Biocontrol and Probiotic Function of Non-Saccharomyces Yeasts: New Insights in Agri-Food Industry
Abstract
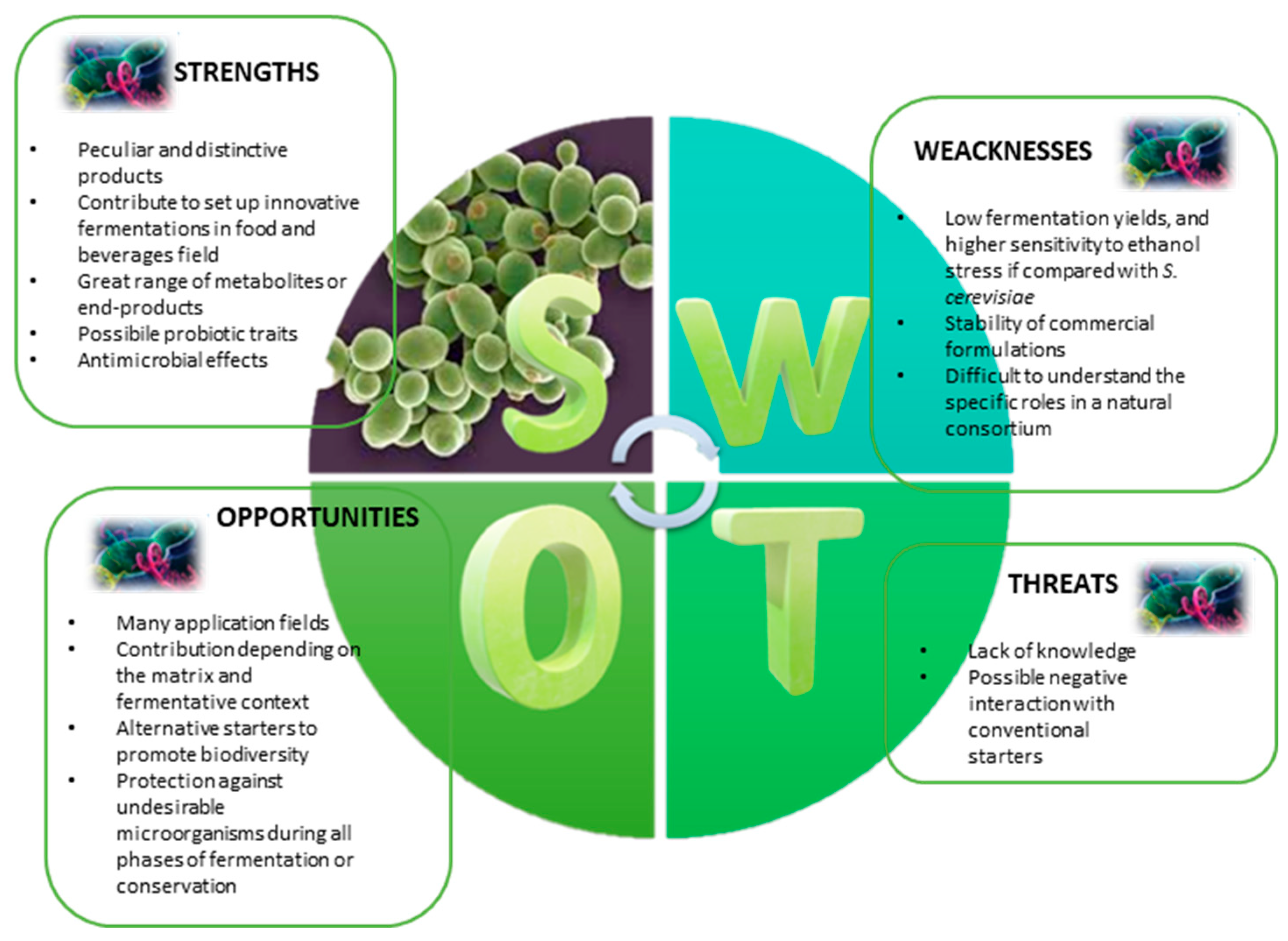
:1. Introduction
2. Antimicrobial Activity of Non-Saccharomyces Yeasts (NSYs)
2.1. Biological Control
2.2. Modalities of Antimicrobial Action of NSYs
2.2.1. Antagonistic Action: Competition of Nutrients and Space
2.2.2. Mycocins
2.2.3. Antimicrobial Peptides (AMPs)
2.2.4. Secreted Enzymes
2.2.5. Mycoparasitism
2.2.6. Volatile Organic Compounds (VOCs)
2.3. Registered Commercial Products of Biocontrol NSYs Species
2.3.1. Candida oleophila
2.3.2. Aureobasidium pullulans
2.3.3. Metschnikowia spp.
2.3.4. Cryptococcus spp.
2.3.5. Torulaspora delbrueckii and Metschnikowia pulcherrima
2.4. Application of NSYs in Biopackaging
3. NSYs as Probiotic Yeasts
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, L.; Horsman, S.R.; Charron-Mazenod, L.; Turnbull, A.L.; Mulcahy, H.; Surette, M.G.; Lewenza, S. Extracellular DNA-induced antimicrobial peptide resistance in Salmonella enterica serovar Typhimurium. BMC Microbiol. 2013, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef] [PubMed]
- Zotta, T.; Di Renzo, T.; Sorrentino, A.; Reale, A.; Boscaino, F. Selection of Non-Saccharomyces Wine Yeasts for the Production of Leavened Doughs. Microorganisms 2022, 10, 1849. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ma, X.; Deng, L.; Zhao, X.; Wei, Y.; Gao, Z.; Sun, C. Fresh garlic extract enhances the antimicrobial activities of antibiotics on resistant strains in vitro. Jundishapur J. Microbiol. 2015, 8, e14814. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with winemaking. World J. Microbiol. Biotechnol. 1997, 14, 199–203. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Manzanares, P.; Ramón, D.; Querol, A. The role of non-Saccharomyces yeasts in industrial winemaking. Int. Microbiol. 1998, 1, 143–148. [Google Scholar] [PubMed]
- de Ullivarri, M.F.; Mendoza, L.M.; Raya, R.R. Characterization of the killer toxin KTCf20 from Wickerhamomyces anomalus, a potential biocontrol agent against wine spoilage yeasts. Biol. Control 2018, 121, 223–228. [Google Scholar] [CrossRef]
- Kuchen, B.; Maturano, Y.P.; Mestre, M.V.; Combina, M.; Toro, M.E.; Vazquez, F. Selection of native non-Saccharomyces yeasts with biocontrol activity against spoilage yeasts in order to produce healthy regional wines. Fermentation 2019, 5, 60. [Google Scholar] [CrossRef]
- Velázquez, A.E.; Elghandour, M.M.; Adegbeye, M.J.; Pilego, A.B.; Vallejo, L.H.; Salem, A.Z.; Salazar, M.C. Influence of dietary inclusion with corn and soybean oils, in combination with live yeast culture, on horse fecal methane, carbon dioxide and hydrogen production. JEVS 2019, 74, 42–50. [Google Scholar] [CrossRef]
- Escott, C.; Del Fresno, J.M.; Loira, I.; Morata, A.; Suárez-Lepe, J.A. Zygosaccharomyces rouxii: Control strategies and applications in food and winemaking. Fermentation 2018, 4, 69. [Google Scholar] [CrossRef]
- Comitini, F.; Ciani, M. Kluyveromyces wickerhamii killer toxin: Purification and activity towards Brettanomyces/Dekkera yeasts in grape must. FEMS Microbiol. Lett. 2011, 316, 77–82. [Google Scholar] [CrossRef]
- Oro, L.; Ciani, M.; Bizzaro, D.; Comitini, F. Evaluation of damage induced by Kwkt and Pikt zymocins against Brettanomyces/Dekkera spoilage yeast, as compared to sulphur dioxide. J. Appl. Microbiol. 2016, 121, 207–214. [Google Scholar] [CrossRef]
- Escott, C.; Loira, I.; Morata, A.; Bañuelos, M.A.; Suárez-Lepe, J.A. Wine spoilage yeasts: Control strategy. Yeast-Ind. Appl. 2017, 89–116. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; González-Muñoz, M.T.; Jimenez-Lopez, C.; Rodriguez-Gallego, M. Bioprotection. In Encyclopedia of Earth Sciences Series; Finkl, C.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 185–189. [Google Scholar]
- Rahman, M.A.; Mostofa, M.G.; Ushimaru, T. The Nem1/Spo7–Pah1/lipin axis is required for autophagy induction after TORC 1 inactivation. FEBS J. 2018, 285, 1840–1860. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Zhang, Z.; Chen, Y.; Tian, S. Antagonistic yeasts: A promising alternative to chemical fungicides for controlling postharvest decay of fruit. J. Fungi 2020, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Oro, L.; Zara, S.; Fancellu, F.; Mannazzu, I.; Budroni, M.; Ciani, M.; Comitini, F. Tp BGL2 codes for a Tetrapisispora phaffii killer toxin active against wine spoilage yeasts. FEMS Yeast Res. 2014, 14, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Oro, L.; Feliziani, E.; Ciani, M.; Romanazzi, G.; Comitini, F. Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. Int. J. Food Microbiol. 2018, 265, 18–22. [Google Scholar] [CrossRef]
