Cold Sulfur Springs—Neglected Niche for Autotrophic Sulfur-Oxidizing Bacteria
Abstract
:1. Introduction
2. Main Body
2.1. The Evolution of Sulfur-Oxidizing Bacteria Was Coupled with the Evolution of Earth
2.2. Sulfur Supporting Life
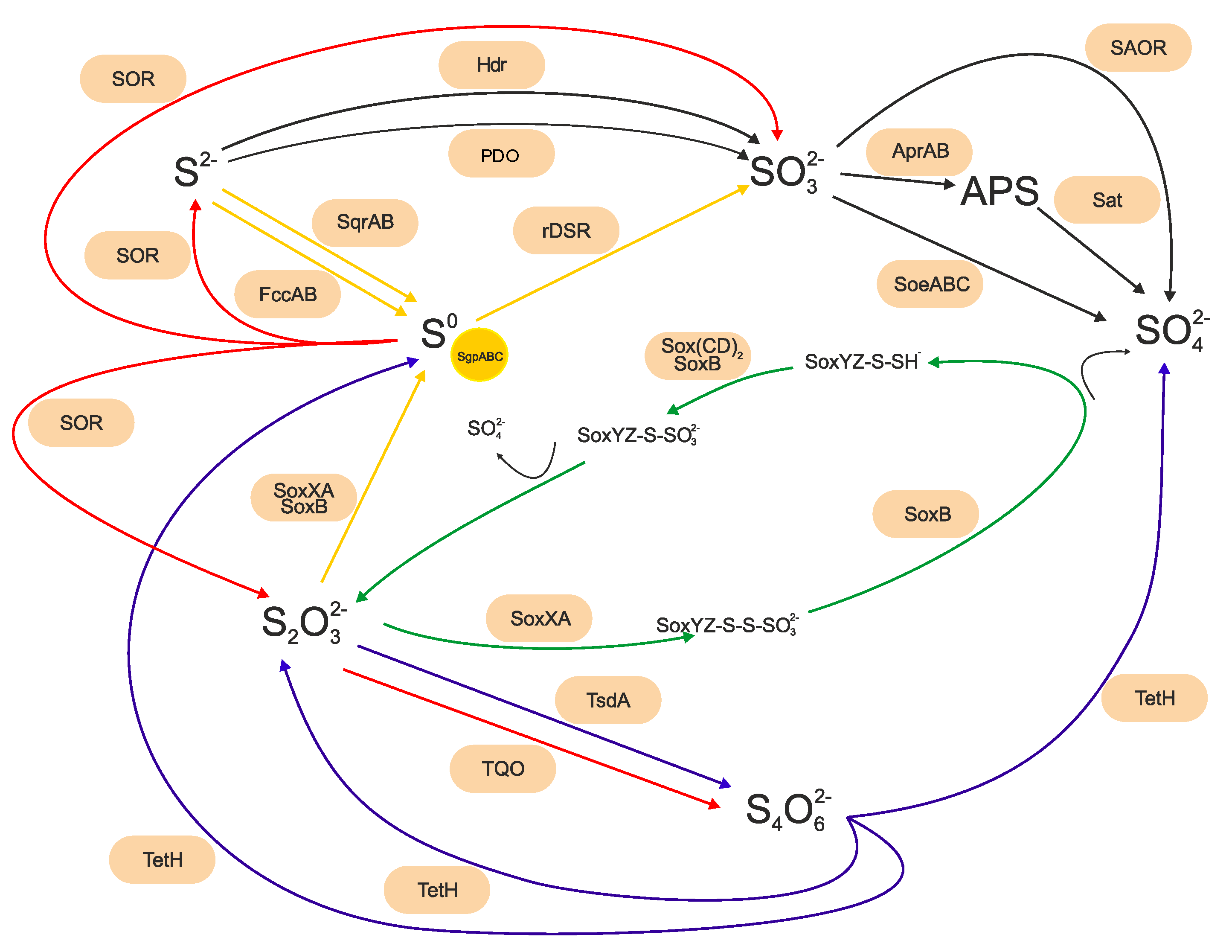
2.3. Sulfur-Oxidizing Bacteria Riding the Biogeochemical Sulfur Cycle Using Different Metabolic Pathways
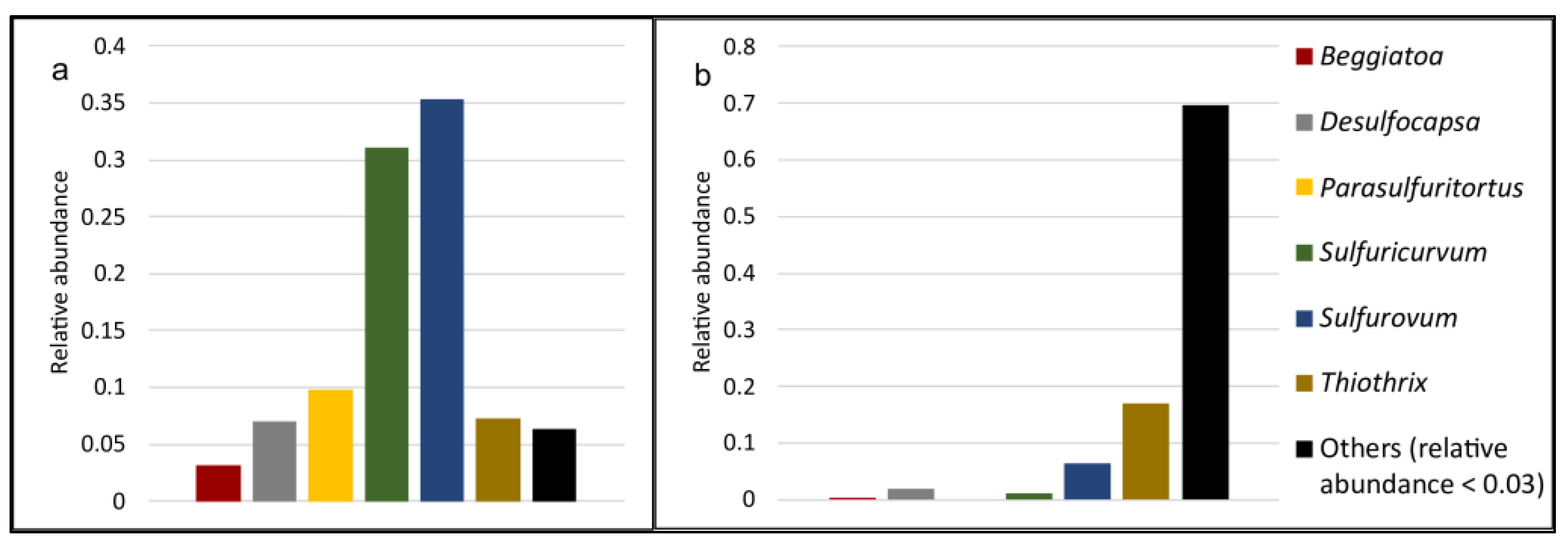
2.4. Cold Sulfur Springs as a Source of Unexplored Life
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sorokin, D.Y.; Kuenen, J.G. Haloalkaliphilic sulfur-oxidizing bacteria in soda lakes. FEMS Microbiol. Rev. 2005, 29, 685–702. [Google Scholar] [CrossRef] [PubMed]
- Fike, D.A.; Bradley, A.S.; Leavitt, W.D. Geomicrobiology of Sulfur. In Ehrlich’s Geomicrobiology, 6th ed.; Ehrlich, H.L., Newman, D.K., Kappler, A., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Zhu, J.; He, J.; Zhu, Y.; Huang, M.; Zhang, Y. Sulfur biogeochemical cycle of marine sediments. Environ. Rev. 2017, 26, 121–132. [Google Scholar] [CrossRef]
- Vavourakis, C.D.; Mehrshad, M.; Balkema, C.; van Hall, R.; Andrei, A.S.; Ghai, R.; Sorokin, D.Y.; Muyzer, G. Metagenomes and metatranscriptomes shed new light on the microbial-mediated sulfur cycle in a Siberian soda lake. BMC Biol. 2019, 17, 69. [Google Scholar] [CrossRef]
- Dahl, C. A biochemical view on the biological sulfur cycle. In Environmental Technologies to Treat Sulfur Pollution: Principles and Engineering, 2nd ed.; Lens, P.N.L., Ed.; IWA Publishing: London, UK, 2020. [Google Scholar] [CrossRef]
- Tourna, M.; Maclean, P.; Condron, L.; O’Callaghan, M.; Wakelin, S.A. Links between sulphur oxidation and sulphur-oxidising bacteria abundance and diversity in soil microcosms based on soxB functional gene analysis. FEMS Microbiol. Ecol. 2014, 88, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Macey, M.C.; Fox-Powell, M.; Ramkissoon, N.K.; Stephens, B.P.; Barton, T.; Schwenzer, S.P.; Pearson, V.K.; Cousins, C.R.; Olsson-Francis, K. The identification of sulfide oxidation as a potential metabolism driving primary production on late Noachian Mars. Sci. Rep. 2020, 10, 10941. [Google Scholar] [CrossRef]
- Vigneron, A.; Cruaud, P.; Culley, A.I.; Couture, R.M.; Lovejoy, C.; Vincent, W.F. Genomic evidence for sulfur intermediates as new biogeochemical hubs in a model aquatic microbial ecosystem. Microbiome 2021, 9, 46. [Google Scholar] [CrossRef]
- Loy, A.; Duller, S.; Wagner, M. Evolution and ecology of microbes dissimilating sulfur compounds: Insights from siroheme sulfite reductases. In Microbial Sulfur Metabolism; Dahl, C., Friedrich, C.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Hahn, C.R.; Farag, I.F.; Murphy, C.L.; Podar, M.; Elshahed, M.S.; Youssef, N.H. Microbial Diversity and Sulfur Cycling in an Early Earth Analogue: From Ancient Novelty to Modern Commonality. mBio 2022, 13, e00016-22. [Google Scholar] [CrossRef]
- Canfield, D.E.; Raiswell, R. The evolution of the sulfur cycle. Am. J. Sci. 1999, 299, 697–723. [Google Scholar] [CrossRef]
- Bailey, J.V.; Corsetti, F.A.; Greene, S.E.; Crosby, C.H.; Liu, P.; Orphan, J.V. Filamentous sulfur bacteria preserved in modern and ancient phosphatic sediments: Implications for the role of oxygen and bacteria in phosphogenesis. Geobiology 2013, 11, 397–405. [Google Scholar] [CrossRef]
- Wacey, D.; Kilburn, M.R.; Saunders, M.; Cliff, J.; Brasier, M.D. Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of Western Australia. Nat. Geosci. 2011, 4, 698–702. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Senko, J.M.; Najar, F.Z.; Kenton, S.M.; Roe, B.A.; Dewers, T.A.; Spear, J.R.; Krumholz, L.R. Bacterial Diversity and Sulfur Cycling in a Mesophilic Sulfide-Rich Spring. Appl. Environ. Microbiol. 2003, 69, 5609–5621. [Google Scholar] [CrossRef] [PubMed]
- Anantharaman, K.; Hausmann, B.; Jungbluth, S.P.; Kantor, R.S.; Lavy, A.; Warren, L.A.; Rappé, M.S.; Pester, M.; Loy, A.; Thomas, B.C.; et al. Expanded diversity of microbial groups that shape the dissimilatory sulfur cycle. ISME J. 2018, 12, 1715–1728. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.; Goswami, S.; Riley, M.; Teske, A.; Sogin, M. Domain Evolution and Functional Diversification of Sulfite Reductases. Astrobiology 2005, 5, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Having, J.R.; Hamilton, T.L.; Bachan, A.; Kump, L.R. Sulfur and carbon isotopic evidence for metabolic pathway evolution and a four-stepped Earth system progression across the Archean and Paleoproterozoic. Earth Sci. Rev. 2017, 174, 1–21. [Google Scholar] [CrossRef]
