Evaluation of the In Vitro Antifungal Activity of Novel Arylsulfonamides against Candida spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Chemistry
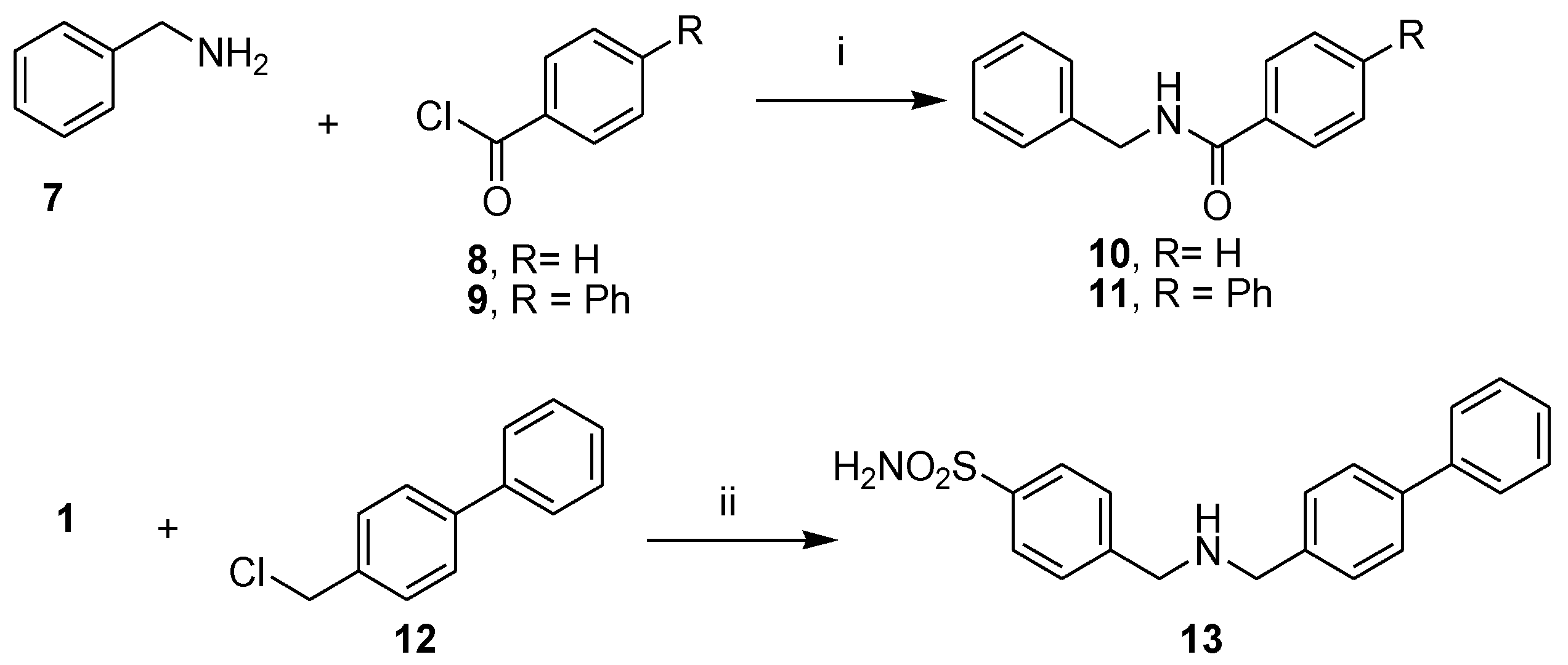
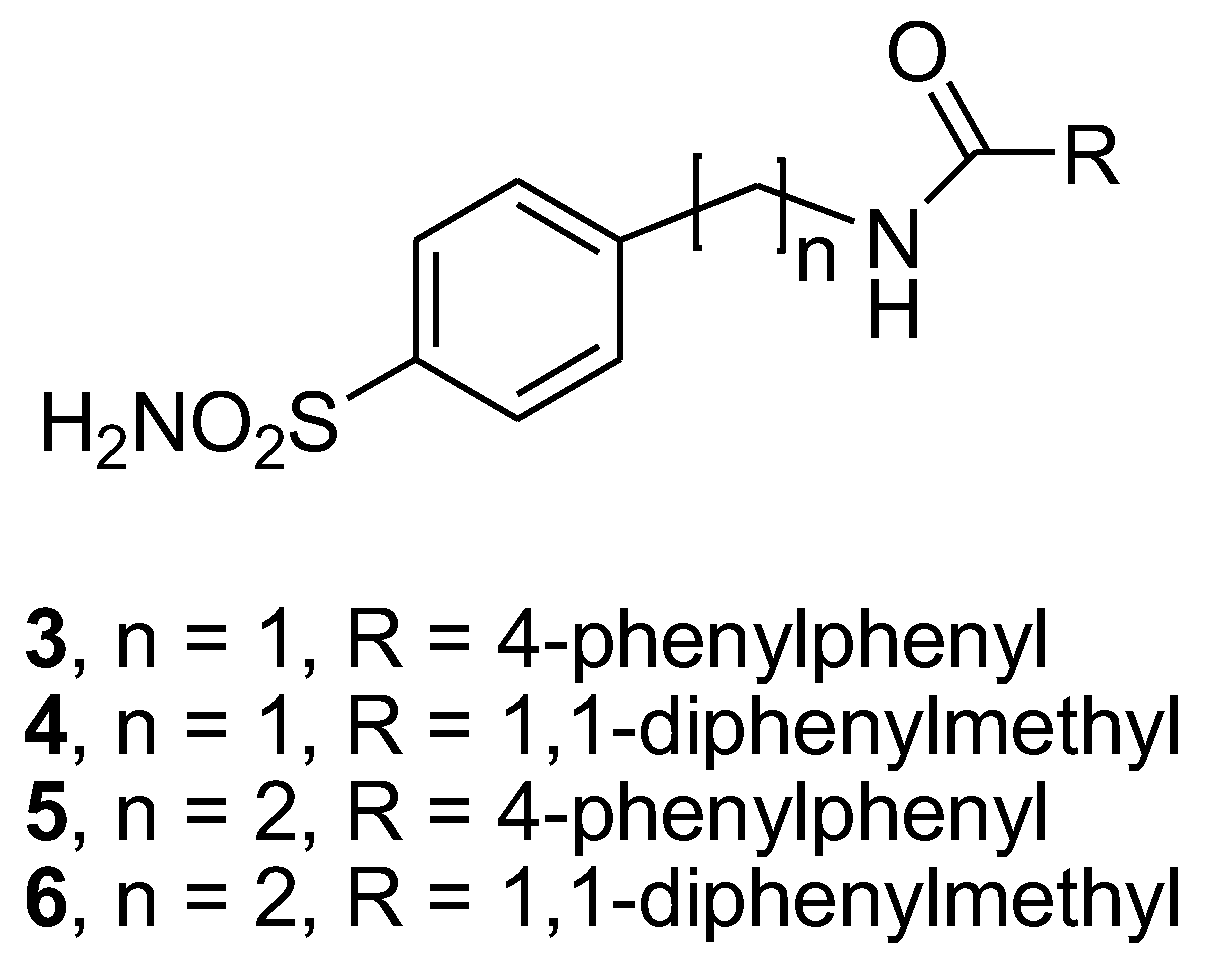
2.2. General Synthetic Procedure for Amide Derivatives 3–6, 10–11
N-benzylbenzamide (10)
N-benzylbiphenyl-4-carboxamide (11)
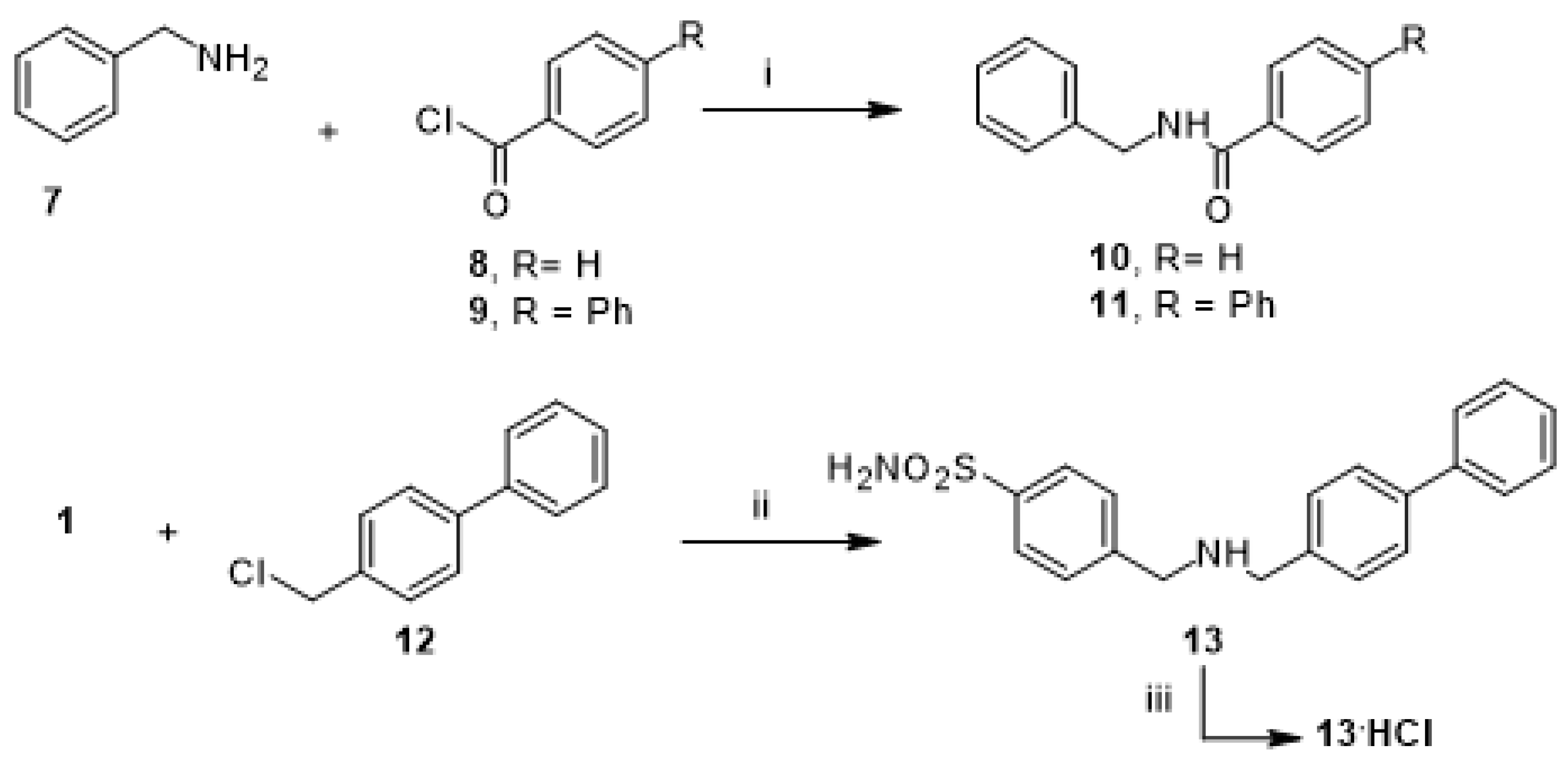
2.3. Synthesis of 4-[[(4-4-((biphenyl-4-ylmethylamino)methyl)benzenesulfonamide (13 and Its Hydrochloride 13.HCl)
4-[[(4-4-((Biphenyl-4-ylmethylamino)methyl)benzenesulfonamide (13)
4-[[(4-4-((Biphenyl-4-ylmethylamino)methyl)benzenesulfonamidehydrocholoride (13.HCl)
2.4. Microbial Strains and Culture Conditions
2.5. Susceptibility Studies
2.6. Evaluation of Cytotoxicity Assay
2.7. Statistical Analysis
3. Results
3.1. Chemistry
3.2. Antifungal Effects
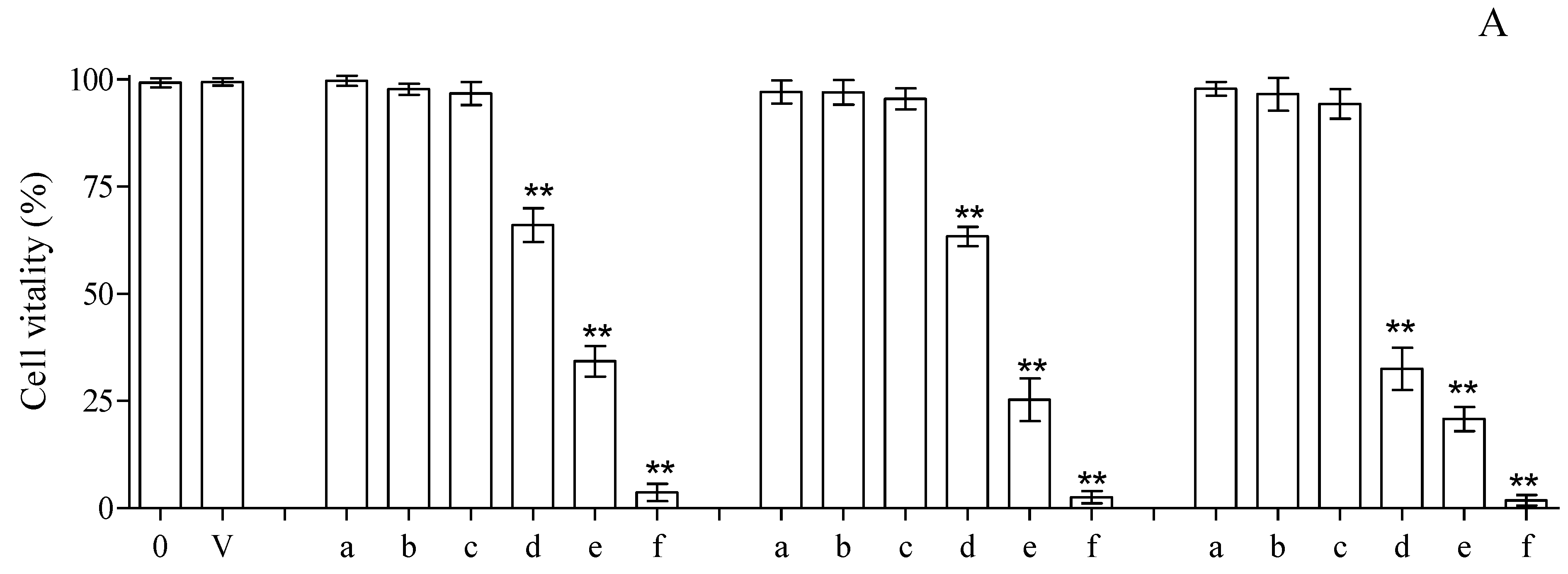
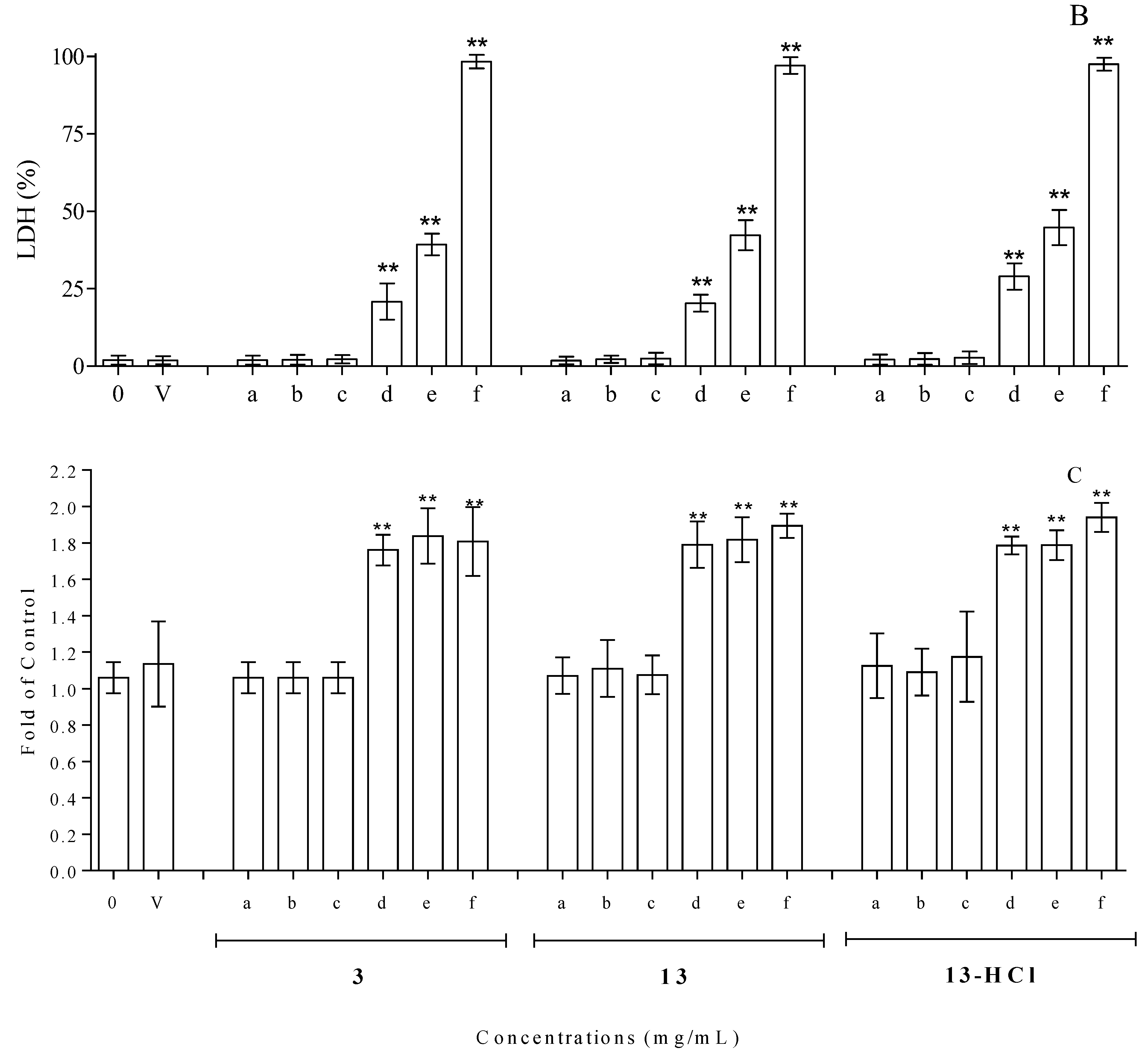
3.3. Cytotoxicity Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zida, A.; Bamba, S.; Yacouba, A.; Ouedraogo-Traore, R.; Guieguemdè, R.T. Anti-Candida albicans natural products: Sources of new antifungal drugs: A review. J. Mycol. Med. 2017, 27, 1–19. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, L.; Wang, S. Recent Progress in the Discovery of Antifungal Agents Targeting the Cell Wall. J. Med. Chem. 2020, 63, 12429–12459. [Google Scholar] [CrossRef] [PubMed]
