Possible Therapeutic Mechanisms and Future Perspectives of Vaginal Microbiota Transplantation
Abstract
:1. Introduction
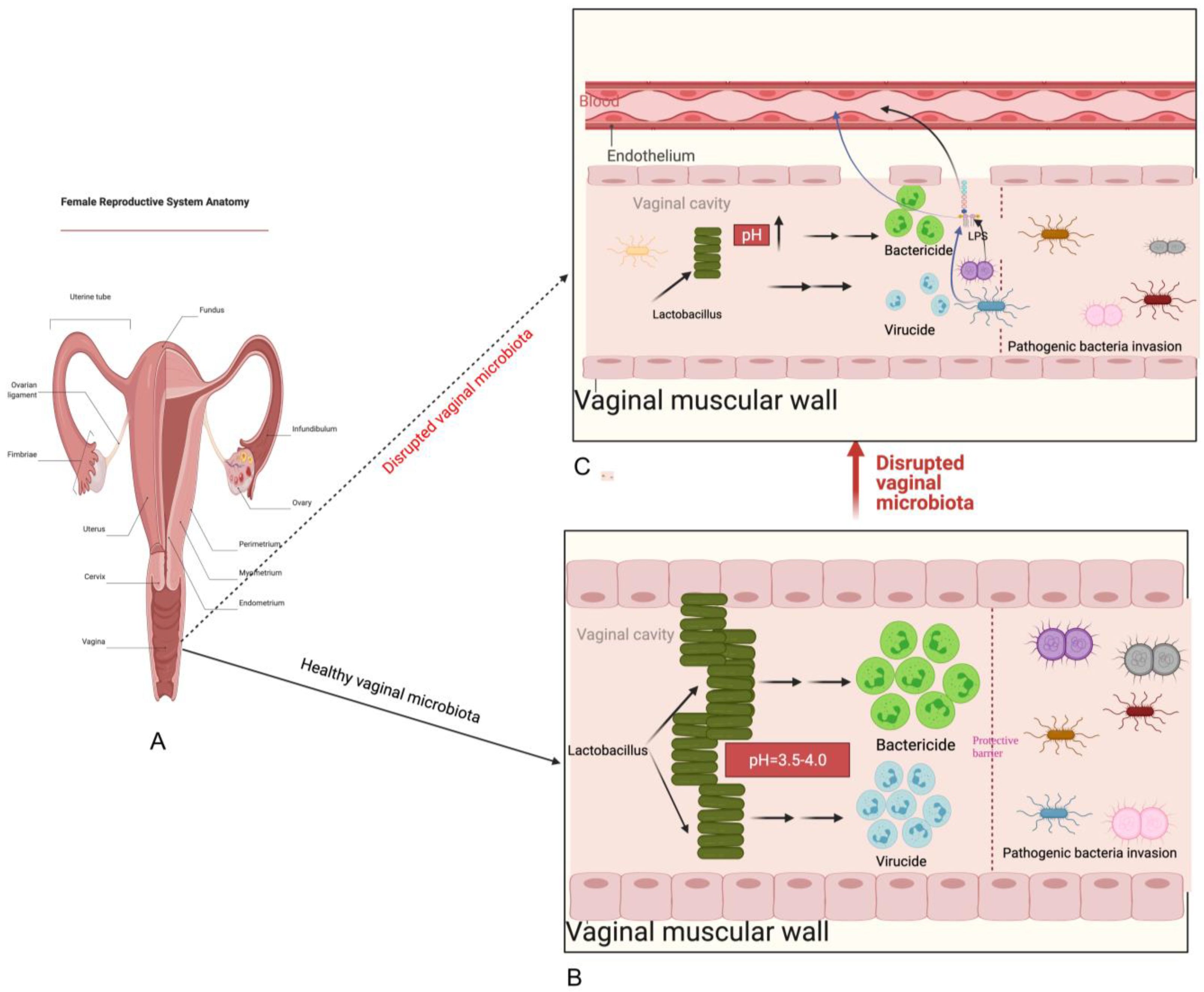
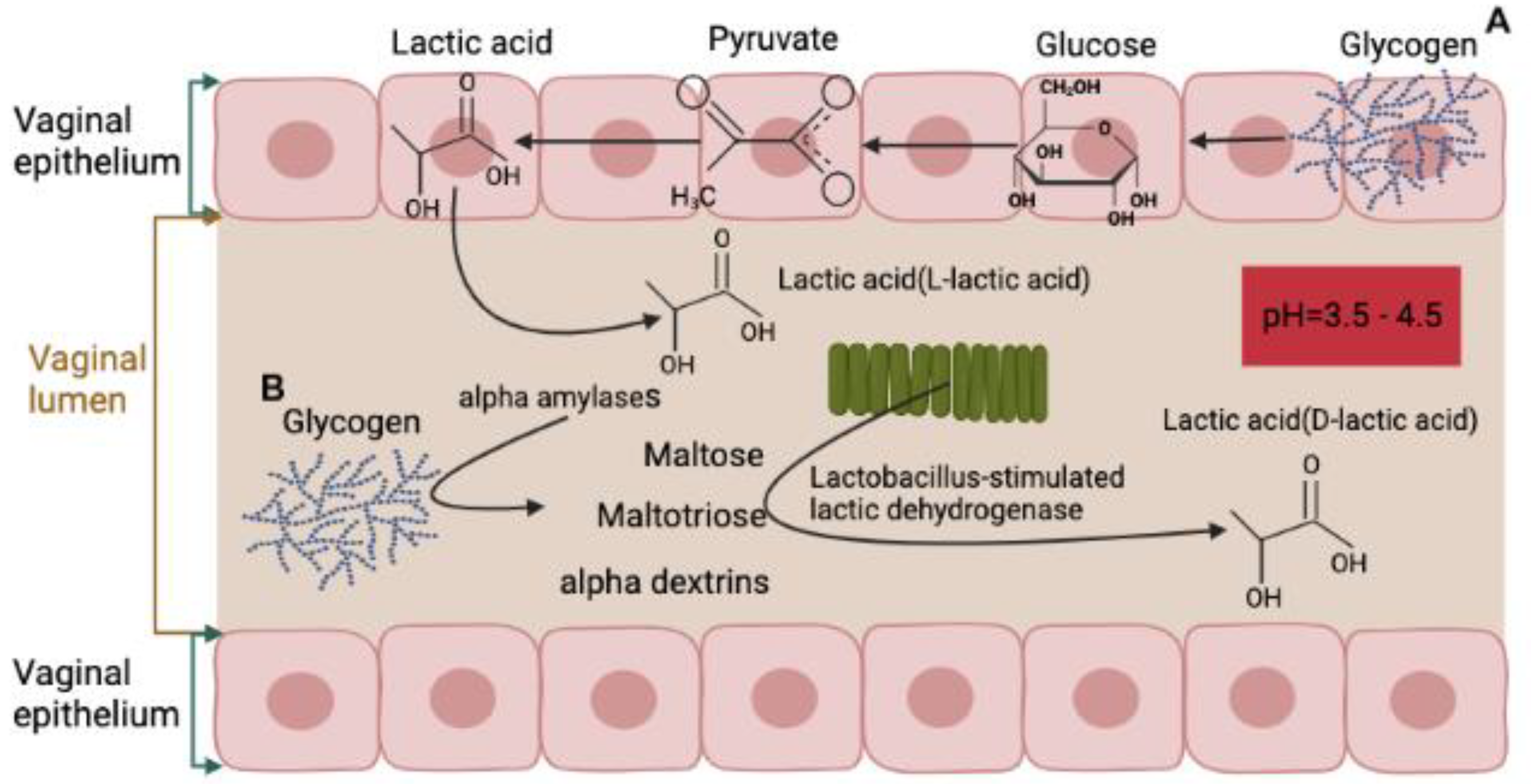
2. Normal Composition and Function of Vaginal Microbiota
3. Development of the Vaginal Microbiota
4. Factors Related to Changes in Vaginal Microbiota
5. Possible Mechanisms of Vaginal Microbiota Transplantation
6. Risks and Limitations
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marchesi, J.R.; Ravel, J. The Vocabulary of Microbiome Research: A Proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Bassis, C.M.; Tang, A.L.; Young, V.B.; Pynnonen, M.A. The Nasal Cavity Microbiota of Healthy Adults. Microbiome 2014, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, M.; Le Noci, V.; Bianchi, F.; Camelliti, S.; Balsari, A.; Tagliabue, E.; Sfondrini, L. The Lung Microbiota: Role in Maintaining Pulmonary Immune Homeostasis and Its Implications in Cancer Development and Therapy. Cell. Mol. Life Sci. 2020, 77, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Egert, M.; Simmering, R. The Microbiota of the Human Skin. Adv. Exp. Med. Biol. 2016, 902, 61–81. [Google Scholar] [CrossRef]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef]
- Mueller, E.R.; Wolfe, A.J.; Brubaker, L. Female Urinary Microbiota. Curr. Opin. Urol. 2017, 27, 282–286. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, G.; Zhao, J.; Chen, J.; Chen, Y.; Huang, W.; Zhong, J.; Zeng, J. Corrigendum: Profiling the Urinary Microbiota in Male Patients With Bladder Cancer in China. Front. Cell. Infect. Microbiol. 2018, 8, 429. [Google Scholar] [CrossRef]
- Moreno, I.; Simon, C. Deciphering the Effect of Reproductive Tract Microbiota on Human Reproduction. Reprod. Med. Biol. 2019, 18, 40–50. [Google Scholar] [CrossRef]
- Bowden, G.H.W. Actinomyces, Propionibacterium Propionicus, and Streptomyces. In Medical Microbiology, 4th ed.; The University of Texas Medical Branch: Galveston, TX, USA, 1996. [Google Scholar]
- Wirusanti, N.I.; Baldridge, M.T.; Harris, V.C. Microbiota Regulation of Viral Infections through Interferon Signaling. Trends Microbiol. 2022, 30, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Nicco, C.; Paule, A.; Konturek, P.; Edeas, M. From Donor to Patient: Collection, Preparation and Cryopreservation of Fecal Samples for Fecal Microbiota Transplantation. Diseases 2020, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Baunwall, S.M.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal Microbiota Transplantation for Recurrent Clostridioides Difficile Infection: An Updated Systematic Review and Meta-Analysis. EClinicalMedicine 2020, 29–30, 100642. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, Y.; Huang, S.; Li, J.; Zhao, K.; Li, X.; Wen, X.; Li, X.-A. Fecal Microbiota Transplantation for Ulcerative Colitis: A Prospective Clinical Study. BMC Gastroenterol. 2019, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Chinna Meyyappan, A.; Forth, E.; Wallace, C.J.K.; Milev, R. Effect of Fecal Microbiota Transplant on Symptoms of Psychiatric Disorders: A Systematic Review. BMC Psychiatry 2020, 20, 299. [Google Scholar] [CrossRef]
- DeLong, K.; Zulfiqar, F.; Hoffmann, D.E.; Tarzian, A.J.; Ensign, L.M. Vaginal Microbiota Transplantation: The Next Frontier. J. Law. Med. Ethics 2019, 47, 555–567. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal Microbiome Transplantation in Women with Intractable Bacterial Vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
- Gardner, H.L.; Dukes, C.D. Haemophilus Vaginalis Vaginitis: A Newly Defined Specific Infection Previously Classified Non-Specific Vaginitis. Am. J. Obstet. Gynecol. 1955, 69, 962–976. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Punzón-Jiménez, P.; Labarta, E. The Impact of the Female Genital Tract Microbiome in Women Health and Reproduction: A Review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541. [Google Scholar] [CrossRef]
- Chadchan, S.B.; Singh, V.; Kommagani, R. Female Reproductive Dysfunctions and the Gut Microbiota. J. Mol. Endocrinol. 2022, 69, R81–R94. [Google Scholar] [CrossRef]
- Quaranta, G.; Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation: A Potential Tool for Treatment of Human Female Reproductive Tract Diseases. Front. Immunol. 2019, 10, 2653. [Google Scholar] [CrossRef] [PubMed]
- Anahtar, M.N.; Gootenberg, D.B.; Mitchell, C.M.; Kwon, D.S. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell. Host Microbe 2018, 23, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- van de Wijgert, J.H.H.M.; Borgdorff, H.; Verhelst, R.; Crucitti, T.; Francis, S.; Verstraelen, H.; Jespers, V. The Vaginal Microbiota: What Have We Learned after a Decade of Molecular Characterization? PLoS ONE 2014, 9, e105998. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The Microbiota Continuum along the Female Reproductive Tract and Its Relation to Uterine-Related Diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef]
- Kyongo, J.K.; Jespers, V.; Goovaerts, O.; Michiels, J.; Menten, J.; Fichorova, R.N.; Crucitti, T.; Vanham, G.; Ariën, K.K. Searching for Lower Female Genital Tract Soluble and Cellular Biomarkers: Defining Levels and Predictors in a Cohort of Healthy Caucasian Women. PLoS ONE 2012, 7, e43951. [Google Scholar] [CrossRef]