- Comitini, F.; Agarbati, A.; Canonico, L.; Galli, E.; Ciani, M. Purification and characterization of WA18, a new mycocin produced by Wickerhamomyces anomalus active in wine against Brettanomyces bruxellensis spoilage yeasts. Microorganisms 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, C.; Nowak, A.I.; Błasiak, P.; Ochman, A.; Pietrowicz, S. Experimental study on a large scale pulsating heat pipe operating at high heat loads, different adiabatic lengths and various filling ratios of acetone, ethanol, and water. Appl. Eng. 2020, 165, 114534. [Google Scholar] [CrossRef]
- Agarbati, A.; Canonico, L.; Pecci, T.; Romanazzi, G.; Ciani, M.; Comitini, F. Biocontrol of non-Saccharomyces yeasts in vineyard against the gray mold disease agent Botrytis cinerea. Microorganisms 2022, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Di Gianvito, P.; Englezos, V.; Rantsiou, K.; Cocolin, L. Bioprotection strategies in winemaking. Int. J. Food Microbiol. 2022, 364, 109532. [Google Scholar] [CrossRef]
- Simonin, S.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Quintanilla-Casas, B.; Vichi, S.; Tourdot-Maréchal, R. Bio-protection as an alternative to sulphites: Impact on chemical and microbial characteristics of red wines. Front. Microbiol. 2020, 11, 1308. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Viana, R.; González-Arenzana, L.; Garijo, P.; Fernández, L.; López, R.; Santamaría, P.; Gutiérrez, A.R. Bioprotective effect of a Torulaspora delbrueckii/Lachancea thermotolerans-mixed inoculum in red winemaking. Fermentation 2022, 8, 337. [Google Scholar] [CrossRef]
- Chacon-Rodriguez, L.; Joseph, C.L.; Nazaris, B.; Coulon, J.; Richardson, S.; Dycus, D.A. Innovative use of non-Saccharomyces in bio-protection: T. delbrueckii and M. pulcherrima applied to a machine harvester. Catal. Discov. Pract. 2020, 4, 82–90. [Google Scholar] [CrossRef]
- Windholtz, S.; Dutilh, L.; Lucas, M.; Maupeu, J.; Vallet-Courbin, A.; Farris, L.; Masneuf-Pomarède, I. Population dynamics and yeast diversity in early winemaking stages without sulfites revealed by three complementary approaches. Appl. Sci. 2021, 11, 2494. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Galli, E.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima as biocontrol agent and wine aroma enhancer in combination with a native Saccharomyces cerevisiae. LWT 2023, 181, 114758. [Google Scholar] [CrossRef]
- Valsaraj, P.; Dubash, T.; Prakash, P.Y. Biocontrol of yeast spoilage in selected food and beverages by yeast mycocin. Acta Biol. Indica 2012, 1, 109–112. [Google Scholar]
- Polonelli, L.; Morace, G. Reevaluation of the yeast killer phenomenon. J. Clin. Microbiol. 1986, 24, 866–869. [Google Scholar] [CrossRef]
- Hatoum, R.M.; Labrie, S.; Fliss, I. Identification and partial characterization of antilisterial compounds produced by dairy yeasts. Probiotics Antimicrob. Proteins 2013, 5, 8–17. [Google Scholar] [CrossRef]
- Wu, W.H.; Hung, W.C.; Lo, K.Y.; Chen, Y.H.; Wan, H.P.; Cheng, K.C. Bioethanol production from taro waste using thermo-tolerant yeast Kluyveromyces marxianus K21. Biores Technol. 2016, 201, 27–32. [Google Scholar] [CrossRef]
- Ceugniez, A.; Drider, D.; Jacques, P.; Coucheney, F. Yeast diversity in a traditional French cheese “Tomme d'orchies” reveals infrequent and frequent species with associated benefits. Food Microbiol. 2015, 52, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Alzaeem, I.; Salama, A.R.; Sedik, M.Z. Incidence of Listeria monocytogenes, Staphylococcus aureus and Escherichia coli in fresh white cheese in Gaza city markets. Asian J. Agric. Food Sci. 2016, 4. [Google Scholar]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Barber, M.F.; Elde, N.C. Buried treasure: Evolutionary perspectives on microbial iron piracy. Trends Genet. 2015, 31, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chi, Z.; Liu, G.; Buzdar, M.A.; Chi, Z.; Gu, Q. Chemical and biological characterization of siderophore produced by themarine-derived Aureobasidium pullulans HN6.2 and its antibacterial activity. Biometals 2009, 22, 965–972. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, A.; Li, H. Using Candida oleophila as a biocontrol agent to prevent foodborne Escherichia coli O157 EHEC infections. Springerplus 2012, 1, 82. [Google Scholar] [CrossRef]
- Gore-Lloyd, D.; Sumann, I.; Brachmann, A.O.; Schneeberger, K.; Ortiz-Merino, R.A.; Moreno-Beltrán, M.; Freimoser, F.M. Snf2 controls pulcherriminic acid biosynthesis and antifungal activity of the biocontrol yeast Metschnikowia pulcherrima. Mol. Microbiol. 2019, 112, 317–332. [Google Scholar] [CrossRef]
- Junker, K.; Chailyan, A.; Hesselbart, A.; Forster, J.; Wendland, J. Multi-omics characterization of the necrotrophic mycoparasite Saccharomycopsis schoenii. PLoS Pathog. 2019, 15, e1007692. [Google Scholar] [CrossRef]