- Fidel, P.L.; Vazquez, J.A.; Sobel, J.D. Candida glabrata: Review of Epidemiology, Pathogenesis, and Clinical Disease with Comparison to C. albicans. Clin. Microbiol. Rev. 1999, 12, 80–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, D.M.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 2002, 70, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trofa, D.; Gácser, A.; Nosanchuk, J.D. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 2008, 21, 606–625. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Muñoz, A.J.; Quindós, G.; Tur, C.; Ruesga, M.; Alonso, R.; Del Valle, O.; Rodriguez, V.; Arévalo, M.P.; Salgado, J.; Martin-Mazuelos, E.; et al. Comparative in vitro antifungal activity of amphotericin B lipid complex, amphotericin B and fluconazole. Chemotherapy 2000, 46, 235–244. [Google Scholar] [CrossRef]
- Lu, H.; Shrivastava, M.; Whiteway, M.; Jiang, Y. Candida albicans targets that potentially synergize with fluconazole. Crit. Rev. Microbiol. 2021, 47, 323–337. [Google Scholar] [CrossRef]
- Gervasi, T.; Ginestra, G.; Mancuso, F.; Barreca, D.; De Luca, L.; Mandalari, G. The in vitro Potential of 1-(1H-indol-3-yl) Derivatives against Candida spp. and Aspergillus niger as Tyrosinase Inhibitors. Microorganisms 2021, 9, 2070. [Google Scholar] [CrossRef]
- Flaherty, D.P.; Seleem, M.N.; Supuran, C.T. Bacterial carbonic anhydrases: Underexploited antibacterial therapeutic targets. Future Med. Chem. 2021, 13, 1619–1622. [Google Scholar] [CrossRef]
- Campestre, C.; De Luca, V.; Carradori, S.; Grande, R.; Carginale, V.; Scaloni, A.; Supuran, C.T.; Capasso, C. Carbonic Anhydrases: New Perspectives on Protein Functional Role and Inhibition in Helicobacter pylori. Front. Microbiol. 2021, 12, 629163. [Google Scholar] [CrossRef]