- Schopf, J.V.; Kudryavtsev, A.B.; Walter, M.R.; Van Kranendonk, M.J.; Williford, K.H.; Kozdon, R.; Valley, J.W.; Gallardo, V.A.; Espinoza, C.; Flannery, D.T. Sulfur-cycling fossil bacteria from the 1.8-Ga Duck Creek Formation provide promising evidence of evolution’s null hypothesis. Proc. Natl. Acad. Sci. USA 2015, 112, 2087–2092. [Google Scholar] [CrossRef]
- Farquhar, J.; Wu, N.; Canfield, D.E.; Oduro, H. Connections between Sulfur Cycle Evolution, Sulfur Isotopes, Sediments, and Base Metal Sulfide Deposits. Econ. Geol. 2010, 105, 509–533. [Google Scholar] [CrossRef]
- Czaja, A.D.; Beukes, N.J.; Osterhout, J.T. Sulfur-oxidizing bacteria prior to the Great Oxidation Event from the 2.52 Ga Gamohaan Formation of South Africa. Geology 2016, 44, 983–986. [Google Scholar] [CrossRef]
- Lens, P.N.L.; Kuenen, J.G. The biological sulfur cycle: Novel opportunities for environmental biotechnology. Water Sci. Technol. 2001, 44, 57–66. [Google Scholar] [CrossRef]
- Luther, G.W., III; Findlay, A.J.; Macdonald, D.J.; Owings, S.M.; Hanson, T.E.; Beinart, R.A.; Girguis, P.R. Thermodynamics and kinetics of sulfide oxidation by oxygen: A look at inorganically controlled reactions and biologically mediated processes in the environment. Front. Microbiol. 2011, 2, 62. [Google Scholar] [CrossRef]
- Morrison, P.R.; Mojzsis, S.J. Tracing the Early Emergence of Microbial Sulfur Metabolisms. Geomicrobiol. J. 2020, 38, 66–86. [Google Scholar] [CrossRef]
- Boucher, Y.; Douady, C.J.; Papke, R.T.; Walsh, D.A.; Boudreau, M.E.R.; Nesbø, C.L.; Case, R.J.; Doolittle, W.F. Lateral gene transfer and the origins of prokaryotic groups. Annu. Rev. Genet. 2003, 37, 283–328. [Google Scholar] [CrossRef]
- Meyer, B.; Kuever, J. Molecular analysis of the distribution and phylogeny of dissimilatory adenosine-5′-phosphosulfate reductase-encoding genes (aprBA) among sulfur-oxidizing prokaryotes. Microbiology 2007, 153, 3478–3498. [Google Scholar] [CrossRef]
- Gosh, W.; Dam, B. Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea. FEMS Microbiol. Rev. 2009, 33, 999–1043. [Google Scholar] [CrossRef] [PubMed]
- Gellatly, A.M.; Lyons, T.W. Trace sulfate in mid-Proterozoic carbonates and the sulfur isotope record of biospheric evolution. Geochim. Cosmochim. Acta 2005, 69, 3813–3829. [Google Scholar] [CrossRef]
- Meyer, B.; Imhoff, J.F.; Kuever, J. Molecular analysis of the distribution and phylogeny of the soxB gene among sulfur-oxidizing bacteria—Evolution of the Sox sulfur oxidation enzyme system. Environ. Microbiol. 2007, 9, 2957–2977. [Google Scholar] [CrossRef] [PubMed]
- Frigaard, N.E.; Dahl, C. Sulfur Metabolism in Phototrophic Sulfur Bacteria. Adv. Microb. Physiol. 2009, 54, 103–200. [Google Scholar] [CrossRef]
- Lomans, B.P.; van der Drift, C.; Pol, A.; Op den Camp, H.J.M. Microbial cycling of volatile organic sulfur compounds. Cell. Mol. Life Sci. 2002, 59, 575–588. [Google Scholar] [CrossRef]
- Benison, K.C.; Bowen, B.B. Extreme sulfur-cycling in acid brine lake environments of Western Australia. Chem. Geol. 2013, 351, 154–167. [Google Scholar] [CrossRef]
- Berben, T.; Overmars, L.; Sorokin, D.Y.; Muyzer, G. Diversity and Distribution of Sulfur Oxidation-Related Genes in Thioalkalivibrio, a Genus of Chemolithoautotrophic and Haloalkaliphilic Sulfur-Oxidizing Bacteria. Front. Microbiol. 2019, 10, 160. [Google Scholar] [CrossRef]
- Rana, K.; Rana, N.; Singh, B. Applications of sulfur oxidizing bacteria. In Physiological and Biotechnological Aspects of Extremophiles, 1st ed.; Salwan, R., Sharma, V., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 131–136. [Google Scholar] [CrossRef]
- Kamyshny, A.; Zerkle, A.L.; Mansaray, Z.F.; Ciglenečki, I.; Bura-Nakić, E.; Farquhar, J.; Ferdelman, T.G. Biogeochemical sulfur cycling in the water column of a shallow stratified sea-water lake: Speciation and quadruple sulfur isotope composition. Mar. Chem. 2011, 127, 144–154. [Google Scholar] [CrossRef]
