Anaplasma phagocytophilum Community-Acquired Pneumonia: Case Report and Literature Review
Abstract
1. Introduction
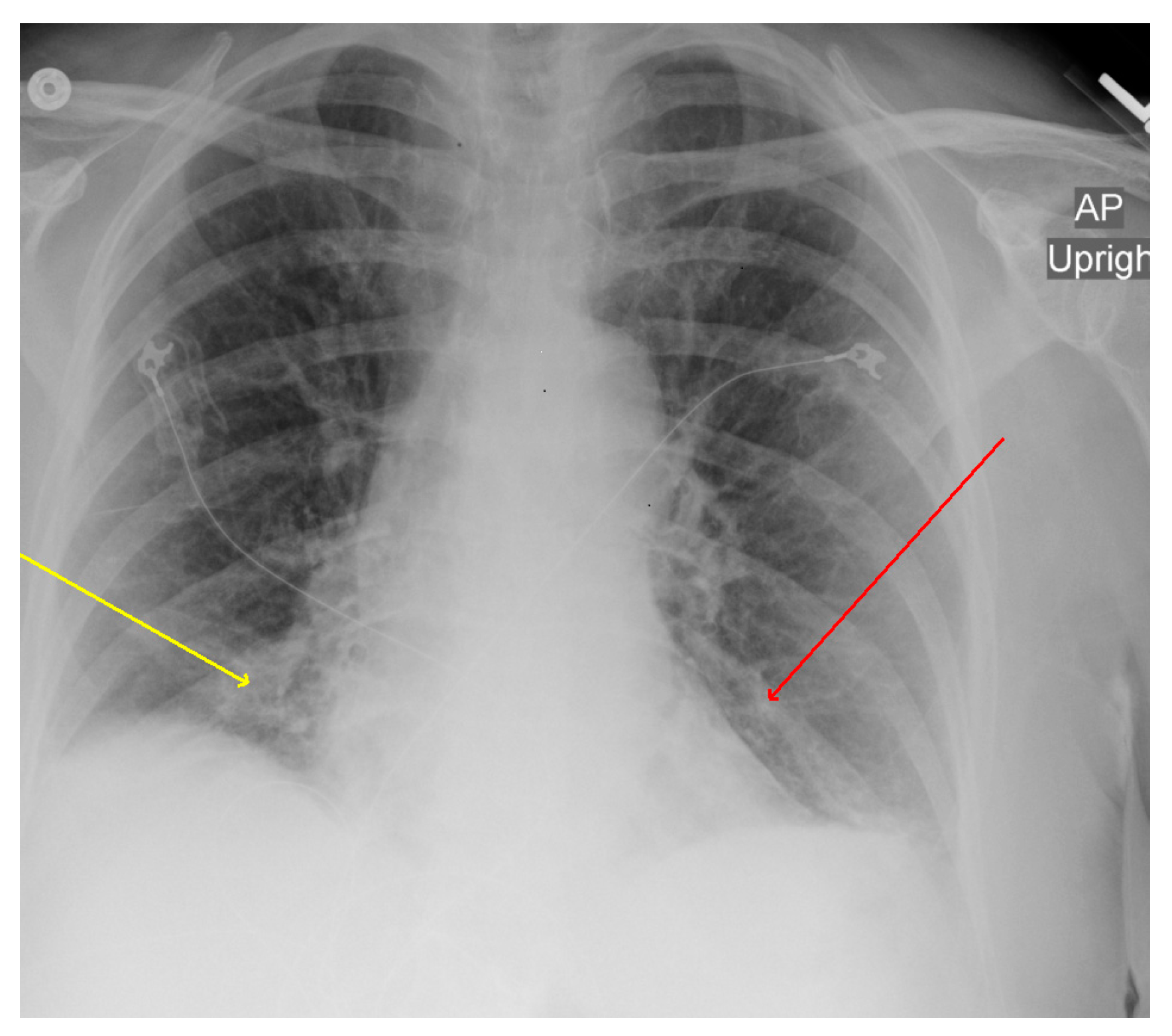
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dumler, J.S.; Choi, K.S.; Garcia-Garcia, J.C.; Barat, N.S.; Scorpio, D.G.; Garyu, J.W.; Grab, D.J.; Bakken, J.S. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 2005, 11, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Dumler, J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. N. Am. 2015, 29, 341–355. [Google Scholar] [CrossRef]
- Dumic, I.; Jevtic, D.; Veselinovic, M.; Nordstrom, C.W.; Jovanovic, M.; Mogulla, V.; Veselinovic, E.M.; Hudson, A.; Simeunovic, G.; Petcu, E.; et al. Human Granulocytic Anaplasmosis-A Systematic Review of Published Cases. Microorganisms 2022, 10, 1433. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Ni, D.; Li, Q.; Yu, Y.; Yu, X.J.; Wan, K.; Li, D.; Liang, G.; Jiang, X.; et al. Nosocomial Transmission of Human Granulocytic Anaplasmosis in China. JAMA 2008, 300, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Fine, A.B.; Sweeney, J.D.; Nixon, C.P.; Knoll, B.M. Transfusion-transmitted anaplasmosis from a leukoreduced platelet pool. Transfusion 2016, 56, 699–704. [Google Scholar] [CrossRef]
- Mir, M.A.; Grant, J. Dysarthria and thrombocytopenia after Tick Bite. Blood 2013, 122, 2538. [Google Scholar] [CrossRef]