- Ginex, T.; Luque, F.J. Searching for effective antiviral small molecules against influenza A virus: A patent review. Expert Opin. Ther. Pat. 2021, 31, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin. Ther. Pat. 2018, 28, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Inhibition of Bacterial Carbonic Anhydrases as a Novel Approach to Escape Drug Resistance. Curr. Top. Med. Chem. 2017, 17, 1237–1248. [Google Scholar] [CrossRef] [Green Version]
- Supuran, C.T.; Capasso, C. A Highlight on the Inhibition of Fungal Carbonic Anhydrases as Drug Targets for the Antifungal Armamentarium. Int. J. Mol. Sci. 2021, 22, 4324. [Google Scholar] [CrossRef]
- De Luca, V.; Angeli, A.; Mazzone, V.; Adelfio, C.; Carginale, V.; Scaloni, A.; Carta, F.; Selleri, S.; Supuran, C.T.; Capasso, C. Heterologous expression and biochemical characterisation of the recombinant β-carbonic anhydrase (MpaCA) from the warm-blooded vertebrate pathogen malasseziapachydermatis. J. Enzyme Inhib. Med. Chem. 2022, 37, 62–68. [Google Scholar] [CrossRef]
- Annunziato, G.; Giovati, L.; Angeli, A.; Pavone, M.; Del Prete, S.; Pieroni, M.; Capasso, C.; Bruno, A.; Conti, S.; Magliani, W.; et al. Discovering a new class of antifungal agents thatselectivelyinhibitsmicrobialcarbonicanhydrases. J. Enzyme Inhib. Med. Chem. 2018, 33, 1537–1544. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, F.; De Luca, L.; Angeli, A.; Berrino, E.; Del Prete, S.; Capasso, C.; Supuran, C.T.; Gitto, R. In Silico-Guided Identification of New Potent Inhibitors of Carbonic Anhydrases Expressed in Vibrio cholerae. ACS Med. Chem. Lett. 2020, 11, 2294–2299. [Google Scholar] [CrossRef]