- Dahl, C. Sulfur Metabolism in Phototrophic Bacteria. In Modern Topics in the Phototrophic Prokaryotes; Hallenbeck, P., Ed.; Springer: Cham, Switzerland; New York, NY, USA, 2017; pp. 27–66. [Google Scholar] [CrossRef]
- Ito, T.; Sugita, K.; Okabe, S. Isolation, Characterization, and In Situ Detection of a Novel Chemolithoautotrophic Sulfur-Oxidizing Bacterium in Wastewater Biofilms Growing under Microaerophilic Conditions. Appl. Environ. Microbiol. 2004, 7, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, B.B.; Findlay, A.J.; Pellerin, A. The Biogeochemical Sulfur Cycle of Marine Sediments. Front. Microbiol. 2019, 10, 849. [Google Scholar] [CrossRef]
- Kletzin, A.; Urich, T.; Müller, F.; Bandeiras, T.M.; Gomes, C.M. Dissimilatory Oxidation and Reduction of Elemental Sulfur in Thermophilic Archaea. J. Bioenerg. Biomembr. 2004, 36, 77–91. [Google Scholar] [CrossRef]
- Friedrich, C.G.; Bradischewsky, F.; Rother, D.; Quentmeier, A.; Fisher, J. Prokaryotic sulfur oxidation. Curr. Opin. Microbiol. 2005, 8, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.M.; Do, P.T.; Pham, Y.B.; Doan, T.O.; Nguyen, X.C.; Lee, W.K.; Nguyen, D.D.; Vadiveloo, A.; Um, M.J.; Ngo, H.H. Roles, mechanism of action, and potential applications of sulfur-oxidizing bacteria for environmental bioremediation. Sci. Total Environ. 2022, 852, 158203. [Google Scholar] [CrossRef] [PubMed]
- Bamford, V.A.; Bruno, S.; Rasmussen, T.; Appia-Ayme, C.; Cheesman, M.R.; Berks, B.C.; Hemmings, A.M. Structural basis for the oxidation of thiosulfate by a sulfur cycle enzyme. EMBO J. 2002, 21, 5599–5610. [Google Scholar] [CrossRef]
- Boden, R.; Kelly, D.P.; Murrell, C.; Schäfer, H. Oxidation of dimethylsulfide to tetrathionate by Methylophaga thiooxidans sp. nov.: A new link in the sulfur cycle. Environ. Microbiol. 2010, 12, 2688–2699. [Google Scholar] [CrossRef]
- Koch, T.; Dahl, C. A novel bacterial sulfur oxidation pathway provides a new link between the cycles of organic and inorganic sulfur compounds. ISME J. 2018, 12, 2479–2491. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Xi, S.; Cai, R.; Zhang, X.; Sun, C. A novel bacterial thiosulfate oxidation pathway provides a new clue about the formation of zero-valent sulfur in deep sea. ISME J. 2020, 14, 261–2274. [Google Scholar] [CrossRef]
- Liu, L.J.; Jiang, Z.; Wang, P.; Qin, Y.L.; Xu, W.; Wang, Y.; Liu, S.J.; Jiang, C.Y. Physiology, Taxonomy, and Sulfur Metabolism of the Sulfolobales, an Order of Thermoacidophilic Archaea. Front. Microbiol. 2021, 12, 768283. [Google Scholar] [CrossRef]
- Slobodkin, A.I.; Slobodkina, G.B. Diversity of Sulfur-Disproportionating Microorganisms. Microbiology 2019, 88, 509–522. [Google Scholar] [CrossRef]
- Muyzer, G.; Kuenen, J.G.; Robertson, L.A. Colorless sulfur bacteria. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 555–588. [Google Scholar] [CrossRef]
- Wasmund, K.; Mußmann, M.; Loy, A. The life sulfuric: Microbial ecology of sulfur cycling in marine sediments. Environ. Microbiol. Rep. 2017, 9, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Tada, C.; Fukuda, Y.; Nakai, Y. Diversity of Sulfur-oxidizing Bacteria at the Surface of Cattle Manure Composting Assessed by an Analysis of the Sulfur Oxidation Gene soxB. Microbes Environ. 2020, 35, ME18066. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Nakamura, S.; Inagaki, F.; Takai, K.; Shirai, N.; Sako, Y. Hydrogenivirga caldilitoris gen. nov., sp. nov., a novel extremely thermophilic, hydrogen- and sulfur-oxidizing bacterium from a coastal hydrothermal field. Int. J. Syst. Evol. Microbiol. 2004, 54, 2079–2084. [Google Scholar] [CrossRef]
- Karavaiko, G.I.; Bogdanova, T.I.; Tourova, T.P.; Kondrat’eva, T.F.; Tsaplina, I.A.; Egorova, M.A.; Krasil’nikova, E.N.; Zakharchuk, L.M. Reclassification of ‘Sulfobacillus thermosulfidooxidans subsp. thermotolerans’ strain K1 as Alicyclobacillus tolerans sp. nov. and Sulfobacillus disulfidooxidans Dufresne et al. 1996 as Alicyclobacillus disulfidooxidans comb. nov., and emended description of the genus Alicyclobacillus. Int. J. Syst. Evol. Microbiol. 2005, 55, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, S.L.; Liu, Y.; Ferrera, I.; Beveridge, T.; Reysenbach, A.L. Thermocrinis minervae sp. nov., a hydrogen- and sulfur-oxidizing, thermophilic member of the Aquificales from a Costa Rican terrestrial hot spring. Int. J. Syst. Evol. Microbiol. 2010, 60, 338–343. [Google Scholar] [CrossRef]
