewski, P.; Herbst-Kralovetz, M.M. Bacterial Vaginosis and Health-Associated Bacteria Modulate the Immunometabolic Landscape in 3D Model of Human Cervix. NPJ Biofilms Microbiomes 2021, 7, 88. [Google Scholar] [CrossRef]
- Fan, Q.; Wu, Y.; Li, M.; An, F.; Yao, L.; Wang, M.; Wang, X.; Yuan, J.; Jiang, K.; Li, W.; et al. Lactobacillus Spp. Create a Protective Micro-Ecological Environment through Regulating the Core Fucosylation of Vaginal Epithelial Cells against Cervical Cancer. Cell Death Dis. 2021, 12, 1094. [Google Scholar] [CrossRef]
- Brusselaers, N.; Shrestha, S.; van de Wijgert, J.; Verstraelen, H. Vaginal Dysbiosis and the Risk of Human Papillomavirus and Cervical Cancer: Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2019, 221, 9–18.e8. [Google Scholar] [CrossRef]
- Di Paola, M.; Sani, C.; Clemente, A.M.; Iossa, A.; Perissi, E.; Castronovo, G.; Tanturli, M.; Rivero, D.; Cozzolino, F.; Cavalieri, D.; et al. Characterization of Cervico-Vaginal Microbiota in Women Developing Persistent High-Risk Human Papillomavirus Infection. Sci. Rep. 2017, 7, 10200. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, Q.; Chen, Y.; Dong, B.; Xue, H.; Lei, H.; Lu, Y.; Wei, X.; Sun, P. Changes of the Vaginal Microbiota in HPV Infection and Cervical Intraepithelial Neoplasia: A Cross-Sectional Analysis. Sci. Rep. 2022, 12, 2812. [Google Scholar] [CrossRef]
- Donmez, H.G.; Sahal, G.; Akgor, U.; Cagan, M.; Ozgul, N.; Beksac, M.S. The Relationship between the Presence of HPV Infection and Biofilm Formation in Cervicovaginal Smears. Infection 2020, 48, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Qingqing, B.; Jie, Z.; Songben, Q.; Juan, C.; Lei, Z.; Mu, X. Cervicovaginal Microbiota Dysbiosis Correlates with HPV Persistent Infection. Microb. Pathog. 2021, 152, 104617. [Google Scholar] [CrossRef] [PubMed]
- So, K.A.; Yang, E.J.; Kim, N.R.; Hong, S.R.; Lee, J.H.; Hwang, C.S.; Shim, S.H.; Lee, S.J.; Kim, T.J. Changes of Vaginal Microbiota during Cervical Carcinogenesis in Women with Human Papillomavirus Infection. PLoS ONE 2020, 15, e0238705. [Google Scholar] [CrossRef] [PubMed]
- Audirac-Chalifour, A.; Torres-Poveda, K.; Bahena-Román, M.; Téllez-Sosa, J.; Martínez-Barnetche, J.; Cortina-Ceballos, B.; López-Estrada, G.; Delgado-Romero, K.; Burguete-García, A.I.; Cantú, D.; et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS ONE 2016, 11, e0153274. [Google Scholar] [CrossRef]
- Gao, W.; Weng, J.; Gao, Y.; Chen, X. Comparison of the Vaginal Microbiota Diversity of Women with and without Human Papillomavirus Infection: A Cross-Sectional Study. BMC Infect. Dis. 2013, 13, 271–310. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, S.; Lee, H.; Song, Y.M.; Lee, K.; Han, M.J.; Sung, J.; Ko, G.P. Association of the Vaginal Microbiota with Human Papillomavirus Infection in a Korean Twin Cohort. PLoS ONE 2013, 8, e0063514. [Google Scholar] [CrossRef] [PubMed]