- Mancuso, F.; Angeli, A.; De Luca, V.; Bucolo, F.; De Luca, L.; Capasso, C.; Supuran, C.T.; Gitto, R. Synthesis and biological evaluation of sulfonamide-based compounds as inhibitors of carbonic anhydrase from Vibrio cholerae. Arch. Pharm. 2022, 355, e2200070. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, K.; Arisawa, M.; Shuto, S. A method for cleaving an allyl protecting group at the amide nitrogen of peptides by one-pot olefin isomerization-oxidation. J. Org. Chem. 2008, 73, 9494–9496. [Google Scholar] [CrossRef]
- Iqbal, N.; Cho, E.J. Visible-Light-Mediated Synthesis of Amides from Aldehydes and Amines via in Situ Acid Chloride Formation. J. Org. Chem. 2016, 81, 1905–1911. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. M27-A3. M27-A3, 4th ed.; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef] [Green Version]
- Barreca, D.; Currò, M.; Bellocco, E.; Ficarra, S.; Laganà, G.; Tellone, E.; Giunta, M.L.; Visalli, G.; Caccamo, D.; Galtieri, A.; et al. Neuroprotective effects of phloretin and its glycosylated derivative on rotenone-induced toxicity in human SH-SY5Y neuronal-like cells. BioFactors 2017, 43, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic content and biological properties of Avola almond (Prunus dulcis Mill. D.A. Webb) skin and its industrial byproducts. Ind. Crops Prod. 2016, 83, 283–293. [Google Scholar] [CrossRef]