- Zhou, E.M.; Xian, W.D.; Mefferd, C.C.; Thomas, S.C.; Adegboruwa, A.L.; Williams, N.; Murugapiran, S.K.; Dodsworth, J.A.; Ganji, R.; Li, M.M.; et al. Thermus sediminis sp. nov., a thiosulfate-oxidizing and arsenate-reducing organism isolated from Little Hot Creek in the Long Valley Caldera, California. Extremophiles 2018, 22, 983–991. [Google Scholar] [CrossRef]
- Sun, X.; Kong, T.; Li, F.; Häggblom, M.M.; Kolton, M.; Lan, L.; Lau Vetter, M.C.Y.; Dong, Y.; Gao, P.; Kostka, J.E.; et al. Desulfurivibrio spp. mediate sulfur-oxidation coupled to Sb(V) reduction, a novel biogeochemical process. ISME J. 2022, 16, 1547–1556. [Google Scholar] [CrossRef]
- Sakurai, H.; Ogawa, T.; Shiga, M.; Inoue, K. Inorganic sulfur oxidizing system in green sulfur bacteria. Photosynth. Res. 2010, 104, 163–176. [Google Scholar] [CrossRef]
- Offre, P.; Spang, A.; Schleper, C. Archaea in biogeochemical cycles. Annu. Rev. Microbiol. 2013, 67, 437–457. [Google Scholar] [CrossRef]
- Liu, Y.; Beer, L.L.; Whitman, W.B. Sulfur metabolism in archaea reveals novel processes. Environ. Microbiol. 2012, 14, 2632–2644. [Google Scholar] [CrossRef] [PubMed]
- Kletzin, A. Oxidation of Sulfur and Inorganic Sulfur Compounds in Acidianus ambivalens. In Microbial Sulfur Metabolism; Dahl, C., Friedrich, C.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Müller, F.H.; Bandeiras, T.M.; Urich, T.; Teixeira, M.; Gomes, C.M.; Kletzin, A. Coupling of the pathway of sulphur oxidation to dioxygen reduction: Characterization of a novel membrane-bound thiosulphate:quinone oxidoreductase. Mol. Microbiol. 2004, 53, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Protze, J.; Müller, F.; Lauber, K.; Naß, B.; Mentele, R.; Lottspeich, F.; Kletzin, A. An Extracellular Tetrathionate Hydrolase from the Thermoacidophilic Archaeon Acidianus ambivalens with an Activity Optimum at pH 1. Front. Microbiol. 2011, 2, 68. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.P.; Shergill, J.K.; Lu, W.P.; Wood, A.P. Oxidative metabolism of inorganic sulfur compounds by bacteria. Antonie Van Leeuwenhoek 1997, 71, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.G.; Rother, D.; Bardischewsky, F.; Quentmeier, A.; Fischer, J. Oxidation of Reduced Inorganic Sulfur Compounds by Bacteria: Emergence of a Common Mechanism? Appl. Environ. Microbiol. 2001, 67, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Dam, B.; Mandal, S.; Gosh, W.; Das Gupta, S.K.; Roy, P. The S4-intermediate pathway for the oxidation of thiosulfate by the chemolithoautotroph Tetrathiobacter kashmirensis and inhibition of tetrathionate oxidation by sulfite. Res. Microbiol. 2007, 158, 330–338. [Google Scholar] [CrossRef]
- Gregersen, L.H.; Bryant, D.A.; Frigaard, N.U. Mechanisms and evolution of oxidative sulfur metabolism in green sulfur bacteria. Front. Microbiol. 2011, 2, 116. [Google Scholar] [CrossRef]
- Friedrich, C.G.; Quentmeier, A.; Bardischewsky, F.; Rother, D.; Kraft, R.; Kostka, S.; Prinz, H. Novel Genes Coding for Lithotrophic Sulfur Oxidation of Paracoccus pantotrophus GB17. J. Bacteriol. 2000, 182, 4677–4687. [Google Scholar] [CrossRef]
- Quentmeier, A.; Friedrich, C.G. The cysteine residue of the SoxY protein as the active site of protein-bound sulfur oxidation of Paracoccus pantotrophus GB17. FEBS Lett. 2001, 503, 168–172. [Google Scholar] [CrossRef]
- Rother, D.; Henrich, H.J.; Quentmeier, A.; Bardischewsky, F.; Friedrich, C.G. Novel Genes of the sox Gene Cluster, Mutagenesis of the Flavoprotein SoxF, and Evidence for a General Sulfur-Oxidizing System in Paracoccus pantotrophus GB17. J. Bacteriol. 2001, 183, 4499–4508. [Google Scholar] [CrossRef]
- Sauvé, V.; Bruno, S.; Berks, B.C.; Hemmings, A.M. The SoxYZ Complex Carries Sulfur Cycle Intermediates on a Peptide Swinging Arm. J. Biol. Chem. 2007, 282, 23194–23204. [Google Scholar] [CrossRef] [PubMed]
- Dahl, C. Inorganic Sulfur Compounds as Electron Donors in Purple Sulfur Bacteria. In Sulfur Metabolism in Phototrophic Organisms; Hell, R., Dahl, C., Knaff, D., Leustek, T., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 289–317. [Google Scholar] [CrossRef]