- Balkus, J.; Agnew, K.; Lawler, R.; Mitchell, C.; Hitti, J. Effects of Pregnancy and Bacterial Vaginosis on Proinflammatory Cytokine and Secretory Leukocyte Protease Inhibitor Concentrations in Vaginal Secretions. J. Pregnancy 2010, 2010, 385981. [Google Scholar] [CrossRef]
- Dezzutti, C.S.; Hendrix, C.W.; Marrazzo, J.M.; Pan, Z.; Wang, L.; Louissaint, N.; Kalyoussef, S.; Torres, N.M.; Hladik, F.; Parikh, U.; et al. Performance of Swabs, Lavage, and Diluents to Quantify Biomarkers of Female Genital Tract Soluble Mucosal Mediators. PLoS ONE 2011, 6, e23136. [Google Scholar] [CrossRef]
- Nikolaitchouk, N.; Andersch, B.; Falsen, E.; Strömbeck, L.; Mattsby-Baltzer, I. The Lower Genital Tract Microbiota in Relation to Cytokine-, SLPI- and Endotoxin Levels: Application of Checkerboard DNA-DNA Hybridization (CDH). APMIS 2008, 116, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Doerflinger, S.Y.; Throop, A.L.; Herbst-Kralovetz, M.M. Bacteria in the Vaginal Microbiome Alter the Innate Immune Response and Barrier Properties of the Human Vaginal Epithelia in a Species-Specific Manner. J. Infect. Dis. 2014, 209, 1989–1999. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.; Bosmans, E.; Dekeersmaecker, A.; Vereecken, A.; Van Bulck, B.; Spitz, B. Pathogenesis of Abnormal Vaginal Bacterial Flora. Am. J. Obstet. Gynecol. 2000, 182, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Stapleton, A.E.; Hooton, T.M.; Roberts, P.L.; Fennell, C.L.; Stamm, W.E. Inverse Association of H2O2-Producing Lactobacilli and Vaginal Escherichia Coli Colonization in Women with Recurrent Urinary Tract Infections. J. Infect. Dis. 1998, 178, 446–450. [Google Scholar] [CrossRef]
- Pybus, V.; Onderdonk, A.B. Microbial Interactions in the Vaginal Ecosystem, with Emphasis on the Pathogenesis of Bacterial Vaginosis. Microbes Infect. 1999, 1, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Cherpes, T.L.; Meyn, L.A.; Krohn, M.A.; Lurie, J.G.; Hillier, S.L. Association between Acquisition of Herpes Simplex Virus Type 2 in Women and Bacterial Vaginosis. Clin. Infect. Dis. 2003, 37, 319–325. [Google Scholar] [CrossRef]
- Martin, H.L.; Richardson, B.A.; Nyange, P.M.; Lavreys, L.; Hillier, S.L.; Chohan, B.; Mandaliya, K.; Ndinya-Achola, J.O.; Bwayo, J.; Kreiss, J. Vaginal Lactobacilli, Microbial Flora, and Risk of Human Immunodeficiency Virus Type 1 and Sexually Transmitted Disease Acquisition. J. Infect. Dis. 1999, 180, 1863–1868. [Google Scholar] [CrossRef]
- Sobel, J.D. Is There a Protective Role for Vaginal Flora? Curr. Infect. Dis. Rep. 1999, 1, 379–383. [Google Scholar] [CrossRef]
- Watts, D.H.; Fazzari, M.; Minkoff, H.; Hillier, S.L.; Sha, B.; Glesby, M.; Levine, A.M.; Burk, R.; Palefsky, J.M.; Moxley, M.; et al. Effects of Bacterial Vaginosis and Other Genital Infections on the Natural History of Human Papillomavirus Infection in HIV-1-Infected and High-Risk HIV-1-Uninfected Women. J. Infect. Dis. 2005, 191, 1129–1139. [Google Scholar] [CrossRef]