- D’Arrigo, M.; Bisignano, C.; Irrera, P.; Smeriglio, A.; Zagami, R.; Trombetta, D.; Romeo, O.; Mandalari, G. In vitro evaluation of the activity of an essential oil from Pistacia vera L. variety Bronte hull against Candida sp. BMC Complement. Altern. Med. 2019, 19, 6. [Google Scholar] [CrossRef]
- Visalli, M.A.; Jacobs, M.R.; Appelbaum, P.C. Activities of three quinolones, alone and in combination with extended-spectrum cephalosporins or gentamicin, against Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 1998, 42, 2002–2005. [Google Scholar] [CrossRef] [Green Version]
- Timofeeva, L.M.; Kleshcheva, N.A.; Moroz, A.F.; Didenko, L.V. Secondary and tertiary polydiallylammonium salts: Novel polymers with high antimicrobial activity. Biomacromolecules 2009, 10, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Swenson, E.R. Carbonic anhydrase inhibitors and hypoxic pulmonary vasoconstriction. Respir. Physiol. Neurobiol. 2006, 151, 209–216. [Google Scholar] [CrossRef]
- Koltai, T.; Reshkin, S.J.; Harguindey, S. Chapter 7—Carbonic Anhydrases. In An Innovative Approach to Understanding and Treating Cancer: Targeting pH; Academic Press: Cambridge, MA, USA, 2020; pp. 157–176. ISBN 9780128190593. [Google Scholar]
- Isik, S.; Guler, O.O.; Kockar, F.; Aydin, M.; Arslan, O.; Supuran, C.T. Saccharomyces cerevisiae beta-carbonic anhydrase: Inhibition and activation studies. Curr. Pharm. Des. 2010, 16, 3327–3336. [Google Scholar] [CrossRef]
- Innocenti, A.; Leewattanapasuk, W.; Muhlschlegel, F.A.; Mastrolorenzo, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of the beta-class enzyme from the pathogenic yeast Candida glabrata with anions. Bioorg. Med. Chem. Lett. 2009, 19, 4802–4805. [Google Scholar] [CrossRef]