- Fiori, S.; Fadda, A.; Giobbe, S.; Berardi, E.; Migheli, Q. Pichia angusta is an effective biocontrol yeast against postharvest decay of apple fruit caused by Botrytis cinerea and Monilia fructicola. FEMS Yeast Res. 2008, 8, 961–963. [Google Scholar] [CrossRef]
- Desai, J.V.; Mitchell, A.P.; Andes, D.R. Fungal biofilms, drug resistance, and recurrent infection. Cold Spring Harb. Perspect. Med. 2014, 4, a019729. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B. Fungal biofilms and polymicrobial diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, M.; Teixeira, M.C. Candida biofilms: Threats, challenges, and promising strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Jingfan, F.; Kai, C.; Chao-an, L.; Yunjiang, C. Phenylethanol promotes adhesion and biofilm formation of the antagonistic yeast Kloeckera apiculata for the control of blue mold on citrus. FEMS Yeast Res. 2014, 14, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Li, G.; Liu, Y.; Liu, G.; Li, M.; Zhang, X.; Liu, J. Increase in antioxidant enzyme activity, stress tolerance and biocontrol efficacy of Pichia kudriavzevii with the transition from a yeast-like to biofilm morphology. Biol. Cont. 2015, 90, 113–119. [Google Scholar] [CrossRef]
- Wachowska, U.; Głowacka, K.; Mikołajczyk, W.; Kucharska, K. Biofilm of Aureobasidium pullulans var. pullulans on winter wheat kernels and its effect on other microorganisms. Microbiology 2016, 85, 523–530. [Google Scholar] [CrossRef]
- Klein, M.N.; Kupper, K.C. Biofilm production by Aureobasidium pullulans improves biocontrol against sour rot in citrus. Food Microbiol. 2018, 69, 1–10. [Google Scholar] [CrossRef]
- Bevan, E.; Makower, M. The physiological basis of the killer character in yeast. In Genetics Today, XIth International Congress on Genetics; Geerts, S.J., Ed.; Pergamon Press: Oxford, UK, 1963; Volume 1, pp. 202–203. [Google Scholar]
- Marquina, D.; Santos, A.; Peinado, J. Biology of killer yeasts. Int. Microbiol. 2002, 5, 65–71. [Google Scholar] [CrossRef]
- Liu, G.L.; Chi, Z.; Wang, G.Y.; Wang, Z.P.; Li, Y.; Chi, Z.M. Yeast killer toxins, molecular mechanisms of their action and their applications. Crit. Rev. Biotechnol. 2015, 35, 222–234. [Google Scholar] [CrossRef]
- Ciani, M.; Fatichenti, F. Killer toxin of Kluyveromyces phaffii DBVPG 6076 as a biopreservative agent to control apiculate wine yeasts. Appl. Environ. Microbiol. 2001, 67, 3058–3063. [Google Scholar] [CrossRef]
- Mazzucco, M.B.; Ganga, M.A.; Sangorrín, M.P. Production of a novel killer toxin from Saccharomyces eubayanus using agro-industrial waste and its application against wine spoilage yeasts. Antonie Van Leeuwenhoek 2019, 112, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; de Ingeniis, J.M.; Pepe, L.; Mannazzu, I.; Ciani, M. Pichia anomala and Kluyveromyces wickerhamii killer toxins as new tools against Dekkera/Brettanomyces spoilage yeasts. FEMS Microb. Lett. 2004, 238, 235–240. [Google Scholar] [CrossRef]
- Comitini, F.; Mannazzu, I.; Ciani, M. Tetrapisispora phaffii killer toxin is a highly specific β-glucanase that disrupts the integrity of the yeast cell wall. Microb. Cell Factories 2009, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Carboni, G.; Fancello, F.; Zara, G.; Zara, S.; Ruiu, L.; Marova, I.; Mannazzu, I. Production of a lyophilized ready-to-use yeast killer toxin with possible applications in the wine and food industries. Int. J. Food Microbiol. 2020, 335, 108883. [Google Scholar] [CrossRef]
- Carboni, G.; Marova, I.; Zara, G.; Zara, S.; Budroni, M.; Mannazzu, I. Evaluation of Recombinant Kpkt Cytotoxicity on HaCaT Cells: Further Steps towards the Biotechnological Exploitation Yeast Killer Toxins. Foods 2021, 10, 556. [Google Scholar] [CrossRef]
- Pinto, L.; Baruzzi, F.; Cocolin, L.; Malfeito-Ferreira, M. Emerging technologies to control Brettanomyces spp. in wine: Recent advances and future trends. Trends Food Sci. Technol. 2020, 99, 88–100. [Google Scholar] [CrossRef]
- De Ingeniis, J.; Raffaelli, N.; Ciani, M.; Mannazzu, I. Pichia anomala DBVPG 3003 secretes a ubiquitin-like protein that has antimicrobial activity. Appl. Environ. Microbiol. 2009, 75, 1129–1134. [Google Scholar] [CrossRef]
- Santos, A.; San Mauro, M.; Bravo, E.; Marquina, D. PMKT2, a new killer toxin from Pichia membranifaciens, and its promising biotechnological properties for control of the spoilage yeast Brettanomyces bruxellensis. Microbiology 2009, 155, 624–634. [Google Scholar] [CrossRef]
- Santos, E.O.; Michelon, M.; Gallas, J.A.; Kalil, S.J.; André Veiga Burkert, C. Raw glycerol as substrate for the production of yeast biomass. Int. J. Food Eng. 2013, 9, 413–420. [Google Scholar] [CrossRef]
- Mehlomakulu, N.N.; Setati, M.E.; Divol, B. Characterization of novel killer toxins secreted by wine-related non-Saccharomyces yeasts and their action on Brettanomyces spp. Int. J. Food Microbiol. 2014, 188, 83–91. [Google Scholar] [CrossRef]