- Cho, J.M.; Chang, J.; Kim, D.-M.; Kwak, Y.G.; Cho, C.R.; Song, J.E. Human granulocytic anaplasmosis combined with rhabdomyolysis: A case report. BMC Infect. Dis. 2021, 21, 1184. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, C.-M.; Kim, D.-M.; Yun, N.R. Manifestation of anaplasmosis as cerebral infarction: A case report. BMC Infect. Dis. 2018, 18, 409. [Google Scholar] [CrossRef]
- Eldaour, Y.; Hariri, R.; Yassin, M. Severe anaplasmosis presenting as possible CVA: Case report and 3-year Anaplasma infection diagnosis data is based on PCR testing and serology. IDCases 2021, 24, e01073. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.R.; Zaki, R.; Zaki, A.; Najjar Mojarrab, J.; Zahid, A. Transient arrhythmia in a patient with human granulocytic anaplasmosis: An uncanny presentation. Cureus 2021, 13, e13241. [Google Scholar] [CrossRef]
- LeDonne, M.; Ahmed, S.A.; Keeney, S.M.; Nadworny, H. Trigeminal neuralgia as the principal manifestation of Anaplasmosis: A case report. Cureus 2022, 14, e21668. [Google Scholar] [CrossRef] [PubMed]
- Hsia, K.; Johnson, J.; Rice, D. Splenomegaly, non-traumatic splenic rupture, and pancytopenia in patient with human granulocytic anaplasmosis. Rhode Isl. Med. J. 2013, 104, 60–62. Available online: https://pubmed.ncbi.nlm.nih.gov/33648322/ (accessed on 26 July 2022).