- Carta, F.; Osman, S.M.; Vullo, D.; AlOthman, Z.; Del Prete, S.; Capasso, C.; Supuran, C.T. Poly(amidoamine) dendrimers show carbonic anhydrase inhibitory activity against alpha-, beta, gamma- and eta-class enzymes. Bioorg. Med. Chem. 2015, 23, 6794–6798. [Google Scholar] [CrossRef]
- Akdemir, A.; Guzel-Akdemir, O.; Karali, N.; Supuran, C.T. Isatin analogs as novel inhibitors of Candida spp. beta-carbonic anhydrase enzymes. Bioorg. Med. Chem. 2016, 24, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Angeli, A.; Ghobril, C.; Hitce, J.; Clavaud, C.; Marat, X.; Supuran, C.T.; Capasso, C. Sulfonamide Inhibition Profile of the β-Carbonic Anhydrase from Malassezia restricta, An Opportunistic Pathogen Triggering Scalp Conditions. Metabolites 2020, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.W.; Lee, K.T.; Kim, S.; Lee, Y.R.; Kim, H.J.; Seo, K.J.; Lee, M.H.; Yeon, S.K.; Jang, B.K.; Park, S.J.; et al. Optimization and Evaluation of Novel Antifungal Agents for the Treatment of Fungal Infection. J. Med. Chem. 2021, 64, 15912–15935. [Google Scholar] [CrossRef] [PubMed]
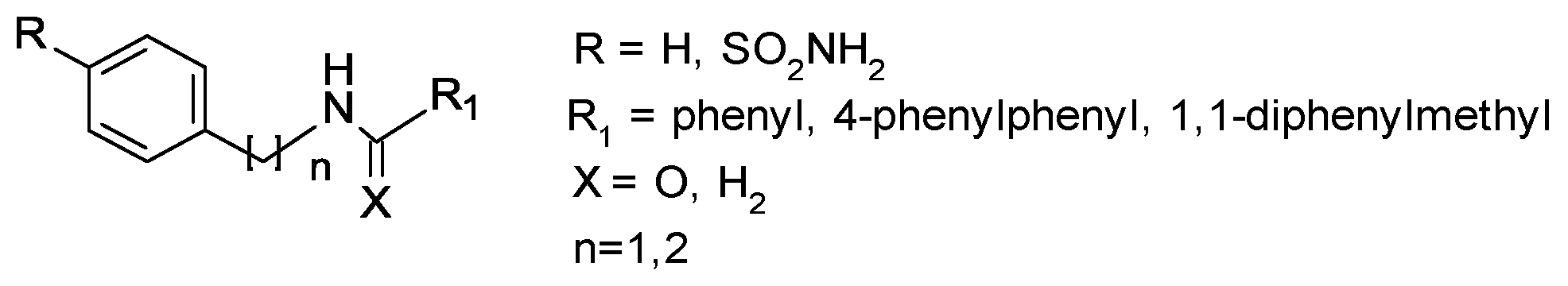

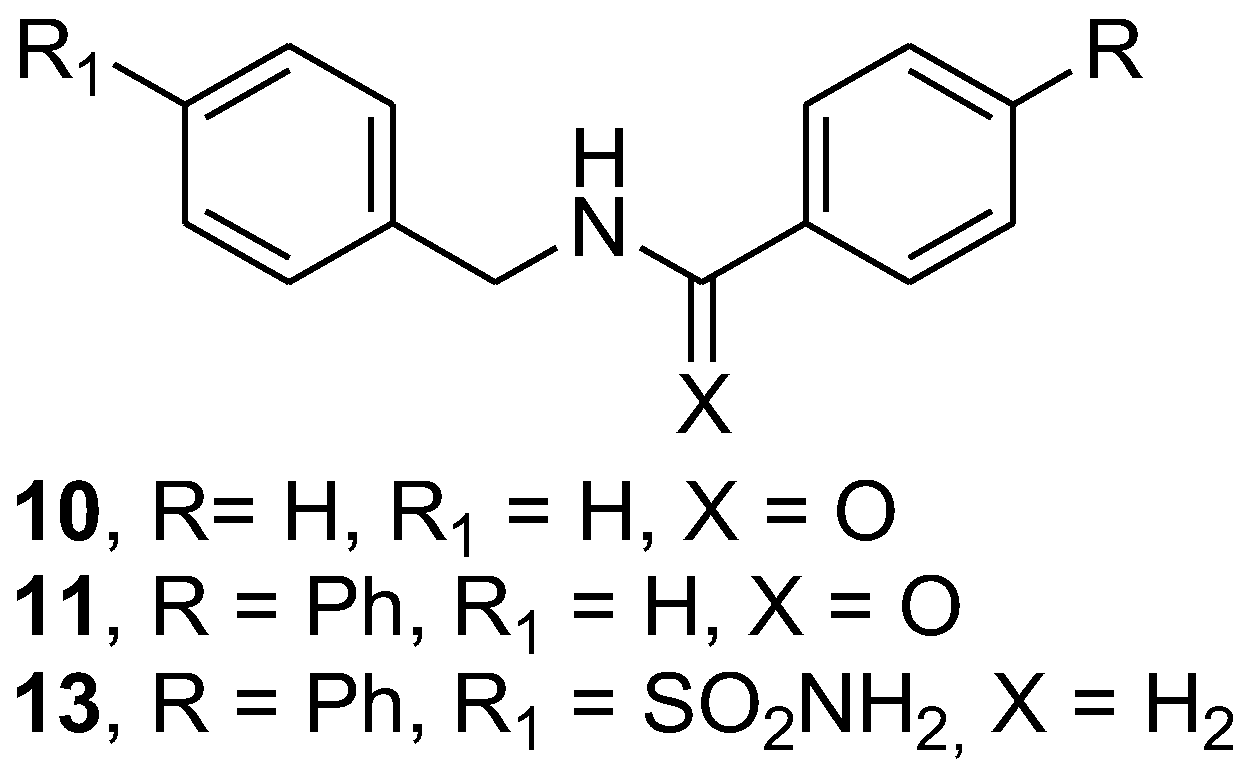
| Strain | 3 | 4 | 5 | 6 |
|---|---|---|---|---|
| Candida glabrata Strain 9 | >1.000 | >1.000 | >1.000 | >1.000 |
| Candida glabrata Strain 33 | 0.125–0.250 | >1.000 | >1.000 | >1.000 |
| Candida glabrata DSM 70614 | >1.000 | >1.000 | >1.000 | >1.000 |
| Candida parapsilosis Strain 30 | 0.250 | >1.000 | >1.000 | >1.000 |