- Kwasniewski, W.; Wolun-Cholewa, M.; Kotarski, J.; Warchol, W.; Kuzma, D.; Kwasniewska, A.; Gozdzicka-Jozefiak, A. Microbiota Dysbiosis Is Associated with HPV-Induced Cervical Carcinogenesis. Oncol. Lett. 2018, 16, 7035–7047. [Google Scholar] [CrossRef]
- Teka, B.; Yoshida-Court, K.; Firdawoke, E.; Chanyalew, Z.; Gizaw, M.; Addissie, A.; Mihret, A.; Colbert, L.E.; Napravnik, T.C.; El Alam, M.B.; et al. Cervicovaginal Microbiota Profiles in Precancerous Lesions and Cervical Cancer among Ethiopian Women. Microorganisms 2023, 11, 833. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical Intraepithelial Neoplasia Disease Progression Is Associated with Increased Vaginal Microbiome Diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef]
- Usyk, M.; Zolnik, C.P.; Castle, P.E.; Porras, C.; Herrero, R.; Gradissimo, A.; Gonzalez, P.; Safaeian, M.; Schiffman, M.; Burk, R.D. Cervicovaginal Microbiome and Natural History of HPV in a Longitudinal Study. PLoS Pathog. 2020, 16, e1008376. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, T.; Forsum, U. Lactobacillus Iners: A Marker of Changes in the Vaginal Flora? J. Clin. Microbiol. 2007, 45, 3145. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.Y.; Kim, B.S.; Seo, S.S.; Kong, J.S.; Lee, J.K.; Park, S.Y.; Hong, K.M.; Kim, H.K.; Kim, M.K. The Association of Uterine Cervical Microbiota with an Increased Risk for Cervical Intraepithelial Neoplasia in Korea. Clin. Microbiol. Infect. 2015, 21, 674.e1–674.e9. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ding, X.; Kong, Y.; Acharya, S.; Wu, H.; Huang, C.; Liang, Y.; Nong, X.; Chen, H. The Feature of Cervical Microbiota Associated with the Progression of Cervical Cancer among Reproductive Females. Gynecol. Oncol. 2021, 163, 348–357. [Google Scholar] [CrossRef]
- Ilhan, Z.E.; Łaniewski, P.; Thomas, N.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Deciphering the Complex Interplay between Microbiota, HPV, Inflammation and Cancer through Cervicovaginal Metabolic Profiling. EBioMedicine 2019, 44, 675–690. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Laniewski, P.; Adamov, A.; Chase, D.M.; Gregory Caporaso, J.; Herbst-Kralovetz, M.M. Multi-Omics Data Integration Reveals Metabolome as the Top Predictor of the Cervicovaginal Microenvironment. PLoS Comput. Biol. 2022, 18, e1009876. [Google Scholar] [CrossRef]
- Kumar, L.; Harish, P.; Malik, P.S.; Khurana, S. Chemotherapy and Targeted Therapy in the Management of Cervical Cancer. Curr. Probl. Cancer 2018, 42, 120–128. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Maleki Dana, P.; Badehnoosh, B.; Asemi, Z.; Hallajzadeh, J.; Mansournia, M.A.; Yousefi, B.; Yousefi, B.; Moazzami, B.; Chaichian, S. Anti-Tumor Activities of Probiotics in Cervical Cancer. J. Ovarian Res. 2020, 13, 68. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Ozen, M.; Dinleyici, E.C. The History of Probiotics: The Untold Story. Benef. Microbes 2015, 6, 159–165. [Google Scholar] [CrossRef]