- Wiesenfeld, H.C.; Hillier, S.L.; Krohn, M.A.; Landers, D.V.; Sweet, R.L. Bacterial Vaginosis Is a Strong Predictor of Neisseria Gonorrhoeae and Chlamydia Trachomatis Infection. Clin. Infect. Dis. 2003, 36, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Hida, K.; Shukair, S.; Wang, Y.-Y.; Figueiredo, A.; Cone, R.; Hope, T.J.; Hanes, J. Human Immunodeficiency Virus Type 1 Is Trapped by Acidic but Not by Neutralized Human Cervicovaginal Mucus. J. Virol. 2009, 83, 11196–11200. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.E.; Hoover, D.R.; Dallabetta, G.A.; Kumwenda, N.I.; Mtimavalye, L.A.; Yang, L.P.; Liomba, G.N.; Broadhead, R.L.; Chiphangwi, J.D.; Miotti, P.G. Bacterial Vaginosis and Disturbances of Vaginal Flora: Association with Increased Acquisition of HIV. AIDS 1998, 12, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Ryckman, K.K.; Simhan, H.N.; Krohn, M.A.; Williams, S.M. Predicting Risk of Bacterial Vaginosis: The Role of Race, Smoking and Corticotropin-Releasing Hormone-Related Genes. Mol. Hum. Reprod. 2009, 15, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Bacterial Vaginosis. Annu. Rev. Med. 2000, 51, 349–356. [Google Scholar] [CrossRef]
- Brotman, R.M. Vaginal Microbiome and Sexually Transmitted Infections: An Epidemiologic Perspective. J. Clin. Investig. 2011, 121, 4610–4617. [Google Scholar] [CrossRef]
- Smith, S.B.; Ravel, J. The Vaginal Microbiota, Host Defence and Reproductive Physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef]
- Boskey, E.R.; Cone, R.A.; Whaley, K.J.; Moench, T.R. Origins of Vaginal Acidity: High d/L Lactate Ratio Is Consistent with Bacteria Being the Primary Source. Hum. Reprod. 2001, 16, 1809–1813. [Google Scholar] [CrossRef]
- Mossop, H.; Linhares, I.M.; Bongiovanni, A.M.; Ledger, W.J.; Witkin, S.S. Influence of Lactic Acid on Endogenous and Viral RNA-Induced Immune Mediator Production by Vaginal Epithelial Cells. Obstet. Gynecol. 2011, 118, 840–846. [Google Scholar] [CrossRef]
- Gartner, L.P.; Hiatt, J.L.; Samperio, J.O. Texto Atlas de Histología; McGraw-Hill Interamericana: New York, NY, USA, 2008. [Google Scholar]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Barrientos-Durán, A.; Fuentes-López, A.; de Salazar, A.; Plaza-Díaz, J.; García, F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients 2020, 12, 419. [Google Scholar] [CrossRef] [PubMed]