| Candida parapsilosis Strain 34 | 0.500 | >1.000 | >1.000 | >1.000 |
| Candida parapsilosis ATCC 22019 | 0.125–0.250 | >1.000 | >1.000 | >1.000 |
| Candida albicans Strain 12 | 0.250 | >1.000 | >1.000 | >1.000 |
| Candida albicans Strain 13 | 0.250 | >1.000 | >1.000 | >1.000 |
| Candida albicans Strain 16 | 0.125–0.250 | >1.000 | >1.000 | >1.000 |
| Candida albicans ATCC 10231 | 0.250 | >1.000 | >1.000 | >1.000 |
| Strain | 3 | 10 | 11 | 13 | 13.HCl |
|---|---|---|---|---|---|
| Candida glabrata Strain 9 | >1.000 | >1.000 | >1.000 | >1.000 | >1.000 |
| Candida glabrata Strain 33 | 0.125–0.250 | >1.000 | >1.000 | 0.500 | 0.250 |
| Candida glabrata DSM 70614 | >1.000 | >1.000 | >1.000 | >1.000 | >1.000 |
| Candida parapsilosis Strain 30 | 0.250 | >1.000 | >1.000 | >1.000 | >1.000 |
| Candida parapsilosis Strain 34 | 0.500 | >1.000 | >1.000 | >1.000 | >1.000 |
| Candida parapsilosis ATCC 22019 | 0.125–0.250 | >1.000 | >1.000 | >1.000 | >1.000 |
| Candida albicans Strain 12 | 0.250 | >1.000 | >1.000 | >1.000 | 0.500 |
| Candida albicans Strain 13 | 0.250 | >1.000 | >1.000 | >1.000 | >1.000 |
| Candida albicans Strain 16 | 0.125–0.250 | >1.000 | >1.000 | 1.000 | 0.500–1.000 |
| Candida albicans ATCC 10231 | 0.250 | >1.000 | >1.000 | >1.000 | >1.000 |
| Mixture | C. glabrata 33 | C. albicans 16 | C. albicans 12 |
|---|---|---|---|
| Compound 3/Fluconazole | 2.00 (FIC A = 1.00, FIC B = 1.00) | 1.01 (FIC A = 0.0156, FIC B = 1.00) | 2.01 (FIC A = 0.0156, FIC B = 2.00) |
| Compound 13/Fluconazole | 1.01 (FIC A = 1.00, FIC B = 0.0078) | 1.12 (FIC A = 0.12, FIC B = 1.00) | 0.99 (FIC A = 0.49, FIC B = 0.50) |