- De Jesus, M.; Lopez, A.; Yabut, J.; Vu, S.; Manne, M.; Ibrahim, L.; Mutneja, R. Anaplasmosis-induced hemophagocytic lymphohistiocytosis. Bayl. Univ. Med. Cent. Proc. 2022, 35, 379–381. [Google Scholar] [CrossRef]
- Halasz, C.L.G.; Niedt, G.W.; Kurtz, C.P.; Scorpio, D.G.; Bakken, J.S.; Dumler, J.S. A Case of Sweet Syndrome Associated with Human Granulocytic Anaplasmosis. Arch. Dermatol. 2005, 141, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.E.; Young, K.; Kwon, T.S.; McKenzie, P.A.; Grant, M.A.; McBride, D.A. Anaplasmosis Presenting with Respiratory Symptoms and Pneumonitis. Open Forum Infect. Dis. 2020, 7, ofaa265. [Google Scholar] [CrossRef]
- Taşdemir, C.; Şimşek, S.; Önal, U.; Çetinkaya, H.; Aydın, L.; Yılmaz, E. A difficult diagnosis of anaplasmosis with pneumonia: A case report. Trop. Doct. 2022, 53, 190–192. [Google Scholar] [CrossRef]
- Remy, V.; Hansmann, Y.; De Martino, S.; Christmann, D.; Brouqui, P. Human anaplasmosis presenting as atypical pneumonitis in France. Clin. Infect. Dis. 2003, 37, 846–848. [Google Scholar] [CrossRef]
- Yawetz, S.; Mark, E.J. Case 37-2001—A 76-year-old man with fever, dyspnea, pulmonary infiltrates, pleural effusions, and confusion. N. Engl. J. Med. 2001, 345, 1627–1634. [Google Scholar] [CrossRef]
- Vyas, J.M.; Castle, A.C.; Bourgouin, P.P.; Turbett, S.E. Case 9-2022: A 56-Year-Old Woman with Fever, Myalgias, Diarrhea, and Cough. N. Engl. J. Med. 2022, 386, 1166–1174. [Google Scholar] [CrossRef]
- Kaphle, U.; Kheir, F.; Thammasitboon, S. A Rare Case of ARDS From Human Anaplasmosis. Respir. Care 2015, 60, e125–e127. [Google Scholar] [CrossRef]
- Tsibris, A.M.; Shepard, J.A.; Zukerberg, L.R. Case records of the Massachusetts General Hospital: Case 6-2011—A 77-year-old man with dyspnea, weakness, and diaphoresis. N. Engl. J. Med. 2011, 364, 759–767. [Google Scholar] [CrossRef] [PubMed]
- CDC. Tickborne Diseases of the United States. 2022. Available online: https://www.cdc.gov/ticks/tickbornediseases/index.html (accessed on 1 May 2023).
- Dumic, I.; Severnini, E. “Ticking Bomb”: The Impact of Climate Change on the Incidence of Lyme Disease. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 5719081. [Google Scholar] [CrossRef]
- Nelder, M.P.; Russell, C.B.; Sheehan, N.J.; Sander, B.; Moore, S.; Li, Y.; Johnson, S.; Patel, S.N.; Sider, D. Human pathogens associated with the blacklegged tick Ixodes scapularis: A systematic review. Parasites Vectors 2016, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Cotté, V.; Bonnet, S.; Le Rhun, D.; Le Naour, E.; Chauvin, A.; Boulouis, H.J.; Lecuelle, B.; Lilin, T.; Vayssier-Taussat, M. Transmission of Bartonella henselae by Ixodes ricinus. Emerg. Infect. Dis. 2008, 14, 1074–1080. [Google Scholar] [CrossRef]
- Lehane, A.; Maes, S.E.; Graham, C.B.; Jones, E.; Delorey, M.; Eisen, R.J. Prevalence of single and coinfections of human pathogens in Ixodes ticks from five geographical regions in the United States, 2013–2019. Ticks Tick Borne Dis. 2021, 12, 101637, Erratum in Ticks Tick Borne Dis. 2021, 12, 101681. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.; Ruben, F.L.; Steere, A.C.; Duray, P.H.; Norden, C.W.; Winkelstein, A. Fatal adult respiratory distress syndrome in a patient with Lyme disease. JAMA 1988, 259, 2737–2739. [Google Scholar] [CrossRef]