- Dei, M.; Di Maggio, F.; Di Paolo, G.; Bruni, V. Vulvovaginitis in Childhood. Best. Pract. Res. Clin. Obstet. Gynaecol. 2010, 24, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ranđelović, G.; Mladenović, V.; Ristić, L.; Otašević, S.; Branković, S.; Mladenović-Antić, S.; Bogdanović, M.; Bogdanović, D. Microbiological Aspects of Vulvovaginitis in Prepubertal Girls. Eur. J. Pediatr. 2012, 171, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Zhou, X.; Williams, C.J.; Hochwalt, A.; Forney, L.J. Bacterial Populations in the Vaginas of Healthy Adolescent Women. J. Pediatr. Adolesc. Gynecol. 2009, 22, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Al-Baghdadi, O.; Ewies, A.A.A. Topical Estrogen Therapy in the Management of Postmenopausal Vaginal Atrophy: An up-to-Date Overview. Climacteric 2009, 12, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus Species as Biomarkers and Agents That Can Promote Various Aspects of Vaginal Health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef]
- Blackwell, A.; Phillips, I.; Fox, A.; Barlow, D. Anaerobic vaginosis (non-specific vaginitis): Clinical, microbiological, and therapeutic findings. Lancet 1983, 322, 1379–1382. [Google Scholar] [CrossRef]
- Donders, G.G.G.; Bellen, G.; Grinceviciene, S.; Ruban, K.; Vieira-Baptista, P. Aerobic Vaginitis: No Longer a Stranger. Res. Microbiol. 2017, 168, 845–858. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The Human Microbiome: At the Interface of Health and Disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Hill, G.B. The Microbiology of Bacterial Vaginosis. Am. J. Obstet. Gynecol. 1993, 169, 450–454. [Google Scholar] [CrossRef]
- Freitas, A.C.; Chaban, B.; Bocking, A.; Rocco, M.; Yang, S.; Hill, J.E.; Money, D.M. VOGUE Research Group The Vaginal Microbiome of Pregnant Women Is Less Rich and Diverse, with Lower Prevalence of Mollicutes, Compared to Non-Pregnant Women. Sci. Rep. 2017, 7, 9212. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. Correction: The Composition and Stability of the Vaginal Microbiota of Normal Pregnant Women Is Different from That of Non-Pregnant Women. Microbiome 2014, 2, 10. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hansmann, M.A.; Davis, C.C.; Suzuki, H.; Brown, C.J.; Schütte, U.; Pierson, J.D.; Forney, L.J. The Vaginal Bacterial Communities of Japanese Women Resemble Those of Women in Other Racial Groups. FEMS Immunol. Med. Microbiol. 2010, 58, 169–181. [Google Scholar] [CrossRef]
- Brotman, R.M.; He, X.; Gajer, P.; Fadrosh, D.; Sharma, E.; Mongodin, E.F.; Ravel, J.; Glover, E.D.; Rath, J.M. Association between Cigarette Smoking and the Vaginal Microbiota: A Pilot Study. BMC Infect. Dis. 2014, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.; da Silva, A.P.; Medeiros, R.; Bicho, M.; Bicho, M.C. Microenvironment in Vagina as a Key-Player on Cervical Cancer: Interaction of Polymorphic Genetic Variants and Vaginal Microbiome as Co-Factors. Cerv. Cancer Screen. Treat. Prev. Univers. Protoc. Ultim. Control. 2018. [Google Scholar] [CrossRef]