- Mehlomakulu, N.N.; Prior, K.J.; Setati, M.E.; Divol, B. Candida pyralidae killer toxin disrupts the cell wall of Brettanomyces bruxellensis in red grape juice. J. Appl. Microbiol. 2017, 122, 747–758. [Google Scholar] [CrossRef]
- Comitini, F.; Agarbati, A.; Canonico, L.; Ciani, M. Yeast interactions and molecular mechanisms in wine fermentation: A comprehensive review. Int. J. Mol. Sci. 2021, 22, 7754. [Google Scholar] [CrossRef] [PubMed]
- Polonelli, L.; Magliani, W.; Ciociola, T.; Giovati, L.; Conti, S. From Pichia anomala killer toxin through killer antibodies to killer peptides for a comprehensive anti-infective strategy. Antonie Leeuwenhoek 2011, 99, 35–41. [Google Scholar] [CrossRef]
- Muccilli, S.; Restuccia, C. Bioprotective role of yeasts. Microorganisms 2015, 3, 588–611. [Google Scholar] [CrossRef]
- Agarbati, A.; Ciani, M.; Esin, S.; Agnolucci, M.; Marcheggiani, F.; Tiano, L.; Comitini, F. Comparative Zymocidial Effect of Three Different Killer Toxins against Brettanomyces bruxellensis spoilage yeasts. Int. J. Mol. Sci. 2023, 24, 1309. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.; Velázquez, R.; Maqueda, M.; López-Piñeiro, A.; Ribas, J.C. A new wine Torulaspora delbrueckii killer strain with broad antifungal activity and its toxin-encoding double-stranded RNA virus. Front. Microbiol. 2015, 6, 983. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.M.; Amézquita, A.; Cardona Jaramillo, J.E.C.; Matiz-Cerón, L.F.; Andrade-Martínez, J.S.; Triana, S.; Cock, H.D. Analysis of Malassezia lipidome disclosed differences among the species and reveals presence of unusual yeast lipids. Front. Cell. Inf. Microbiol. 2020, 10, 338. [Google Scholar] [CrossRef]
- Peña, R.; Vílches, J.; Poblete, C.; Ganga, M.A. Effect of Candida intermedia LAMAP1790 antimicrobial peptides against wine-spoilage yeasts Brettanomyces bruxellensis and Pichia guilliermondii. Fermentation 2020, 6, 65. [Google Scholar] [CrossRef]
- Acuña-Fontecilla, A.; Silva-Moreno, E.; Ganga, M.A.; Godoy, L. Evaluation of antimicrobial activity from native wine yeast against food industry pathogenic microorganisms. CyTA-J. Food 2017, 15, 457–465. [Google Scholar] [CrossRef]
- Younis, G.; Awad, A.; Dawod, R.E.; Yousef, N.E. Antimicrobial activity of yeasts against some pathogenic bacteria. Vet. World 2017, 10, 979. [Google Scholar] [CrossRef]
- Mencher, A.; Morales, P.; Valero, E.; Tronchoni, J.; Patil, K.R.; Gonzalez, R. Proteomic characterization of extracellular vesicles produced by several wine yeast species. Microb. Biotechnol. 2020, 13, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Zajc, J.; Gostincar, C.; Cernosa, A.; Gunde-Cimerman, N. Stress tolerant yeasts: Opportunistic pathogenicity versus biocontrol potential. Genes 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Roach, W.; Sun, T.; Jain, T.; Prinz, B.; Yu, T.Y.; Krauland, E. Addressing polyspecificity of antibodies selected from an in vitro yeast presentation system: A FACS-based, high-throughput selection and analytical tool. ProteinEng. Des. Sel. 2013, 26, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shimon, M.; Yehuda, H.; Cohen, L.; Weiss, B.; Kobeshnikov, A.; Daus, A.; Droby, S. Characterization of extracellular lytic enzymes produced by the yeast biocontrol agent Candida oleophila. Curr. Genet. 2004, 45, 140–148. [Google Scholar] [PubMed]
- Friel, D.; Pessoa, N.M.G.; Vandenbol, M.; Jijakli, M.H. Separate and Combined Disruptions of Two Exo-β-1, 3-Glucanase Genes Decrease the Efficiency of Pichia Anomala (Strain K) Biocontrol against Botrytis Cinerea on Apple. Mol. Plant-Microbe Interact. 2007, 20, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Castoria, R.; De Curtis, F.; Lima, G.; De Cicco, V. β-1, 3-glucanase activity of two saprophytic yeasts and possible mode of action as biocontrol agents against postharvest diseases. Postharvest Biol. Technol. 1997, 12, 293–300. [Google Scholar] [CrossRef]
- Park, H.W.; Choi, K.D.; Shin, I.S. Antimicrobial activity of isothiocyanates (ITCs) extracted from horseradish (Armoracia rusticana) root against oral microorganisms. Biocontrol Sci. 2013, 18, 163–168. [Google Scholar]
- Banani, H.; Spadaro, D.; Zhang, D.; Matic, S.; Garibaldi, A.; Gullino, M.L. Postharvest application of a novel chitinase cloned from Metschnikowia fructicola and overexpressed in Pichia pastoris to control brown rot of peaches. Int. J. Food Microbiol. 2015, 199, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.; Biles, C.; Droby, S.R.; Wilson, C.; Chalutz, E. Mode of action of the postharvest biocontrol yeast, Pichia guilliermondii. Characterization of attachment to Botrytis cinerea. Physiol. Mol. Plant. 1991, 39, 245–258. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Performance evaluation of volatile organic compounds by antagonistic yeasts immobilized on hydrogel spheres against gray, green and blue postharvest decays. Food Microbiol. 2017, 63, 191–198. [Google Scholar] [CrossRef]