- Mohaparta, B.R.; Gould, W.D.; Dinardo, O.; Koren, D.W. An Overview of the Biochemical and Molecular Aspects of Microbial Oxidation of Inorganic Sulfur Compounds. Clean Soil Air Water 2008, 36, 823–829. [Google Scholar] [CrossRef]
- Mukhopadhyaya, P.N.; Deb, C.; Lahiri, C.; Roy, P. A soxA Gene, Encoding a Diheme Cytochrome c, and a sox Locus, Essential for Sulfur Oxidation in a New Sulfur Lithotrophic Bacterium. J. Bacteriol. 2000, 182, 4278–4287. [Google Scholar] [CrossRef]
- Appia-Ayme, C.; Little, P.J.; Matsumoto, Y.; Leech, A.P.; Berks, B.C. Cytochrome complex essential for photosynthetic oxidation of both thiosulfate and sulfide in Rhodovulum sulfidophilum. J. Bacteriol. 2001, 183, 6107–6118. [Google Scholar] [CrossRef]
- Anandham, R.; Indiragandhi, P.; Madhaiyan, M.; Ryu, K.Y.; Jee, H.J.; Sa, T.M. Chemolithoautotrophic oxidation of thiosulfate and phylogenetic distribution of sulfur oxidation gene (soxB) in rhizobacteria isolated from crop plants. Res. Microbiol. 2008, 159, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Zander, U.; Faust, A.; Klink, B.U.; de Sanctis, D.; Panjikar, S.; Quentmeier, A.; Bardischewsky, F.; Friedrich, C.G.; Scheidig, A.J. Structural basis for the oxidation of protein-bound sulfur by the sulfur cycle molybdohemo-enzyme sulfane dehydrogenase SoxCD. J. Biol. Chem. 2011, 286, 8349–8360. [Google Scholar] [CrossRef]
- Grabarczyk, D.B.; Berks, B.C. Intermediates in the Sox sulfur oxidation pathway are bound to a sulfane conjugate of the carrier protein SoxYZ. PLoS ONE 2017, 12, e0173395. [Google Scholar] [CrossRef]
- Grimm, F.; Franz, B.; Dahl, C. Thiosulfate and sulfur oxidation in purple sulfur bacteria. In Microbial Sulfur Metabolism; Dahl, C., Friedrich, C.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Dahl, C. Sulfite oxidation in the purple sulfur bacterium Allochromatium vinosum: Identification of SoeABC as a major player and relevance of SoxYZ in the process. Microbiology 2013, 159, 2626–2638. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.; Sylverster, M.; Kröninger, L.; Dahl, C. A Comparative Quantitative Proteomic Study Identifies New Proteins Relevant for Sulfur Oxidation in the Purple Sulfur Bacterium Allochromatium vinosum. Appl. Environ. Microbiol. 2014, 80, 2279–2292. [Google Scholar] [CrossRef]
- Kanao, T.; Kamimura, K.; Sugio, T. Identification of a gene encoding a tetrathionate hydrolase in Acidithiobacillus ferrooxidans. J. Biotechnol. 2007, 132, 16–22. [Google Scholar] [CrossRef]
- Rzhepishevska, O.I.; Valdés, J.; Marcinkeviciene, L.; Gallardo, C.A.; Meskys, R.; Bonnefoy, V.; Holmes, D.S.; Dopson, M. Regulation of a Novel Acidithiobacillus caldus Gene Cluster Involved in Metabolism of Reduced Inorganic Sulfur Compounds. Appl. Environ. Microbiol. 2007, 73, 7367–7372. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, L.J.; van Munster, J.M.; Rawlings, D.E. Construction of arsB and tetH Mutants of the Sulfur-Oxidizing Bacterium Acidithiobacillus caldus by Marker Exchange. Appl. Environ. Microbiol. 2008, 74, 5686–5694. [Google Scholar] [CrossRef]
- Denkmann, K.; Grein, F.; Zigann, R.; Siemen, A.; Bergmann, J.; van Helmont, S.; Nicolai, A.; Pereira, I.A.C.; Dahl, C. Thiosulfate dehydrogenase: A widespread unusual acidophilic c-type cytochrome. Environ. Microbiol. 2012, 14, 2673–2688. [Google Scholar] [CrossRef]
- Brito, J.A.; Denkmann, K.; Pereira, I.A.C.; Archer, M.; Dahl, C. Thiosulfate dehydrogenase (TsdA) from Allochromatium vinosum: Structural and functional insights into thiosulfate oxidation. J. Biol. Chem. 2015, 290, 9222–9238. [Google Scholar] [CrossRef] [PubMed]
- Orlova, M.V.; Tarlachkov, S.V.; Dubinina, G.A.; Belousova, E.V.; Tutukina, M.N.; Grabovich, M.Y. Genomic insights into metabolic versatility of a lithotrophic sulfur-oxidizing diazotrophic Alphaproteobacterium Azospirillum thiophilum. FEMS Microbiol. Ecol. 2016, 92, fiw199. [Google Scholar] [CrossRef]
- Kikumoto, M.; Nogami, S.; Kanao, T.; Takada, J.; Kamimura, K. Tetrathionate-Forming Thiosulfate Dehydrogenase from the Acidophilic, Chemolithoautotrophic Bacterium Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2013, 79, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Pyne, P.; Alam, M.; Rameez, M.J.; Mandal, S.; Sar, A.; Mondal, N.; Debnath, U.; Mathew, B.; Misra, A.K.; Mandal, A.K.; et al. Homologs from sulfur oxidation (Sox) and methanol dehydrogenation (Xox) enzyme systems collaborate to give rise to a novel pathway of chemolithotrophic tetrathionate oxidation. Mol. Microbiol. 2018, 109, 169–191. [Google Scholar] [CrossRef]