- Di Paola, M.; Sani, C.; Clemente, A.M.; Iossa, A.; Perissi, E.; Castronovo, G.; Tanturli, M.; Rivero, D.; Cozzolino, F.; Cavalieri, D.; et al. Characterization of Cervico-Vaginal Microbiota in Women Developing Persistent High-Risk Human Papillomavirus Infection. Sci. Rep. 2017, 7, 10200. [Google Scholar] [CrossRef]
- Balle, C.; Lennard, K.; Dabee, S.; Barnabas, S.L.; Jaumdally, S.Z.; Gasper, M.A.; Maseko, V.; Mbulawa, Z.Z.A.; Williamson, A.-L.; Bekker, L.-G.; et al. Endocervical and Vaginal Microbiota in South African Adolescents with Asymptomatic Chlamydia Trachomatis Infection. Sci. Rep. 2018, 8, 11109. [Google Scholar] [CrossRef]
- Ng, S.C.; Kamm, M.A.; Yeoh, Y.K.; Chan, P.K.S.; Zuo, T.; Tang, W.; Sood, A.; Andoh, A.; Ohmiya, N.; Zhou, Y.; et al. Scientific Frontiers in Faecal Microbiota Transplantation: Joint Document of Asia-Pacific Association of Gastroenterology (APAGE) and Asia-Pacific Society for Digestive Endoscopy (APSDE). Gut 2020, 69, 83–91. [Google Scholar] [CrossRef]
- Tuniyazi, M.; Hu, X.; Fu, Y.; Zhang, N. Canine Fecal Microbiota Transplantation: Current Application and Possible Mechanisms. Vet. Sci. 2022, 9, 396. [Google Scholar] [CrossRef]
- Mullish, B.H.; Quraishi, M.N.; Segal, J.P.; McCune, V.L.; Baxter, M.; Marsden, G.L.; Moore, D.; Colville, A.; Bhala, N.; Iqbal, T.H.; et al. The Use of Faecal Microbiota Transplant as Treatment for Recurrent or Refractory Clostridium Difficile Infection and Other Potential Indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) Guidelines. J. Hosp. Infect. 2018, 100, S1–S31. [Google Scholar] [CrossRef] [PubMed]
- Boskey, E.R.; Telsch, K.M.; Whaley, K.J.; Moench, T.R.; Cone, R.A. Acid Production by Vaginal Flora in Vitro Is Consistent with the Rate and Extent of Vaginal Acidification. Infect. Immun. 1999, 67, 5170–5175. [Google Scholar] [CrossRef] [PubMed]
- Rajawat, A.S.; Shrivastava, V.; Shrivastava, A.; Singh, V. In Vitro Evaluation of Inhibitory Activity of Probiotic Lactobacilli against Candida Species Isolated from the Vaginal Flora of Immunocompro-Mised Patients. South Asian J. Exp. Biol. 2014, 3, 325–329. [Google Scholar] [CrossRef]
- Osset, J.; Bartolomé, R.M.; García, E.; Andreu, A. Assessment of the Capacity of Lactobacillus to Inhibit the Growth of Uropathogens and Block Their Adhesion to Vaginal Epithelial Cells. J. Infect. Dis. 2001, 183, 485–491. [Google Scholar] [CrossRef]
- Mastromarino, P.; Brigidi, P.; Macchia, S.; Maggi, L.; Pirovano, F.; Trinchieri, V.; Conte, U.; Matteuzzi, D. Characterization and Selection of Vaginal Lactobacillus Strains for the Preparation of Vaginal Tablets. J. Appl. Microbiol. 2002, 93, 884–893. [Google Scholar] [CrossRef]