- Markowiak, P.; Ślizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Cha, M.; Lee, D.; An, H.; Lee, S.; Shin, S.; Kwon, J.; Kim, K.; Ha, N. Antiviral Activity of Bifidobacterium Adolescentis SPM1005-A on Human Papillomavirus Type 16. BMC Med. 2012, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef] [PubMed]
- Van Baarlen, P.; Troost, F.; Van Der Meer, C.; Hooiveld, G.; Boekschoten, M.; Brummer, R.J.M.; Kleerebezem, M. Human Mucosal in Vivo Transcriptome Responses to Three Lactobacilli Indicate How Probiotics May Modulate Human Cellular Pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 4562–4569. [Google Scholar] [CrossRef]
- Wang, K.D.; Xu, D.J.; Wang, B.Y.; Yan, D.H.; Lv, Z.; Su, J.R. Inhibitory Effect of Vaginal Lactobacillus Supernatants on Cervical Cancer Cells. Probiotics Antimicrob. Proteins 2018, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Sungur, T.; Aslim, B.; Karaaslan, C.; Aktas, B. Impact of Exopolysaccharides (EPSs) of Lactobacillus Gasseri Strains Isolated from Human Vagina on Cervical Tumor Cells (HeLa). Anaerobe 2017, 47, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.; Aranha, C. Lactobacilli Metabolites Restore E-Cadherin and Suppress MMP9 in Cervical Cancer Cells. Curr. Res. Toxicol. 2022, 3, 100088. [Google Scholar] [CrossRef]
- Ou, Y.C.; Fu, H.C.; Tseng, C.W.; Wu, C.H.; Tsai, C.C.; Lin, H. The Influence of Probiotics on Genital High-Risk Human Papilloma Virus Clearance and Quality of Cervical Smear: A Randomized Placebo-Controlled Trial. BMC Women’s Health 2019, 19, 103. [Google Scholar] [CrossRef]
- van de Wijgert, J.H.H.M.; Verwijs, M.C. Lactobacilli-Containing Vaginal Probiotics to Cure or Prevent Bacterial or Fungal Vaginal Dysbiosis: A Systematic Review and Recommendations for Future Trial Designs. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 287–299. [Google Scholar] [CrossRef]
- Tomusiak, A.; Strus, M.; Heczko, P.B.; Adamski, P.; Stefański, G.; Mikołajczyk-Cichońska, A.; Suda-Szczurek, M. Efficacy and Safety of a Vaginal Medicinal Product Containing Three Strains of Probiotic Bacteria: A Multicenter, Randomized, Double-Blind, and Placebo-Controlled Trial. Drug Des. Dev. Ther. 2015, 9, 5345–5354. [Google Scholar] [CrossRef]
- Palma, E.; Recine, N.; Domenici, L.; Giorgini, M.; Pierangeli, A.; Panici, P.B. Long-Term Lactobacillus Rhamnosus BMX 54 Application to Restore a Balanced Vaginal Ecosystem: A Promising Solution against HPV-Infection. BMC Infect. Dis. 2018, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, S. Antibacterial Activity and Mechanism of Lacidophilin From Lactobacillus Pentosus Against Staphylococcus Aureus and Escherichia Coli. Front. Microbiol. 2020, 11, 582349. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, J.; Zhou, H.; You, L.; Zhu, Y. Influence of Lacidophilin Vaginal Capsules plus Rh-IFN- α 2b on Efficacy, Vaginal Microecology, and Safety of Patients with HPV Infection. Evid.-Based Complement. Altern. Med. 2022, 2022, 3632053. [Google Scholar] [CrossRef]