- Lemos, W.J.; Treu, L.; da Silva Duarte, V.; Carlot, M.; Nadai, C.; Campanaro, S.; Giacomini, A.; Corich, V. Biocontrol ability and action mechanism of Starmerella bacillaris (synonym Candida zemplinina) isolated from wine musts against gray mold disease agent Botrytis cinerea on grape and their effects on alcoholic fermentation. Front. Microbiol. 2016, 7, 1249. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Farbo, M.G.; Urgeghe, P.P.; Fiori, S.; Marcello, A.; Oggiano, S.; Balmas, V.; Hassan, Z.U.I.; Jaoua, S.; Migheli, Q. Effect of yeast volatile organic compounds on ochratoxin A-producing Aspergillus carbonarius and A. ochraceus. Int. J. Food Microbiol. 2018, 284, 1–10. [Google Scholar] [CrossRef]
- Fiori, S.; Urgeghe, P.P.; Hammami, W.; Razzu, S.; Jaoua, S.; Migheli, Q. Biocontrol activity of four non- and low-fermenting yeast strains against Aspergillus carbonarius and their ability to remove ochratoxin A from grape juice. Int. J. Food Microbiol. 2014, 189, 45–50. [Google Scholar] [CrossRef]
- Hua, S.S.; Beck, J.J.; Sarreal, S.B.; Gee, W. The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 2014, 30, 71–78. [Google Scholar] [CrossRef]
- Arrarte, E.; Garmendia, G.; Rossini, C.; Wisniewski, M.; Vero, S. Volatile organic compounds produced by Antarctic strains of Candida sake play a role in the control of postharvest pathogens of apples. Biol. Control 2017, 109, 14–20. [Google Scholar] [CrossRef]
- Masoud, W.; Poll, L.; Jakobsen, M. Influence of volatile compounds produced by yeasts predominant during processing of Coffea arabica in East Africa on growth and ochratoxin A (OTA) production by Aspergillus ochraceus. Yeast 2005, 22, 1133–1142. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Hernández, A.; Galvan, A.I.; Córdoba, M.G.; Casquete, R.; Serradilla, M.J.; Martín, A. Selection and application of antifungal VOCs-producing yeasts as biocontrol agents of grey mould in fruits. Food Microbiol. 2020, 92, 103556. [Google Scholar] [CrossRef]
- Bosqueiro, A.S.; Bizarria, R., Jr.; Rosa-Magri, M.M. Biocontrol of post-harvest tomato rot caused by Alternaria arborescens using Torulaspora indica. Biocontrol Sci. Technol. 2023, 33, 115–132. [Google Scholar] [CrossRef]
- Nasanit, R.; Jaibangyang, S.; Onwibunsiri, T.; Khunnamwong, P. Screening of Volatile Organic Compound-Producing Yeasts and Yeast-Like Fungi against Aflatoxigenic Aspergillus Flavus. Microbiol. Biotechnol. Lett. 2022, 50, 202–210. [Google Scholar] [CrossRef]
- Zou, X.; Wei, Y.; Jiang, S.; Cao, Z.; Xu, F.; Wang, H.; Zhan, P.; Shao, X. Volatile organic compounds and rapid proliferation of Candida pseudolambica W16 are modes of action against gray mold in peach fruit. Postharvest Biol. Technol. 2022, 183, 111751. [Google Scholar] [CrossRef]
- Liu, J.; Li, G.; Sui, Y. Optimization of culture medium enhances viable biomass production and biocontrol efficacy of the antagonistic yeast, Candida diversa. Front. Microbiol. 2017, 8, 2021. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Guo, J.H.; Liu, P.; Cheng, Y.J.; Wang, B.Q.; Long, C.A.; Deng, B.X. Inhibitory activity of tea polyphenol and Candida ernobii against Diplodia natalensis infections. J. Appl. Microbiol. 2010, 108, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Papon, N.; Savini, V.; Lanoue, A.; Simkin, A.J.; Crèche, J.; Giglioli-Guivarc’h, N.; Clastre, M.; Courdavault, V.; Sibirny, A.A. Candida guilliermondii: Biotechnological applications, perspectives for biological control, emerging clinical importance and recent advances in genetics. Curr. Genet. 2013, 59, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Carbo, A.; Torres, R.; Teixido, N.; Usall, J.; Medina, A.; Magan, N. Impact of climate change environmental conditions on the resilience of different formulations of the biocontrol agent Candida sake CPA-1 on grapes. Lett. Appl. Microbiol. 2018, 67, 2–8. [Google Scholar] [CrossRef]
- Huang, R.; Li, G.Q.; Zhang, J.; Yang, L.; Che, H.J.; Jiang, D.H.; Huang, H.C. Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology 2011, 101, 859–869. [Google Scholar] [CrossRef]
- Wisniewski, M.; Wilson, C.; Droby, S.; Chalutz, E.; El Ghaouth, A.; Stevens, C. Postharvest biocontrol: New concepts and applications. In Biological Control: A Global Perspective; CABI: Wallingford, UK, 2007; pp. 262–273. [Google Scholar]
- Scherm, B.; Ortu, G.; Muzzu, A.; Budroni, M.; Arras, G.; Migheli, Q. Biocontrol activity of antagonistic yeasts against Penicillium expansum on apple. J. Plant Pathol. 2003, 85, 205–213. [Google Scholar]
- European Food Safety Authority (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance Candida oleophila strain O. EFSA J. 2015, 10, 2944.