- Zárate, G.; Nader-Macias, M.E. Influence of Probiotic Vaginal Lactobacilli on In Vitro Adhesion of Urogenital Pathogens to Vaginal Epithelial Cells. Lett. Appl. Microbiol. 2006, 43, 174–180. [Google Scholar] [CrossRef]
- Phukan, N.; Parsamand, T.; Brooks, A.E.S.; Nguyen, T.N.M.; Simoes-Barbosa, A. The Adherence of Trichomonas Vaginalis to Host Ectocervical Cells Is Influenced by Lactobacilli. Sex. Transm. Infect. 2013, 89, 455–459. [Google Scholar] [CrossRef]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.-J.M.; Wells, J.M. Regulation of Human Epithelial Tight Junction Proteins by Lactobacillus Plantarum in Vivo and Protective Effects on the Epithelial Barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli Inactivate Chlamydia Trachomatis through Lactic Acid but Not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef]
- Juárez Tomás, M.S.; Ocaña, V.S.; Wiese, B.; Nader-Macías, M.E. Growth and Lactic Acid Production by Vaginal Lactobacillus Acidophilus CRL 1259, and Inhibition of Uropathogenic Escherichia Coli. J. Med. Microbiol. 2003, 52, 1117–1124. [Google Scholar] [CrossRef]
- Graver, M.A.; Wade, J.J. The Role of Acidification in the Inhibition of Neisseria Gonorrhoeae by Vaginal Lactobacilli during Anaerobic Growth. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Shukair, S.A.; Allen, S.A.; Cianci, G.C.; Stieh, D.J.; Anderson, M.R.; Baig, S.M.; Gioia, C.J.; Spongberg, E.J.; Kauffman, S.M.; McRaven, M.D.; et al. Human Cervicovaginal Mucus Contains an Activity That Hinders HIV-1 Movement. Mucosal Immunol. 2013, 6, 427–434. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal PH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Gómez, G.; Prado-Audelo, M.L.D.; Ortega-Peña, S.; Mendoza-Muñoz, N.; Urbán-Morlán, Z.; González-Torres, M.; González-Del Carmen, M.; Figueroa-González, G.; Reyes-Hernández, O.D.; Cortés, H. Modifications in Vaginal Microbiota and Their Influence on Drug Release: Challenges and Opportunities. Pharmaceutics 2019, 11, 217. [Google Scholar] [CrossRef]
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human Microbiome: An Academic Update on Human Body Site Specific Surveillance and Its Possible Role. Arch. Microbiol. 2020, 202, 2147–2167. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the Human Microbiome. Nutr. Rev. 2012, 70 (Suppl. 1), S38–S44. [Google Scholar] [CrossRef]
- Lehtoranta, L.; Hibberd, A.A.; Yeung, N.; Laitila, A.; Maukonen, J.; Ouwehand, A.C. Characterization of Vaginal Fungal Communities in Healthy Women and Women with Bacterial Vaginosis (BV); a Pilot Study. Microb. Pathog. 2021, 161, 105055. [Google Scholar] [CrossRef]