- Rodriguez-Arrastia, M.; Martinez-Ortigosa, A.; Rueda-Ruzafa, L.; Ayora, A.F.; Ropero-Padilla, C. Probiotic Supplements on Oncology Patients’ Treatment-Related Side Effects: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 4265. [Google Scholar] [CrossRef] [PubMed]
- Negi, D.; Singh, A.; Joshi, N.; Mishra, N. Cisplatin and Probiotic Biomass Loaded Pessaries for the Management of Cervical Cancer. Anticancer Agents Med. Chem. 2019, 20, 589–598. [Google Scholar] [CrossRef]
- Haghighi, D.; Yazdani, S.; Farzanehpour, M.; Esmaeili Gouvarchinghaleh, H. Combined Extract of Heated TC1, a Heat-Killed Preparation of Lactobacillus Casei and Alpha-Galactosyl Ceramide in a Mouse Model of Cervical Cancer. Infect. Agent. Cancer 2022, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, J. Gastrointestinal Complications of Pelvic Radiotherapy: Are They of Any Importance? Gut 2005, 54, 1051–1054. [Google Scholar] [CrossRef]
- Linn, Y.H.; Thu, K.K.; Win, N.H.H. Effect of Probiotics for the Prevention of Acute Radiation-Induced Diarrhoea Among Cervical Cancer Patients: A Randomized Double-Blind Placebo-Controlled Study. Probiotics Antimicrob. Proteins 2019, 11, 638–647. [Google Scholar] [CrossRef]
- Yockey, L.J.; Hussain, F.A.; Bergerat, A.; Reissis, A.; Worrall, D.; Xu, J.; Gomez, I.; Bloom, S.M.; Mafunda, N.A.; Kelly, J.; et al. Screening and Characterization of Vaginal Fluid Donations for Vaginal Microbiota Transplantation. Sci. Rep. 2022, 12, 17948. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal Microbiome Transplantation in Women with Intractable Bacterial Vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
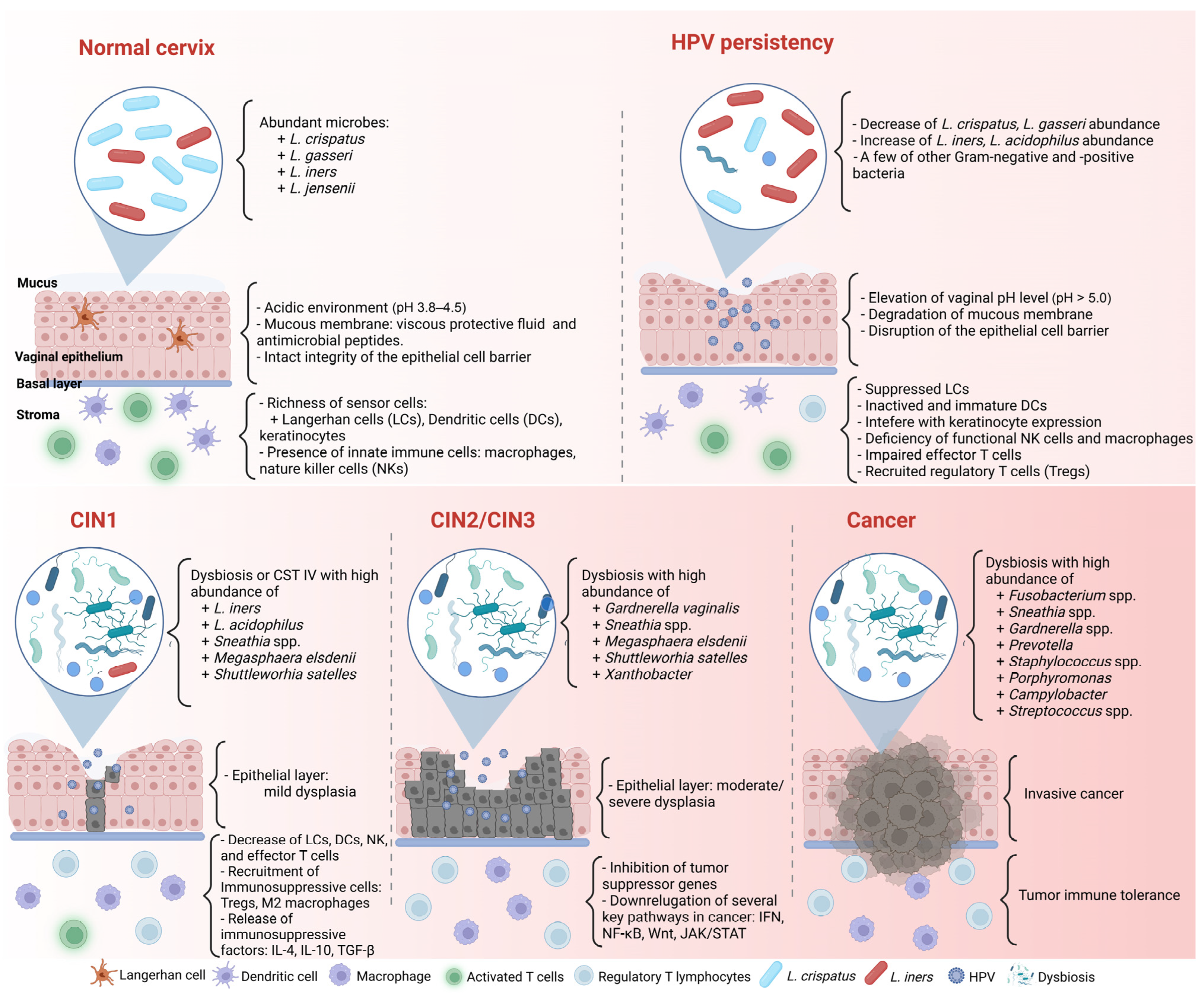
| Author | Participants | Sample Types | Microbial Analysis | * Healthy | * CIN1 or LSIL- HPV(+) | * CIN2/3 or HSIL- HPV(+) | * ICC |
|---|---|---|---|---|---|---|---|
| Audirac-Chalifour et al. (2016) [85] | 32 Mexican women | Cervical epithelial scraping swabs, fresh biopsy | V3-V4 regions of 16S rRNA | L. crispatus, L. iners | Sneathia spp., Megasphaera elsdenii, Shuttleworhia satelles | Sneathia spp., M. elsdenii, S. satelles | Fusobacterium spp. |
| Kwasniewski et al. (2018) [89] | 250 Polish women | Cervical swabs | V4 region of 16S rRNA | L. crispatus, L. iners, L. taiwanensis | L. acidophilus, L. iners | G. vaginalis, L. acidophilus | |
| Laniewski et al. (2018) [73] | 100 Hispanic women (USA) | Cervical (swabs, lavage) | V4 region of 16S rRNA | Lactobacillus spp. | L. iners | L. iners | Sneathia spp. |
| Kang et al. (2021) [12] | 23 Korean women | Cervicovaginal swabs | V3 region of 16S rRNA | Lactobacillus spp. | Gardnerella, Prevotella | Streptococcus spp. | |