- European Commission Health & Consumers Directorate-General. Review Report for the Active Substance Candida oleophila strain O; vol SANCO/10395/2013 rev 1; European Commission: Brussels, Belgium, 2013. [Google Scholar] [CrossRef]
- Kunz, S.; Schmitt, A.; Haug, P. Field testing of strategies for fire blight control in organic fruit growing. Acta Hortic. 2011, 896, 431–436. [Google Scholar] [CrossRef]
- Weiss, A.; Mögel, G.; Kunz, S. Development of “Boni-Protect”—A yeast preparation for use in the control of post-harvest diseases of apples. In Proceedings of the 12th International Conference on Cultivation Technique and Psychopathological Problems in Organic Fruit-Growing, Weinsberg, Germany, 1 January–2 February 2006; pp. 113–117. [Google Scholar]
- Hilber-Bodmer, M.; Schmid, M.; Ahrens, C.H.; Freimoser, F.M. Competition assays and physiological experiments of soil and phyllosphere yeasts identify Candida subhashii as a novel antagonist of filamentous fungi. BMC Microbiol. 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Turkel, S.; Korukluoglu, M.; Yavuz, M. Biocontrol activity of the local strain of Metschnikowia pulcherrima on different postharvest pathogens. Biotechnol. Res. Int. 2014, 2014, 397167. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.; Droby, S. Biopreservation of food and feed by postharvest biocontrol with microorganisms. In Beneficial Microorganisms in Agriculture, Food and the Environment; Sundh, I., Wilcks, A., Goettel, M.S., Eds.; CABI International: Oxfordshire, UK, 2012; pp. 1234–1254. [Google Scholar]
- Droby, S.; El-Gerberia, B. Yeast Metschnikowia fructicola NRRL Y-30752 for Inhibiting Deleterious Microorganisms on Plants. U.S. Patent 6,994,849, 7 February 2006. [Google Scholar]
- Tian, S.P.; Qin, G.Z.; Xu, Y.; Wang, Y.S. Application of antagonistic yeasts under field conditions and their biocontrol ability against postharvest diseases of sweet cherry. Acta Bot. Sin. 2004, 46, 1324–1330. [Google Scholar]
- Zhang, H.Y.; Zheng, X.D.; Wang, L.; Li, S.S.; Liu, R.F. Effect of yeast antagonist in combination with hot water dips on postharvest Rhizopus rot of strawberries. J. Food Eng. 2007, 78, 281–287. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zheng, X.D.; Yu, T. Biological control of postharvest diseases of peach with Cryptococcus laurentii. Food Control 2007, 18, 287–291. [Google Scholar] [CrossRef]
- Kowalska, J.; Drożdżyński, D.; Remlein-Starosta, D.; Sas-Paszt, L.; Malusá, E. Use of Cryptococcus albidus for controlling grey mould in the production and storage of organically grown strawberries. J. Plant. Dis. Prot. 2012, 119, 174–178. [Google Scholar] [CrossRef]
- Lutz, M.C.; Lopes, C.A.; Rodriguez, M.E.; Sosa, M.C.; Sangorrin, M.P. Efficacy and putative mode of action of native and commercial antagonistic yeasts against postharvest pathogens of pear. Int. J. Food Microbiol. 2013, 164, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, D.; Wacławek, S.; Venkateshaiah, A.; Krawczyk, K.; Sobel, B.; Padil, V.V.; Varma, R.S. Synthesis of Ag nanoparticles by a chitosan-poly (3-hydroxybutyrate) polymer conjugate and their superb catalytic activity. Carbohydr. Polym. 2020, 232, 115806. [Google Scholar] [CrossRef]
- Di Matteo, G.; Di Matteo, P.; Sambucci, M.; Tirillò, J.; Giusti, A.M.; Vinci, G.; Valente, M. Commercial bio-packaging to preserve the quality and extend the shelf-life of vegetables: The case-study of pumpkin samples studied by a multimethodological approach. Foods 2012, 10, 2440. [Google Scholar] [CrossRef]
- Castelán, E.; Rodríguez-Hernández, A.I.; Chavarría-Hernández, N.; López-Ortega, M.A.; del Rocio López-Cuellar, M. Natural antimicrobial systems protected by complex polyhydroxyalkanoate matrices for food biopackaging applications—A review. Int. J. Biol. Macromol. 2023, 233, 123418. [Google Scholar] [CrossRef]
- Maisanaba, S.; Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Puerto, M.; Prieto, A.I.; Jos, A.; Cameán, A.M. New advances in active packaging incorporated with essential oils or their main components for food preservation. Food Rev. Int. 2017, 33, 447–515. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N.B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Pediocin applications in antimicrobial food packaging systems. In Antimicrobial Food Packaging; Academic Press: Cambridge, MA, USA, 2016; pp. 445–454. [Google Scholar]
- dos Santos Pires, A.C.; de Fátima Ferreira Soares, N.; de Andrade, N.J.; da Silva, L.H.M.; Camilloto, G.P.; Bernardes, P.C. Development and evaluation of active packaging for sliced mozzarella preservation. Packag. Technol. Sci. 2008, 21, 375–383. [Google Scholar] [CrossRef]
- Hammami, R.; Oueslati, M.; Smiri, M.; Nefzi, S.; Ruissi, M.; Comitini, F.; Sadfi Zouaoui, N. Epiphytic yeasts and bacteria as candidate biocontrol agents of green and blue molds of citrus fruits. J. Fungi 2022, 8, 818. [Google Scholar] [CrossRef] [PubMed]
- Bertini, E.V.; Leguina, A.C.D.V.; Castellanos, L.I.; Nieto Peñalver, C.G. Endophytic microorganisms Agrobacterium tumefaciens 6N2 and Meyerozyma guilliermondii 6N serve as models for the study of microbial interactions in colony biofilms. Rev. Argent. Microbiol. 2019, 2, 234–237. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Di Cagno, R.; Trani, A.; Cardinali, G.; Gobbetti, M. Antifungal activity of Meyerozyma guilliermondii: Identification of active compounds synthesized during dough fermentation and their effect on long-term storage of wheat bread. Food Microbiol. 2013, 33, 243–251. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Ul-Islam, M.; Ahmed, A.A.Q.; Ullah, M.W.; Yang, G. Silver decorated bacterial cellulose nanocomposites as antimicrobial food packaging materials. ES Food Agrofor. 2021, 6, 12–26. [Google Scholar] [CrossRef]