- Faul, J.L.; Doyle, R.L.; Kao, P.N.; Ruoss, S.J. Tick-borne pulmonary disease: Update on diagnosis and management. Chest 1999, 116, 222–230. [Google Scholar] [CrossRef]
- Abbott, R.A.; Hammans, S.; Margarson, M.; Aji, B.M. Diaphragmatic paralysis and respiratory failure as a complication of Lyme disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1306–1307. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Cunha, B.A.; Nausheen, S.; Szalda, D. Pulmonary complications of babesiosis: Case report and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 505–508. [Google Scholar] [CrossRef]
- Pedati, C.; House, J.; Hancock-Allen, J.; Colton, L.; Bryan, K.; Ortbahn, D.; Kightlinger, L.; Kugeler, K.; Petersen, J.; Mead, P.; et al. Notes from the Field: Increase in Human Cases of Tularemia—Colorado, Nebraska, South Dakota, and Wyoming, January–September 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1317–1318, Erratum in MMWR Morb. Mortal. Wkly. Rep. 2016, 64, 1409. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef]
- Jay, R.; Armstrong, P.A. Clinical characteristics of Rocky Mountain spotted fever in the United States: A literature review. J. Vector Borne Dis. 2020, 57, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Lantos, P.M.; Rumbaugh, J.; Bockenstedt, L.K.; Falck-Ytter, Y.T.; Aguero-Rosenfeld, M.E.; Auwaerter, P.G.; Baldwin, K.; Bannuru, R.R.; Belani, K.K.; Bowie, W.R.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis and Treatment of Lyme Disease. Clin. Infect. Dis. 2021, 72, e1–e48. [Google Scholar] [CrossRef] [PubMed]

| Admission | Day 2 | Discharge | |
|---|---|---|---|
| Hemoglobin | 12.4 | 11.9 | 11.6 |
| Platelet Count | 22 | 38 | 69 |
| White Blood Cell Count | 4.3 | 9.2 | 7.9 |
| Neutrophils | 2.15 | 1.07 | 0.91 |
| Lymphocytes | 1.94 | 6.72 | 6.21 |
| Bilirubin, Total | 2.2 | 0.3 | 0.8 |
| Alanine Aminotransferase | 129 | 107 | 109 |
| Aspartate Aminotransferase | 128 | 114 | 100 |
| C-reactive Protein | 250.4 | 75.9 | 41.2 |
| Test | Blood Specimen | Urine Specimen | Sputum Specimen | Respiratory Nasopharyngeal Swab |
|---|---|---|---|---|
| Cultures | Negative | Negative | Negative | |
| Legionella pneumophila antigen | - | Negative | - | - |
| Streptococcus pneumonia antigen | - | Negative | - | - |
| Peripheral smear | Intra neutrophilic inclusion | - | - | - |
| Mycoplasma pneumonia antibodies | IgM and IgG negative | - | - | - |
| Chlamydia pneumoniae antibodies | IgM negative | - | - | - |
| Chlamydia trachomatis antibodies | IgM and IgG negative | - | - | - |
| Chlamydia psittaci antibodies | IgM and IgG negative | - | - | - |
| Adenovirus | - | - | - | Negative |
| Coronavirus 229E | - | - | - | Negative |
| Coronavirus HKU1 | - | - | - | Negative |
| Coronavirus NL63 | - | - | - | Negative |
| Coronavirus OC43 | - | - | - | Negative |
| SARS Coronavirus-2 | - | - | - | Negative |
| Human Metapneumovirus | - | - | - | Negative |
| Human Rhinovirus/Enterovirus | - | - | - | Negative |
| Influenza A Virus | - | - | - | Negative |
| Influenza B Virus | - | - | - | Negative |
| Parainfluenza Virus 1 | - | - | - | Negative |
| Parainfluenza Virus 2 | - | - | - | Negative |
| Parainfluenza Virus 3 | - | - | - | Negative |
| Parainfluenza Virus 4 | - | - | - | Negative |