- Rameez, M.J.; Pyne, P.; Mandal, S.; Chatterjee, S.; Alam, M.; Bhattacharya, S.; Mondal, N.; Sarkar, J.; Gosh, W. Two pathways for thiosulfate oxidation in the alphaproteobacterial chemolithotroph Paracoccus thiocyanatus SST. Microbiol. Res. 2020, 230, 126345. [Google Scholar] [CrossRef]
- Sorokin, D.Y. Oxidation of Inorganic Sulfur Compounds by Obligately Organotrophic Bacteria. Microbiology 2003, 72, 641–653. [Google Scholar] [CrossRef]
- Van Vliet, D.M.; von Meijenfeldt, B.; Dutilh, B.E.; Vilanueva, L.; Damsté, J.S.S.; Stams, A.J.M.; Sánchez-Andrea, I. The bacterial sulfur cycle in expanding dysoxic and euxinic marine waters. Environ. Microbiol. 2020, 23, 2834–2857. [Google Scholar] [CrossRef]
- Kappler, U.; Dahl, C. Enzymology and molecular biology of prokaryotic sulfite oxidation. FEMS Microb. Lett. 2001, 203, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kappler, U. Bacterial sulfite-oxidizing enzymes. Biochim. Biophys. Acta 2011, 1807, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lin, J.Q.; Liu, X.M.; Pang, X.; Zhang, C.J.; Yang, C.L.; Gao, X.Y.; Lin, C.M.; Li, Y.Q.; Li, Y.; et al. Sulfur Oxidation in the Acidophilic Autotrophic Acidithiobacillus spp. Front. Microbiol. 2019, 10, 3290. [Google Scholar] [CrossRef]
- Boughanemi, S.; Infossi, P.; Giudici-Orticoni, M.T.; Schoepp-Cothenet, B.; Guiral, M. Sulfite oxidation by the quinone-reducing molybdenum sulfite dehydrogenase SoeABC from the bacterium Aquifex aeolicus. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148279. [Google Scholar] [CrossRef]
- Hou, N.; Xia, Y.; Wang, X.; Liu, H.; Liu, H.; Xun, L. H2S biotreatment with sulfide-oxidizing heterotrophic bacteria. Biodegradation 2018, 29, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Rühl, P.; Haas, P.; Seipel, D.; Becker, J.; Kletzin, A. Persulfide Dioxygenase from Acidithiobacillus caldus: Variable Roles of Cysteine Residues and Hydrogen Bond Networks of the Active Site. Front. Microbiol. 2018, 9, 1610. [Google Scholar] [CrossRef]
- Xin, Y.; Gao, R.; Cui, F.; Lü, C.; Liu, H.; Liu, H.; Xia, Y.; Xun, L. The Heterotrophic Bacterium Cupriavidus pinatubonensis JMP134 Oxidizes Sulfide to Sulfate with Thiosulfate as a Key Intermediate. Appl. Environ. Microbiol. 2020, 86, e01835-20. [Google Scholar] [CrossRef]
- Pfeffer, C.; Larsen, S.; Song, J.; Dong, M.; Besenbacher, F.; Meyer, R.L.; Kjeldsen, K.U.; Schreiber, L.; Gorby, Y.A.; El-Naggar, M.Y.; et al. Filamentous bacteria transport electrons over centimeter distances. Nature 2012, 491, 218–221. [Google Scholar] [CrossRef]
- Dopson, M.; Johnson, B. Biodiversity, metabolism and applications of acidophilic sulfur-metabolizing microorganisms. Environ. Microbiol. 2012, 14, 2620–2631. [Google Scholar] [CrossRef]
- Camacho, A. Sulfur bacteria. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 261–278. [Google Scholar] [CrossRef]
- Lindström, E.S.; Kamst-Van Agterveld, M.P.; Zwart, G. Distribution of Typical Freshwater Bacterial Groups Is Associated with pH, Temperature, and Lake Water Retention Time. Appl. Environ. Microbiol. 2005, 71, 8201–8206. [Google Scholar] [CrossRef]
- Watanabe, T.; Kojima, H.; Takano, Y.; Fukui, M. Diversity of sulfur-cycle prokaryotes in freshwater lake sediments investigated using aprA as the functional marker gene. Syst. Appl. Microbiol. 2013, 36, 436–443. [Google Scholar] [CrossRef]
- Satoh, S.; Tanaka, R.; Yokono, M.; Endoh, D.; Yabuki, T.; Tanaka, A. Phylogeny analysis of whole protein-coding genes in metagenomic data detected an environmental gradient for the microbiota. PLoS ONE 2023, 18, e0281288. [Google Scholar] [CrossRef]
- Minckley, W.; Unmack, P. Western springs: Their faunas, and threats to their existence. In Freshwater Ecoregions of North America; Abell, R.A., Olson, D.M., Dinerstein, E., Hurley, P.T., Eds.; Island Press: Washington, DC, USA, 2000; pp. 52–53. [Google Scholar]
- Headd, B.; Engel, A.S. Biogeographic congruency among bacterial communities from terrestrial sulfidic springs. Front. Microbiol. 2014, 5, 473. [Google Scholar] [CrossRef]