- da Costa Guimarães, N.; Freitas-de-Sousa, L.A.; de Souza, M.C.S.; de Almeida, P.D.O.; Dos-Santos, M.C.; Nunez, C.V.; de Moura, V.M. Evaluation of the anti-snakebite, antimicrobial and antioxidant potential of Philodendron megalophyllum Schott (Araceae), traditionally used in accidents caused by snakes in the western region of Pará, Brazil. Toxicon 2020, 184, 99–108. [Google Scholar] [CrossRef]
- Aloui, H.; Licciardello, F.; Khwaldia, K.; Hamdi, M.; Restuccia, C. Physical properties and antifungal activity of bioactive films containing Wickerhamomyces anomalus killer yeast and their application for preservation of oranges and control of postharvest green mold caused by Penicillium digitatum. Int. J. Food Microbiol. 2015, 200, 22–30. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- McGuire, R.G.; Baldwin, E.A. Compositions of cellulose coatings affect populations of yeasts in the liquid formulation and on coated grapefruits. In Proceedings-Florida State Horticultural Society; Florida State Horticultural Society: Lake Alfred, FL, USA, 1994; Volume 107, pp. 293–297. [Google Scholar]
- Sharma, N.; Brandis, K.A.; Herrera, S.K.; Johnson, B.E.; Vaidya, T.; Shrestha, R.; DebBurman, S.K. α-Synuclein budding yeast model: Toxicity enhanced by impaired proteasome and oxidative stress. JMB 2006, 28, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Sokorai, K.J.; Liao, C.H.; Cooke, P.; Zhang, H.Q. Antibrowing and antimicrobial properties of sodium acid sulfate in apple slices. J. Food Sci. 2009, 74, M485–M492. [Google Scholar] [CrossRef] [PubMed]
- Yinzhe, R.; Shaoying, Z. Effect of carboxymethyl cellulose and alginate coating combined with brewer yeast on postharvest grape preservation. Int. Sch. Res. Not. 2013, 2013, 871396. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. The effect of locust bean gum (LBG)-based edible coatings carrying biocontrol yeasts against Penicillium digitatum and Penicillium italicum causal agents of postharvest decay of mandarin fruit. Food Microbiol. 2016, 58, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Gadir, A.A.; Dhir, R. Probiotics: Reiterating what they are and what they are not. Front. Microbiol. 2019, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic and potentially probiotic yeasts—Characteristics and food application. Foods 2021, 10, 1306. [Google Scholar] [CrossRef]
- McFarland, L.V. From yaks to yogurt: The history, development, and current use of probiotics. Clin. Infect. Dis. 2015, 60, S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Simões, L.A.; Cristina de Souza, A.; Ferreira, I.; Melo, D.S.; Lopes, L.A.A.; Magnani, M.; Dias, D.R. Probiotic properties of yeasts isolated from Brazilian fermented table olives. J. Appl. Microbiol. 2021, 131, 1983–1997. [Google Scholar] [CrossRef]
- Wang, J.; Da, R.; Tuo, X. Probiotic and safety properties screening of Enterococcus faecalis from healthy Chinese infants. Probiotics Antimicrob. Proteins 2020, 12, 1115–1125. [Google Scholar] [CrossRef]
- Pereira, R.P.; Jadhav, R.; Baghela, A.; Barretto, D.A. In Vitro Assessment of probiotic potential of Saccharomyces cerevisiae DABRP5 isolated from bollo batter, a traditional Goan fermented food. Probiotics Antimicrob. Proteins 2021, 13, 796–808. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; World Health Organization, Food and Agriculture organization of the United Nations: Geneva, Switzerland; Rome, Italy, 2002. [Google Scholar]
- Shruthi, B.; Deepa, N.; Somashekaraiah, R.; Adithi, G.; Divyashree, S.; Sreenivasa, M.Y. Exploring biotechnological and functional characteristics of probiotic yeasts: A review. Biotechnol. Rep. 2022, 34, e00716. [Google Scholar] [CrossRef] [PubMed]
- Khatri, I.; Tomar, R.; Ganesan, K.; Prasad, G.S.; Subramanian, S. Complete genome sequence and comparative genomics of the probiotic yeast Saccharomyces boulardii. Sci. Rep. 2017, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What makes it tick as successful probiotic? J. Fungus 2020, 6, 78. [Google Scholar] [CrossRef]
- Lazo-Vélez, M.A.; Serna-Saldívar, S.O.; Rosales-Medina, M.F.; Tinoco-Alvear, M.; Briones-García, M. Application of Saccharomyces cerevisiae var. boulardii in food processing: A review. J. Appl. Microbiol. 2018, 125, 943–951. [Google Scholar] [CrossRef]
- Martirosyan, D.M.; Leem, C. The bioactive compounds of probiotic foods/supplements and their application in managing mental disorders. BCHD 2019, 2, 206–220. [Google Scholar] [CrossRef]
- Rai, A.K.; Pandey, A.; Sahoo, D. Biotechnological potential of yeasts in functional food industry. Trends Food Sci. Technol. 2019, 83, 129–137. [Google Scholar] [CrossRef]
- Li, Y.; Sadiq, F.A.; Liu, T.; Chen, J.; He, G. Purification and identification of novel peptides with inhibitory effect against angiotensin I-converting enzyme and optimization of process conditions in milk fermented with the yeast Kluyveromyces marxianus. J. Funct. Foods 2015, 16, 278–288. [Google Scholar] [CrossRef]
- Agarbati, A.; Marini, E.; Galli, E.; Canonico, L.; Ciani, M.; Comitini, F. Characterization of wild yeasts isolated from artisan dairies in the Marche region, Italy, for selection of promising functional starters. LWT 2021, 139, 110531. [Google Scholar] [CrossRef]
- Fernández-Pacheco, P.; García-Béjar, B.; Jiménez-del Castillo, M.; Carreño-Domínguez, J.; Briones Perez, A.; Arévalo-Villena, M. Potential probiotic and food protection role of wild yeasts isolated from pistachio fruits (Pistacia vera). J. Sci. Food Agric. 2021, 101, 2201–2209. [Google Scholar] [CrossRef]
- Agarbati, A.; Canonico, L.; Marini, E.; Zannini, E.; Ciani, M.; Comitini, F. Potential probiotic yeasts sourced from natural environmental and spontaneous processed foods. Foods 2020, 9, 287. [Google Scholar] [CrossRef]
- Fadda, M.E.V.; Mossa, M.; Deplano, M.B.; Pisano, S.; Cosentino, D. In vitro screening of Kluyveromyces strains isolated from Fiore Sardo cheese for potential use as probiotics. LWT 2017, 75, 100–106. [Google Scholar] [CrossRef]