- Jakobsen, R.R.; Haahr, T.; Humaidan, P.; Jensen, J.S.; Kot, W.P.; Castro-Mejia, J.L.; Deng, L.; Leser, T.D.; Nielsen, D.S. Characterization of the Vaginal DNA Virome in Health and Dysbiosis. Viruses 2020, 12, 1143. [Google Scholar] [CrossRef]
- Youngster, I.; Russell, G.H.; Pindar, C.; Ziv-Baran, T.; Sauk, J.; Hohmann, E.L. Oral, Capsulized, Frozen Fecal Microbiota Transplantation for Relapsing Clostridium Difficile Infection. JAMA 2014, 312, 1772–1778. [Google Scholar] [CrossRef]
- Burz, S.D.; Abraham, A.-L.; Fonseca, F.; David, O.; Chapron, A.; Béguet-Crespel, F.; Cénard, S.; Le Roux, K.; Patrascu, O.; Levenez, F.; et al. A Guide for Ex Vivo Handling and Storage of Stool Samples Intended for Fecal Microbiota Transplantation. Sci. Rep. 2019, 9, 8897. [Google Scholar] [CrossRef]
- Patel, N.; Patel, N.; Patel, S.; Nathani, N.; Pandit, R.; Patel, M.; Patel, N.; Joshi, C.; Parekh, B. Distinct Gut and Vaginal Microbiota Profile in Women with Recurrent Implantation Failure and Unexplained Infertility. BMC Women’s Health 2022, 22, 113. [Google Scholar] [CrossRef] [PubMed]
- Amato, V.; Papaleo, E.; Pasciuta, R.; Viganò, P.; Ferrarese, R.; Clementi, N.; Sanchez, A.M.; Quaranta, L.; Burioni, R.; Ambrosi, A.; et al. Differential Composition of Vaginal Microbiome, but Not of Seminal Microbiome, Is Associated With Successful Intrauterine Insemination in Couples With Idiopathic Infertility: A Prospective Observational Study. Open. Forum Infect. Dis. 2020, 7, ofz525. [Google Scholar] [CrossRef] [PubMed]
- Okwelogu, S.I.; Ikechebelu, J.I.; Agbakoba, N.R.; Anukam, K.C. Microbiome Compositions From Infertile Couples Seeking In Vitro Fertilization, Using 16S RRNA Gene Sequencing Methods: Any Correlation to Clinical Outcomes? Front. Cell. Infect. Microbiol. 2021, 11, 709372. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuniyazi, M.; Zhang, N. Possible Therapeutic Mechanisms and Future Perspectives of Vaginal Microbiota Transplantation. Microorganisms 2023, 11, 1427. https://doi.org/10.3390/microorganisms11061427
Tuniyazi M, Zhang N. Possible Therapeutic Mechanisms and Future Perspectives of Vaginal Microbiota Transplantation. Microorganisms. 2023; 11(6):1427. https://doi.org/10.3390/microorganisms11061427
Chicago/Turabian StyleTuniyazi, Maimaiti, and Naisheng Zhang. 2023. "Possible Therapeutic Mechanisms and Future Perspectives of Vaginal Microbiota Transplantation" Microorganisms 11, no. 6: 1427. https://doi.org/10.3390/microorganisms11061427
APA StyleTuniyazi, M., & Zhang, N. (2023). Possible Therapeutic Mechanisms and Future Perspectives of Vaginal Microbiota Transplantation. Microorganisms, 11(6), 1427. https://doi.org/10.3390/microorganisms11061427