- Camacho, A.; Rochera, C.; Silvestre, J.J.; Vicente, E.; Hahn, M.W. Spatial Dominance and Inorganic Carbon Assimilation by Conspicuous Autotrophic Biofilms in a Physical and Chemical Gradient of a Cold Sulfurous Spring: The Role of Differential Ecological Strategies. Microb. Ecol. 2005, 50, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, C.; Moissl, C.; Henneberger, R.; Huber, R. Ecology and microbial structures of archaeal/bacterial strings-of-pearls communities and archaeal relatives thriving in cold sulfidic springs. FEMS Microbiol. Ecol. 2004, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sahm, K.; John, P.; Nacke, H.; Wemheuer, B.; Grote, R.; Daniel, R.; Antranikian, G. High abundance of heterotrophic prokaryotes in hydrothermal springs of the Azores as revealed by a network of 16S rRNA gene-based methods. Extremophiles 2013, 17, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.V.; Pjevac, P.; Bach, W.; Hourdez, S.; Girguis, P.R.; Vidoudez, C.; Amann, R.; Meyerdierks, A. Niche partitioning of diverse sulfur-oxidizing bacteria at hydrothermal vents. ISME J. 2017, 11, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.; Douglas, D.D. Structural and Geomicrobiological Characteristics of a Microbial Community from a Cold Sulfide Spring. Geomicrobiol. J. 2001, 18, 401–422. [Google Scholar] [CrossRef]
- Chaudhary, A.; Haack, S.K.; Duris, J.W.; Marsh, T.L. Bacterial and Archaeal Phylogenetic Diversity of a Cold Sulfur-RichSpring on the Shoreline of Lake Erie, Michigan. Appl. Environ. Microbiol. 2009, 75, 5025–5036. [Google Scholar] [CrossRef]
- Cantonati, M.; Komáre, J.; Montejano, G. Cyanobacteria in ambient springs. Biodivers. Conserv. 2015, 24, 865–888. [Google Scholar] [CrossRef]
- Engel, A.S.; Lee, N.; Porter, M.L.; Stern, L.A.; Bennett, P.C.; Wagner, M. Filamentous “Epsilonproteobacteria” Dominate Microbial Mats from Sulfidic Cave Springs. Appl. Environ. Microbiol. 2003, 69, 5503–5511. [Google Scholar] [CrossRef] [PubMed]
- Fenchel, T.; King, G.; Blackburn, T.H. Aquatic Sediments. In Bacterial Biogeochemistry, 3rd ed.; Academic Press: London, UK, 2012; pp. 121–142. [Google Scholar] [CrossRef]
- Rudolph, C.; Wanner, G.; Huber, R. Natural communities of novel archaea and bacteria growing in cold sulfurous springs with a string-of-pearls-like morphology. Appl. Environ. Microbiol. 2001, 67, 2336–2344. [Google Scholar] [CrossRef]
- Moissl, C.; Rudolph, C.; Huber, R. Natural Communities of Novel Archaea and Bacteria with a String-of-Pearls-Like Morphology: Molecular Analysis of the Bacterial Partners. Appl. Environ. Microbiol. 2002, 68, 933–937. [Google Scholar] [CrossRef]
- Grasby, S.E.; Allen, C.C.; Longazo, T.G.; Lisle, J.T.; Griffin, D.W.; Beauchamp, B. Supraglacial Sulfur Springs and Associated Biological Activity in the Canadian High Arctic—Signs of Life Beneath the Ice. Astrobiology 2003, 3, 583–596. [Google Scholar] [CrossRef]
- Perreault, N.N.; Andersen, D.T.; Pollard, W.H.; Greer, C.W.; Whyte, L.G. Characterization of the Prokaryotic Diversity in Cold Saline Perennial Springs of the Canadian High Arctic. Appl. Environ. Microbiol. 2008, 73, 1532–1543. [Google Scholar] [CrossRef]
- Wright, K.E.; Williamson, C.; Grasby, S.E.; Spear, J.R.; Templeton, A.S. Metagenomic evidence for sulfur lithotrophy by Epsilonproteobacteria as the major energy source for primary productivity in a sub-aerial arctic glacial deposit, Borup Fiord Pass. Front. Microbiol. 2013, 4, 63. [Google Scholar] [CrossRef]
- Boyd, E.S.; Hamilton, T.L.; Havig, J.R.; Skidmore, M.L.; Shock, E.L. Chemolithotrophic Primary Production in a Subglacial Ecosystem. Appl. Environ. Microbiol. 2014, 80, 6146–6453. [Google Scholar] [CrossRef] [PubMed]
- Harrold, Z.R.; Skidmore, M.L.; Hamilton, T.L.; Desch, L.; Amada, K.; van Gelder, W.; Glover, K.; Roden, E.E.; Boyd, E.S. Aerobic and Anaerobic Thiosulfate Oxidation by a Cold-Adapted, Subglacial Chemoautotroph. Appl. Environ. Microbiol. 2016, 82, 1486–1495. [Google Scholar] [CrossRef]
- Trivedi, C.B.; Lau, G.E.; Grasby, S.E.; Templeton, A.S.; Spear, J.R. Low-Temperature Sulfidic-Ice Microbial Communities, Borup Fiord Pass, Canadian High Arctic. Front. Microbiol. 2018, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, C.B.; Stamps, B.W.; Lau, G.E.; Grasby, S.E.; Templeton, A.S.; Spear, J.R. Microbial Metabolic Redundancy Is a Key Mechanism in a Sulfur-Rich Glacial Ecosystem. mSystems 2020, 5, e00504-20. [Google Scholar] [CrossRef]