- Alvarez, S.C.V.; Alaniz, M.J.L.; Furlani, M.V.M.; Vazquez, F.; Agresti, P.M.; Nally, M.C.; Maturano, Y.P. Bioprospecting of the probiotic potential of yeasts isolated from a wine environment. Fungal Genet. Biol. 2023, 164, 103767. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pacheco, C.; Cueva, M.; Ar´evalo-Villena, M.V.; Moreno-Arribas, A.; Briones P´erez, G. Saccharomyces cerevisiae and Hanseniaspora osmophila strains as yeast active cultures for potential probiotic applications. Food Funct. 2019, 10, 4924–4931. [Google Scholar] [CrossRef]
- Zahoor, F.C.; Sooklim, P.; Songdech, O.; Duangpakdee, N.; Soontorngun, O. Selection of potential yeast probiotics and a cell factory for xylitol or acid production from honeybee samples. Metabolites 2021, 11, 312. [Google Scholar] [CrossRef]
- Fernández-Pacheco, P.; Pintado, C.; Briones Pérez, A.; Arévalo-Villena, M. Potential probiotic strains of Saccharomyces and non-Saccharomyces: Functional and biotechnological characteristics. J. Fungus 2021, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, A.F.; El-Enshasy, H.A.; Shetaia, Y.M.; Mahrous, H.; Othman, N.Z.; Yousef, A.E. Semi-industrial scale production of a new yeast with probiotic traits, Cryptococcus sp. YMHS, isolated from the Red Sea. Probiotics Antimicrob. 2018, 10, 77–88. [Google Scholar] [CrossRef]
- França, R.C.; Conceição, F.R.; Mendonça, M.; Haubert, L.; Sabadin, G.; de Oliveira, P.D.; Moreira, Â.N. Pichia pastoris X-33 has probiotic properties with remarkable antibacterial activity against Salmonella typhimurium. Appl. Microbiol. Biotechnol. 2015, 99, 7953–7961. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Zannini, E.; Ciani, M.; Comitini, F. Assessment of non-conventional yeasts with potential probiotic for protein-fortified craft beer production. LWT 2021, 145, 111361. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Cantatore, V.; Polo, A.; Celano, G.; Martinovic, A.; Gobbetti, M. Design of potential probiotic yeast starters tailored for making a cornelian cherry (Cornus mas L.) functional beverage. Int. J. Food Microbiol. 2020, 323, 108591. [Google Scholar] [CrossRef]

| NSYs | Products | Distinctive Antimicrobial Features | References |
|---|---|---|---|
| Aureobasidium pullulans | Guava fruit | Antibacterial activity mediated by fusarinin C | [37] |
| Aureobasidium pullulans | Citrus | Antimicrobial biofilm formation | [48] |
| Aureobasidium pullulans | Plants | Biocontrol mediated by nutrient, space or VOC | [18,52,54,55,56] |
| Aureobasidium pullulans | Mandarins’ edible coating | Anti-mold activity | [80] |
| Aureobasidium pullulans | Table grape berries | Biocontrol VOC mediated | [80] |
| Aureobasidium pullulans | Fruits | Biocontrol VOC mediated against A. flavus | [86] |
| Candida spp. | Top, hazel and citrus fruits | Antagonist of filamentous fungi | [104] |
| Candida albicans | Medical field | Inhibitory effect on S. aureus and E. coli | [33] |
| Candida guillermondii | Oranges’ biopackaging | Anti-mold activity | [128] |
| Candida inetrmedia | Milk | Probiotic activity | [139] |
| Candida intermedia | Wine environment | Anti-spoilage yeasts (wide spectrum) AMPs mediated | [69] |
| Candida intermedia | Medical field | Antimicrobial against E. coli, S. aureus AMPs mediated | [70,71] |
| Candida laurentii | Strawberries’ edible coating | Antimold activity | [129] |
| Candida oleophyla | Fruits | Antimold activity (P. digitatum) EVs mediated | [75] |
| Candida oleophyla | Table grapefuits’ biopackaging | Antimold activity | [127] |
| Candida orthopsilosis | Table olives | Probiotic activity | [135] |
| Candida pseudolambica | Peach | Biocontrol VOC mediated against B. cinerea | [92] |
| Candida pyralidale | Wine | Anti-Brettanomyces activity mycocin mediated | [63] |
| Candida quercitresa | Indigenous fermented food | Probiotic activity | [139] |
| Candida sake | Apple rot | Biocontrol VOC mediated against P. expansum and B. cinerea | [87] |
| Candida sake | Milk products | Probiotic activity | [139] |
| Candida tropicalis | Medical field | Bacterial lysis | [30] |
| Candida tropicalis | Table olives | Probiotic activity | [135] |
| Candida utilis | Tomatoes biopackaging | Anti-mold activity | [128] |
| Debaryomyces hansenii | Medical field | Bacterial lysis | [30] |
| Debaryomyces hansenii | Table olives | Probiotic activity | [135] |
| Hanseniaspora guillermondii | N.D. 1 | Probiotic activity | [139] |
| Hanseniaspora uvarum | Coffee | Biocontrol VOC mediated against A. ochraceus | [88] |
| Hanseniaspora uvarum | N.D. 1 | Probiotic activity | [139] |
| Kluyveromyces lactis | Medical field | L. monocytogenes and C. albicans growth inhibition | [32] |
| Kluyveromyces marxianus | Medical field | E. coli diseases prevention in mice | [31] |
| Kluyveromyces marxianus | Medical field | L. monocytogenes and C. albicans growth inhibition | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comitini, F.; Canonico, L.; Agarbati, A.; Ciani, M. Biocontrol and Probiotic Function of Non-Saccharomyces Yeasts: New Insights in Agri-Food Industry. Microorganisms 2023, 11, 1450. https://doi.org/10.3390/microorganisms11061450
Comitini F, Canonico L, Agarbati A, Ciani M. Biocontrol and Probiotic Function of Non-Saccharomyces Yeasts: New Insights in Agri-Food Industry. Microorganisms. 2023; 11(6):1450. https://doi.org/10.3390/microorganisms11061450
Chicago/Turabian StyleComitini, Francesca, Laura Canonico, Alice Agarbati, and Maurizio Ciani. 2023. "Biocontrol and Probiotic Function of Non-Saccharomyces Yeasts: New Insights in Agri-Food Industry" Microorganisms 11, no. 6: 1450. https://doi.org/10.3390/microorganisms11061450
APA StyleComitini, F., Canonico, L., Agarbati, A., & Ciani, M. (2023). Biocontrol and Probiotic Function of Non-Saccharomyces Yeasts: New Insights in Agri-Food Industry. Microorganisms, 11(6), 1450. https://doi.org/10.3390/microorganisms11061450