| Compound 13.HCl/Fluconazole | 2.00 (FIC A = 1.00, FIC B = 1.00) | 0.75 (FIC A = 0.49, FIC B = 0.26) | 1.03 (FIC A = 0.0312, FIC B = 1.00) |
| Compound 3/Amphotericin B | 1.01 (FIC A = 1.00, FIC B = 1.0076) | 1.01 (FIC A = 0.0078, FIC B = 1.00) | 1.01 (FIC A = 0.0078, FIC B = 1.00) |
| Compound 13/Amphotericin B | 1.02 (FIC A = 1.00, FIC B = 0.0156) | 2.24 (FIC A = 2.00, FIC B = 0.24) | 1.50 (FIC A = 1.00, FIC B = 0.50) |
| Compound 13.HCl/Amphotericin B | 1.01 (FIC A = 1.00, FIC B = 0.0076) | 1.01 (FIC A = 0.0156, FIC B = 1.00) | 1.01 (FIC A = 0.0156, FIC B = 1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginestra, G.; Gervasi, T.; Mancuso, F.; Bucolo, F.; De Luca, L.; Gitto, R.; Barreca, D.; Mandalari, G. Evaluation of the In Vitro Antifungal Activity of Novel Arylsulfonamides against Candida spp. Microorganisms 2023, 11, 1522. https://doi.org/10.3390/microorganisms11061522
Ginestra G, Gervasi T, Mancuso F, Bucolo F, De Luca L, Gitto R, Barreca D, Mandalari G. Evaluation of the In Vitro Antifungal Activity of Novel Arylsulfonamides against Candida spp. Microorganisms. 2023; 11(6):1522. https://doi.org/10.3390/microorganisms11061522
Chicago/Turabian StyleGinestra, Giovanna, Teresa Gervasi, Francesca Mancuso, Federica Bucolo, Laura De Luca, Rosaria Gitto, Davide Barreca, and Giuseppina Mandalari. 2023. "Evaluation of the In Vitro Antifungal Activity of Novel Arylsulfonamides against Candida spp." Microorganisms 11, no. 6: 1522. https://doi.org/10.3390/microorganisms11061522