- Engel, A.S.; Porter, M.L.; Stern, L.A.; Quinlan, S.; Bennett, P.C. Bacterial diversity and ecosystem function of filamentous microbial mats from aphotic (cave) sulfidic springs dominated by chemolithoautotrophic “Epsilonproteobacteria”. FEMS Microbiol. Ecol. 2004, 51, 31–53. [Google Scholar] [CrossRef]
- Macalady, J.L.; Lyon, E.H.; Koffman, B.; Albertson, L.K.; Meyer, K.; Galdenzi, S.; Mariani, S. Dominant microbial populations in limestone-corroding stream biofilms, Frasassi cave system, Italy. Appl. Environ. Microbiol. 2006, 72, 5596–5609. [Google Scholar] [CrossRef]
- Hamilton, T.L.; Jones, D.S.; Schaperdoth, I.; Macalady, J.L. Metagenomic insights into S(0) precipitation in a terrestrial subsurface lithoautotrophic ecosystem. Front. Microbiol. 2015, 5, 756. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.J.; Engel, A.S.; Porter, M.L.; Takai, K. The versatile ε-proteobacteria: Key players in sulphidic habitats. Nat. Rev. Microbiol. 2006, 4, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Nosalova, L.; Piknova, M.; Bonova, K.; Pristas, P. Deep Subsurface Hypersaline Environment as a Source of Novel Species of Halophilic Sulfur-Oxidizing Bacteria. Microorganisms 2022, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Nosalova, L.; Kiskova, J.; Fecskeova, L.K.; Piknova, M.; Pristas, P. Bacterial Community Structure of Two Cold Sulfur Springs in Slovakia (Central Europe). Curr. Microbiol. 2023, 80, 145. [Google Scholar] [CrossRef]
- Nosalova, L.; Fecskeova, L.K.; Piknova, M.; Bonova, K.; Pristas, P. Unique Populations of Sulfur-Oxidizing Bacteria in Natural Cold Sulfur Springs in Slovakia. Geomicrobiol. J. 2023, 40, 315–324. [Google Scholar] [CrossRef]
- Dubinina, G.; Savvichev, A.; Orlova, M.; Gavrish, E.; Verbarg, S.; Grabovich, M. Beggiatoa leptomitoformis sp. nov., the first freshwater member of the genus capable of chemolithoautotrophic growth. Int. J. Syst. Evol. Microbiol. 2017, 67, 197–204. [Google Scholar] [CrossRef]
- Hinck, S.; Neu, T.R.; Lavik, G.; Mussmann, M.; de Beer, D.; Jonkers, H.M. Physiological Adaptation of a Nitrate-Storing Beggiatoa sp. to Diel Cycling in a Phototrophic Hypersaline Mat. Appl. Environ. Microbiol. 2007, 73, 7013–7022. [Google Scholar] [CrossRef]
- Kumar, U.; Panneerselvam, P.; Gupta, V.V.S.R.; Manjunath, M.; Priyadarshinee, P.; Sahoo, A.; Dash, S.R.; Kaviraj, M.; Annapurna, K. Diversity of sulfur-oxidizing and sulfur-reducing microbes in diverse ecosystems. In Advances in Soil Microbiology: Recent Trends and Future Prospects; Microorganisms for Sustainability; Adhya, T., Lal, B., Mohapatra, B., Paul, D., Das, S., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Niederberger, T.D.; Perreault, N.N.; Lawrence, J.R.; Nadeau, J.L.; Mielke, R.E.; Greer, C.W.; Andersen, D.T.; Whyte, L.G. Novel sulfur-oxidizing streamers thriving in perennial cold saline springs of the Canadian high Arctic. Environ. Microbiol. 2009, 11, 616–629. [Google Scholar] [CrossRef]
- Magnuson, E.; Mykytczuk, M.C.S.; Pellerin, A.; Goordial, J.; Twine, S.M.; Wing, B.; Foote, S.J.; Fulton, K.; Whyte, L.G. Thiomicrorhabdus streamers and sulfur cycling in perennial hypersaline cold springs in the Canadian high Arctic. Environ. Microbiol. 2020, 23, 3384–3400. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosalova, L.; Piknova, M.; Kolesarova, M.; Pristas, P. Cold Sulfur Springs—Neglected Niche for Autotrophic Sulfur-Oxidizing Bacteria. Microorganisms 2023, 11, 1436. https://doi.org/10.3390/microorganisms11061436
Nosalova L, Piknova M, Kolesarova M, Pristas P. Cold Sulfur Springs—Neglected Niche for Autotrophic Sulfur-Oxidizing Bacteria. Microorganisms. 2023; 11(6):1436. https://doi.org/10.3390/microorganisms11061436
Chicago/Turabian StyleNosalova, Lea, Maria Piknova, Mariana Kolesarova, and Peter Pristas. 2023. "Cold Sulfur Springs—Neglected Niche for Autotrophic Sulfur-Oxidizing Bacteria" Microorganisms 11, no. 6: 1436. https://doi.org/10.3390/microorganisms11061436
APA StyleNosalova, L., Piknova, M., Kolesarova, M., & Pristas, P. (2023). Cold Sulfur Springs—Neglected Niche for Autotrophic Sulfur-Oxidizing Bacteria. Microorganisms, 11(6), 1436. https://doi.org/10.3390/microorganisms11061436