| Respiratory Syncytial Virus | - | - | - | Negative |
| Bordetella parapertussis | - | - | - | Negative |
| Bordetella pertussis | - | - | - | Negative |
| Chlamydia pneumoniae | - | - | - | Negative |
| Mycoplasma pneumoniae | - | - | - | Negative |
| Borrelia burgdorferi | IgM positive | - | - | - |
| Anaplasma phagocytophilum | Positive IgG, IgM, and PCR | - | - | - |
| Borrelia miyamotoi, PCR, B | Negative | - | - | - |
| Ehrlichia chaffeensis | Negative | - | - | - |
| Ehrlichia ewingii/canis | Negative | - | - | - |
| Ehrlichia muris eauclairensis | Negative | - | - | - |
| Babesia divergens/MO-1 | Negative | - | - | - |
| Babesia duncani | Negative | - | - | - |
| Case Reference | Age | Sex | Country Year | Respiratory Symptoms | Imaging | Treatment (Duration in Days) | Appropriate Empiric Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 [18] | 76 | M | USA 2001 | Nonproductive Cough | Bilateral pleural effusions and patchy GGO | DOX (3) LEV(8) | Yes | Improved |
| 2 [17] | 47 | M | France 2003 | Cough | Bilateral interstitial infiltrates | Spiramycin Cefpodoxime (15) | No | Improved |
| 3 [21] | 77 | M | USA 2011 | Dyspnea Hypoxia | Left lung atelectasis, left pleural effusion | DOX (10) | No | Improved |
| 4 [20] | 62 | M | USA 2015 | Dyspnea | Bilateral patchy GGO, ARDS | DOX IV steroids | Yes | Improved |
| 5 [15] | 70 | F | USA 2020 | Cough Dyspnea | Bilateral GGO | DOX (10) | No | Improved |
| 6 [15] | 63 | F | USA 2020 | Cough Dyspnea | Right upper and middle lobe GGO | DOX (10) | No | Improved |
| 7 [15] | 64 | M | USA 2020 | Dyspnea Chest Pain | Peripheral GGO bilaterally | DOX (10) | No | Improved |
| 8 [15] | 78 | M | USA 2020 | Productive cough Dyspnea | Bilateral pleural effusion | Dox (21) | No | Improved |
| 9 [16] | 57 | M | Turkey 2022 | Cough Tachypnea | Bilateral opacities | DOX (18) | No | Improved |
| 10 [19] | 56 | F | USA 2022 | Dry Cough | Bilateral GGO and pleural effusions | DOX (10) | No | Improved |
| Patient from this report | 78 | F | USA 2020 | Cough Hypoxia | Bilateral infiltrates and bilateral pleural effusion | DOX (21) | Yes | Improved |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumic, I.; Person, E.; Igandan, O.; Adetimehin, O.; Nordstrom, C.W.; Williams, C.; Shweta, F. Anaplasma phagocytophilum Community-Acquired Pneumonia: Case Report and Literature Review. Microorganisms 2023, 11, 1483. https://doi.org/10.3390/microorganisms11061483
Dumic I, Person E, Igandan O, Adetimehin O, Nordstrom CW, Williams C, Shweta F. Anaplasma phagocytophilum Community-Acquired Pneumonia: Case Report and Literature Review. Microorganisms. 2023; 11(6):1483. https://doi.org/10.3390/microorganisms11061483
Chicago/Turabian StyleDumic, Igor, Emily Person, Oladapo Igandan, Omobolanle Adetimehin, Charles W. Nordstrom, Christopher Williams, and Fnu Shweta. 2023. "Anaplasma phagocytophilum Community-Acquired Pneumonia: Case Report and Literature Review" Microorganisms 11, no. 6: 1483. https://doi.org/10.3390/microorganisms11061483
APA StyleDumic, I., Person, E., Igandan, O., Adetimehin, O., Nordstrom, C. W., Williams, C., & Shweta, F. (2023). Anaplasma phagocytophilum Community-Acquired Pneumonia: Case Report and Literature Review. Microorganisms, 11(6), 1483. https://doi.org/10.3390/microorganisms11061483