| Wu et al. (2021) [95] | 94 Chinese women | Cervical mucus discharge | V4 region of 16S rRNA | Lactobacillus spp. | Lactobacillus, Xanthobacter, Thermus, FlavisolibacterSphingopyxis, Sediminibacterium, Geobacillus | Sneathia, Gardnerella, Megasphera | Porphyromonas, Prevotella, and Campylobacter |
| Teka B. et al. (2023) [90] | 120 Ethiopian women | Cervical (swabs, brush) | V4 region of 16S rRNA | Lactobacillus spp. | L. iners | L.iners | Porphyromonas somerae, Prevotella timonensis, Porphyromonas asaccharolytica |
| Studies | NCT Number | Participants | Strain | Treatment | Results in CC | HPV Clearance |
|---|---|---|---|---|---|---|
| Veronique et al. (2013) [14] | NCT01097356 | 54 women with HPV-positive LSILs | L. casei strain Sheroni (Yakult) | Oral daily for 6 months | Probiotics significantly contributed to the resolution of cytological abnormalities | HPV clearance was higher in probiotic takers than in the control group, but not statistically significant |
| Out et al. (2019) [109] | NCT01599416 | 121 women 62 HR-HPV(+), 59 control | L. rhamnosus GR-1 L. reuteri RC-14 | Oral daily until HPV- | Decreased, mildly abnormal, and unsatisfactory cervical smears in group probiotic takers | Noninfluence HR-HPV |
| Pierro et al. (2021) [15] | Not specified | 35 HPV-positive women | L. crispatus M247 | Oral, 90 days | Change in CST status to CST I after using probiotics | Increased HPV clearance in group probiotic takers |
| Dellino et al. (2022) [13] | Not specified | 80 women with HPV infection and 80 control | L. crispatus M247 | Oral, follow-up 12 months | Reducing HPV-related cytological anomalies in long-term oral probiotic users | The proportion of HPV-negative women was not significantly different |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.D.T.; Le, T.M.; Lee, E.; Lee, D.; Choi, Y.; Cho, J.; Park, N.J.-Y.; Chong, G.O.; Seo, I.; Han, H.S. Relationship between Human Papillomavirus Status and the Cervicovaginal Microbiome in Cervical Cancer. Microorganisms 2023, 11, 1417. https://doi.org/10.3390/microorganisms11061417
Nguyen HDT, Le TM, Lee E, Lee D, Choi Y, Cho J, Park NJ-Y, Chong GO, Seo I, Han HS. Relationship between Human Papillomavirus Status and the Cervicovaginal Microbiome in Cervical Cancer. Microorganisms. 2023; 11(6):1417. https://doi.org/10.3390/microorganisms11061417
Chicago/Turabian StyleNguyen, Hong Duc Thi, Tan Minh Le, Eunmi Lee, Donghyeon Lee, Yeseul Choi, Junghwan Cho, Nora Jee-Young Park, Gun Oh Chong, Incheol Seo, and Hyung Soo Han. 2023. "Relationship between Human Papillomavirus Status and the Cervicovaginal Microbiome in Cervical Cancer" Microorganisms 11, no. 6: 1417. https://doi.org/10.3390/microorganisms11061417
APA StyleNguyen, H. D. T., Le, T. M., Lee, E., Lee, D., Choi, Y., Cho, J., Park, N. J.-Y., Chong, G. O., Seo, I., & Han, H. S. (2023). Relationship between Human Papillomavirus Status and the Cervicovaginal Microbiome in Cervical Cancer. Microorganisms, 11(6), 1417. https://doi.org/10.3390/microorganisms11061417






